The Use of Artificial Neural Networks and a General Discriminant Analysis for Predicting Culling Reasons in Holstein-Friesian Cows Based on First-Lactation Performance Records
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Data Splitting
2.3. Estimation of Wood’s Model Parameters
2.4. Data Editing
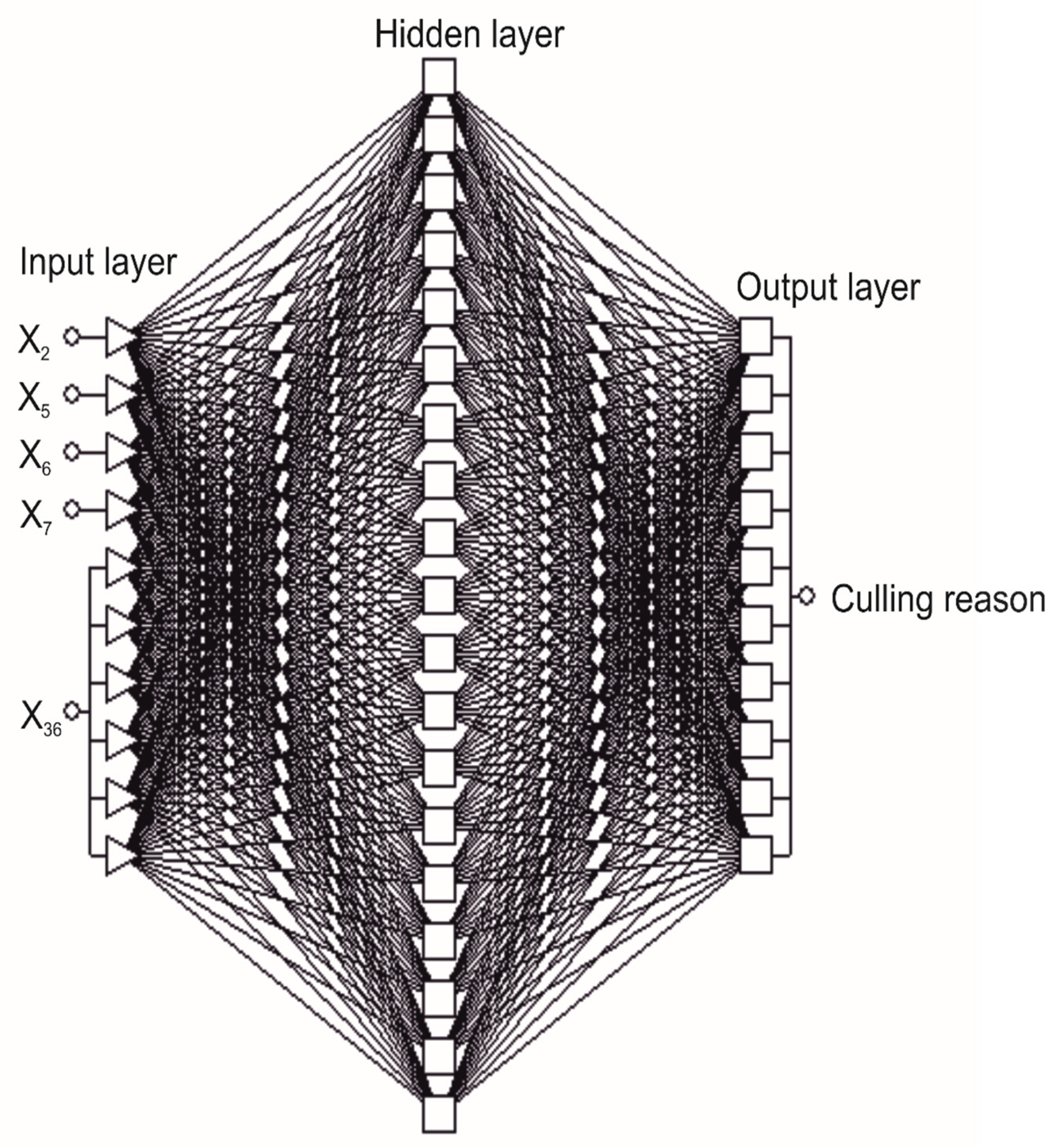
2.5. Neural Network Analysis
2.6. Training of the Neural Model with the Most Discriminative Predictors
2.7. Discriminant Analysis
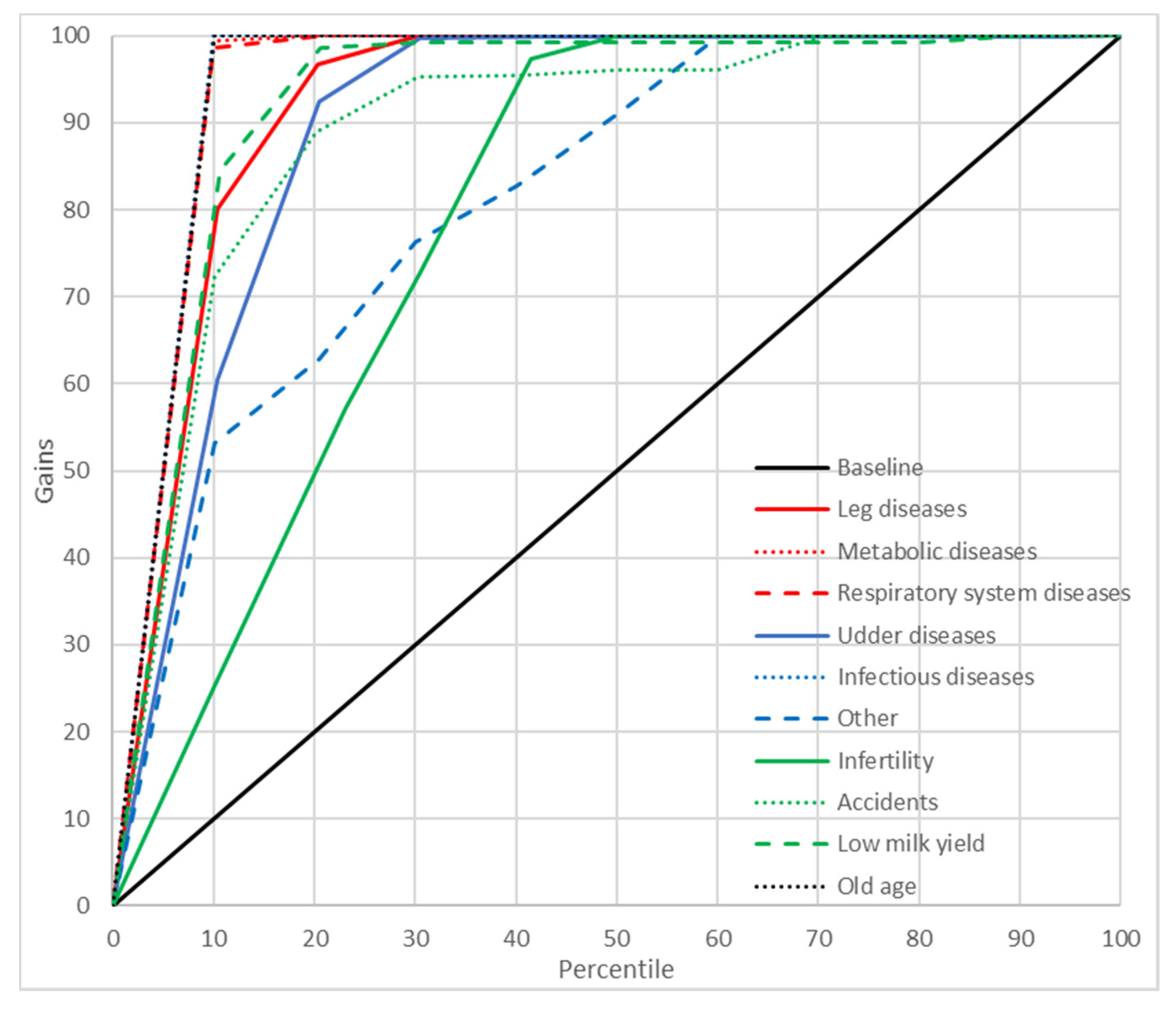
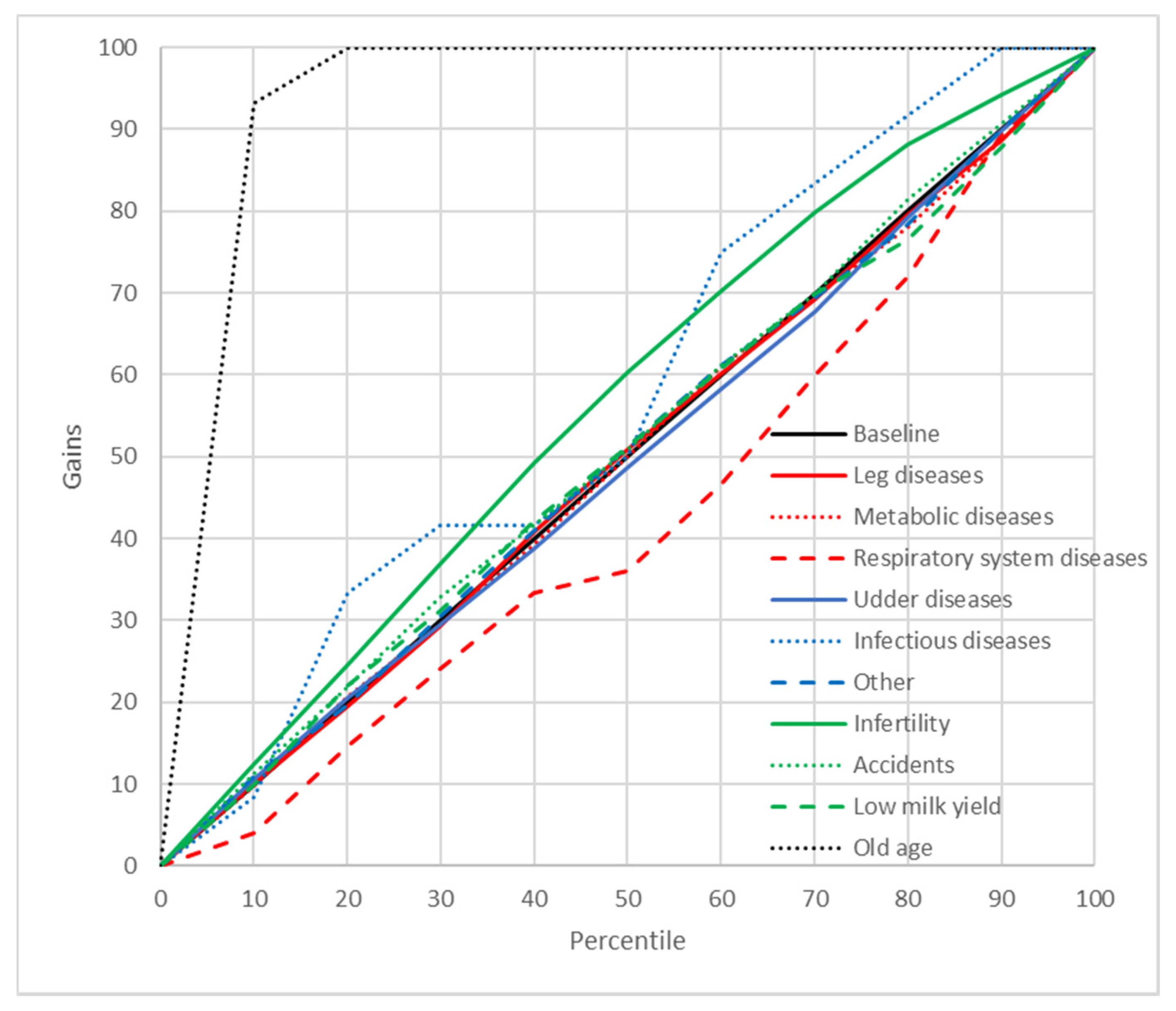
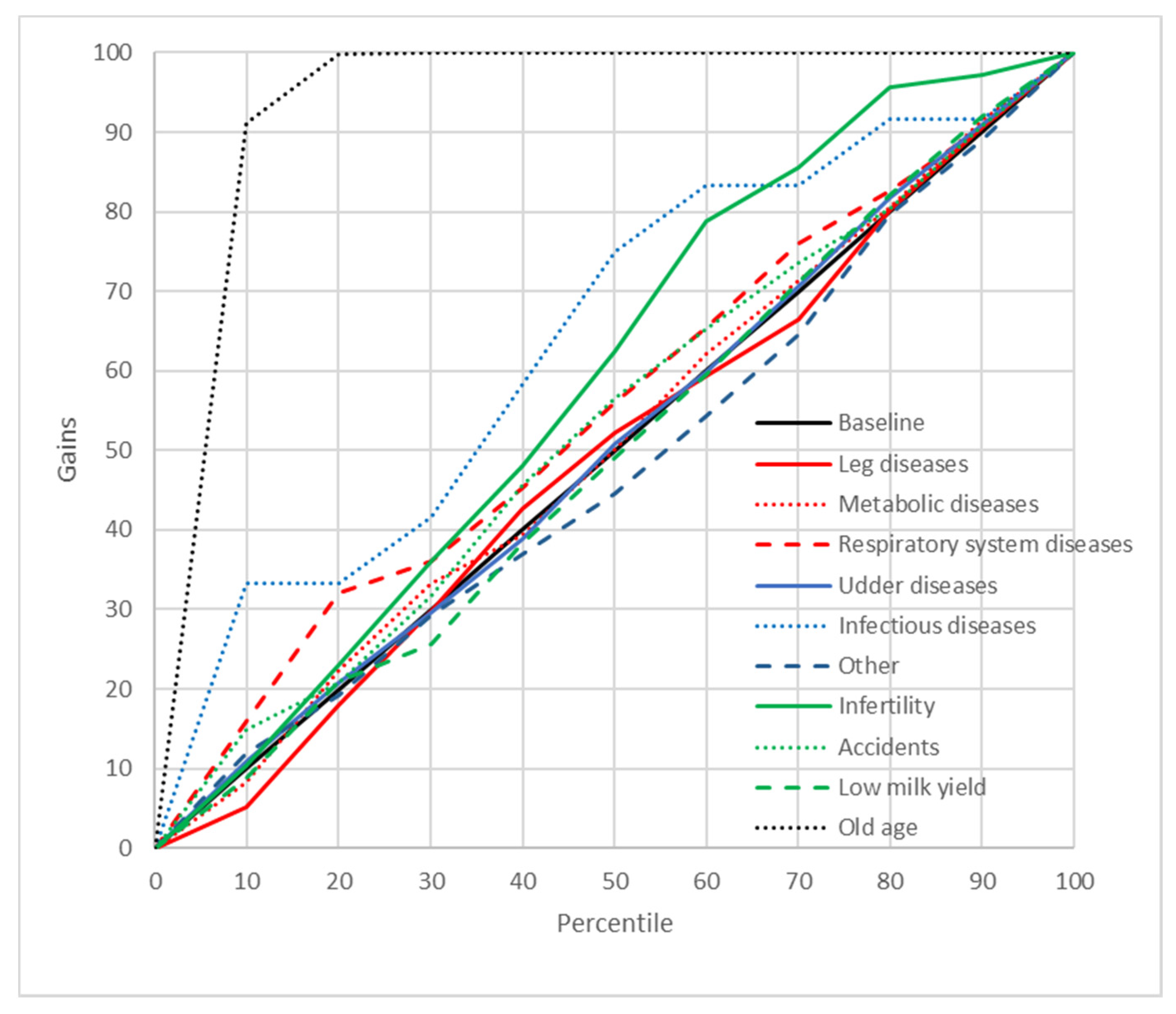
2.8. Gains Charts
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- De Vries, A. Cow longevity economics: The cost benefit of keeping the cow in the herd. In Proceedings of the “Cow Longevity Conference”, Hamra Farm/Tumba, Sweden, 28–29 August 2013; pp. 22–52. [Google Scholar]
- Brouwer, H.; Stegeman, J.A.; Straatsma, J.W.; Hooijer, G.A.; van Schaik, G. The validity of a monitoring system based on routinely collected dairy cattle health data relative to a standardized herd check. Prev. Vet. Med. 2015, 122, 76–82. [Google Scholar] [CrossRef]
- Congleton, W.R. Dairy cow culling decision. 3. Risk of culling on predicted income (an application of Bayes criterion) 1. J. Dairy Sci. 1988, 71, 1916–1925. [Google Scholar] [CrossRef]
- Weigel, K.A.; Lawlor, T.J., Jr.; VanRaden, P.M.; Wiggans, G.R. Use of linear type and production data to supplement early predicted transmitting abilities for productive life. J. Dairy Sci. 1998, 81, 2040–2044. [Google Scholar] [CrossRef]
- Caraviello, D.Z.; Weigel, K.A.; Gianola, D. Comparison between a Weibull proportional hazards model and a linear model for predicting the genetic merit of US Jersey sires for daughter longevity. J. Dairy Sci. 2004, 87, 1469–1476. [Google Scholar] [CrossRef]
- Caraviello, D.Z.; Weigel, K.A.; Gianola, D. Prediction of longevity breeding values for US Holstein sires using survival analysis methodology. J. Dairy Sci. 2004, 87, 3518–3525. [Google Scholar] [CrossRef]
- Roberts, T.; Chapinal, N.; LeBlanc, S.J.; Kelton, D.F.; Dubuc, J.; Duffield, T.F. Metabolic parameters in transition cows as indicators for early-lactation culling risk. J. Dairy Sci. 2012, 95, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.A.; LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic predictors of post-partum disease and culling risk in dairy cattle. Vet. J. 2011, 188, 216–220. [Google Scholar] [CrossRef]
- Szyda, J.; Morek-Kopeć, M.; Komisarek, J.; Żarnecki, A. Evaluating markers in selected genes for association with functional longevity of dairy cattle. BMC Genet. 2011, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Nordlund, K. The Transition cow needs space and comfort. In Proceedings of the “Cow Longevity Conference”, Hamra Farm/Tumba, Sweden, 28–29 August 2013; pp. 166–177. [Google Scholar]
- Dallago, G.M.; de Figueiredo, D.M.; de Resende Andrade, P.C.; dos Santos, R.A.; Lacroix, R.; Santschi, D.E.; Lefebvre, D.M. Predicting first test day milk yield of dairy heifers. Comput. Electron. Agric. 2019, 166, 105032. [Google Scholar] [CrossRef]
- Lacroix, R.; Salehi, F.; Yang, X.Z.; Wade, K.M. Effects of data preprocessing on the performance of artificial neural networks for dairy yield prediction and cow culling classification. Trans. ASAE 1997, 40, 839–846. [Google Scholar] [CrossRef]
- Adamczyk, K.; Zaborski, D.; Grzesiak, W.; Makulska, J.; Jagusiak, W. Recognition of culling reasons in Polish dairy cows using data mining methods. Comput. Electron. Agric. 2016, 127, 26–37. [Google Scholar] [CrossRef]
- Krug, C.; Haskell, M.J.; Nunes, T.; Stilwell, G. Creating a model to detect dairy cattle farms with poor welfare using a national database. Prev. Vet. Med. 2015, 122, 280–286. [Google Scholar] [CrossRef]
- Bach, A. Associations between several aspects of heifer development and dairy cow survivability to second lactation. J. Dairy Sci. 2011, 94, 1052–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrichs, A.J.; Zanton, G.I.; Lascano, G.J.; Jones, C.M. A 100-Year Review: A century of dairy heifer research. J. Dairy Sci. 2017, 100, 10173–10188. [Google Scholar] [CrossRef]
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal 2014, 8, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Lohakare, J.D.; Südekum, K.-H.; Pattanaik, A.K. Nutrition-induced changes of growth from birth to first calving and its impact on mammary development and first-lactation milk yield in dairy heifers: A review. Asian. Austral. J. Anim. 2012, 25, 1338–1350. [Google Scholar] [CrossRef] [Green Version]
- Wall, E.; Coffey, M.P.; Brotherstone, S. The relationship between body energy traits and production and fitness traits in first-lactation dairy cows. J. Dairy Sci. 2007, 90, 1527–1537. [Google Scholar] [CrossRef]
- Haworth, G.M.; Tranter, W.P.; Chuck, J.N.; Cheng, Z.; Wathes, D.C. Relationships between age at first calving and first lactation milk yield, and lifetime productivity and longevity in dairy cows. Vet. Rec. 2008, 162, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Jairath, L.K.; Hayes, J.F.; Cue, R.I. Correlations between first lactation and lifetime performance traits of Canadian Holsteins. J. Dairy Sci. 1995, 78, 438–448. [Google Scholar] [CrossRef]
- Janus, E.; Borkowska, D. Correlations between milk yield in primiparous PHF cows and selected lifetime performance and fertility indicators as well as reasons for culling. Acta Sci. Pol. Zootechnica 2012, 11, 23–32. [Google Scholar]
- Sawa, A.; Siatka, K.; Krężel-Czopek, S. Effect of age at first calving on first lactation milk yield, lifetime milk production and longevity of cows. Ann. Anim. Sci. 2019, 19, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Jeretina, J.; Babnik, D.; Škorjanc, D. Modeling lactation curve standards for test-day milk yield in Holstein, Brown Swiss and Simmental cows. J. Anim. Plant. Sci. 2013, 23, 754–762. [Google Scholar]
- Silvestre, A.M.; Martins, A.M.; Santos, V.A.; Ginja, M.M.; Colaço, J.A. Lactation curves for milk, fat and protein in dairy cows: A full approach. Livest. Sci. 2009, 122, 308–313. [Google Scholar] [CrossRef]
- Samarasinghe, S. Neural Networks for Applied Sciences and Engineering: From Fundamentals to Complex. Pattern Recognition; Auerbach: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-1306-1. [Google Scholar]
- Grzesiak, W.; Zaborski, D. Examples of the use of data mining methods in animal breeding. In Data Mining Applications in Engineering and Medicine; Karahoca, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 303–324. [Google Scholar] [CrossRef] [Green Version]
- Behkami, S.; Zain, S.M.; Gholami, M.; Khir, M.F.A. Classification of cow milk using artificial neural network developed from the spectral data of single-and three-detector spectrophotometers. Food Chem. 2019, 294, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Elfadl, E.A.A.; Abdallah, F.D. Using discriminant analysis and artificial neural network models for classification and prediction of fertility status of Friesian cattle. Am. J. Appl. Math. Stat. 2017, 5, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Fenlon, C.; O’Grady, L.; Mee, J.F.; Butler, S.T.; Doherty, M.L.; Dunnion, J. A comparison of 4 predictive models of calving assistance and difficulty in dairy heifers and cows. J. Dairy Sci. 2017, 100, 9746–9758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-H.; Lee, S.-H.; Cho, B.-K.; Wakholi, C.; Seo, Y.-W.; Cho, S.-H.; Kang, T.-H.; Lee, W.-H. Estimation of carcass weight of Hanwoo (Korean Native Cattle) as a function of body measurements using statistical models and a neural network. Asian-Australas. J. Anim. Sci. 2020, 33, 1633. [Google Scholar] [CrossRef] [Green Version]
- Hill, T.; Lewicki, P. Statistics: Methods and Applications; StatSoft: Tulsa, OK, USA, 2007; ISBN 1-884233-59-7. [Google Scholar]
- Maddala, G.S. Introduction to Econometrics, 3rd ed.; Wiley India Pvt. Limited: New Delhi, India, 2007; ISBN 978-81-265-1095-5. [Google Scholar]
- Zaborski, D.; Proskura, W.S.; Grzesiak, W. The Use of data mining methods for dystocia detection in Polish Holstein-Friesian Black-and-White Cattle. Asian-Australas. J. Anim. Sci. 2018, 31, 1700. [Google Scholar] [CrossRef] [Green Version]
- Sifre, L.; Berge, P.; Engel, E.; Martin, J.-F.; Bonny, J.-M.; Listrat, A.; Taylor, R.; Culioli, J. Influence of the spatial organization of the perimysium on beef tenderness. J. Agric. Food Chem. 2005, 53, 8390–8399. [Google Scholar] [CrossRef]
- Wood, P.D.P. Algebraic model of the lactation curve in cattle. Nature 1967, 216, 164. [Google Scholar] [CrossRef]
- Olori, V.E.; Brotherstone, S.; Hill, W.G.; McGuirk, B.J. Fit of standard models of the lactation curve to weekly records of milk production of cows in a single herd. Livest. Prod. Sci. 1999, 58, 55–63. [Google Scholar] [CrossRef]
- Nawi, N.M.; Ransing, M.R.; Ransing, R.S. An improved learning algorithm based on the Broyden-Fletcher-Goldfarb-Shanno (BFGS) method for back propagation neural networks. In Proceedings of the Sixth International Conference on Intelligent Systems Design and Applications, Jinan, China, 16–18 October 2006; Volume 1, pp. 152–157. [Google Scholar]
- Burez, J.; Van den Poel, D. Handling class imbalance in customer churn prediction. Expert Syst. Appl. 2009, 36, 4626–4636. [Google Scholar] [CrossRef] [Green Version]
- Ha, K.; Cho, S.; MacLachlan, D. Response models based on bagging neural networks. J. Interact. Mark. 2005, 19, 17–30. [Google Scholar] [CrossRef]
- Krpálková, L.; Cabrera, V.E.; Kvapilík, J.; Burdych, J.; Crump, P. Associations between age at first calving, rearing average daily weight gain, herd milk yield and dairy herd production, reproduction, and profitability. J. Dairy Sci. 2014, 97, 6573–6582. [Google Scholar] [CrossRef] [PubMed]
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-year review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef]
- Oltenacu, P.A.; Broom, D.M. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim. Welfare 2010, 19, 39–49. [Google Scholar]
- Vasseur, E. Animal Behavior and Well-Being Symposium: Optimizing outcome measures of welfare in dairy cattle assessment. J. Anim. Sci. 2017, 95, 1365–1371. [Google Scholar] [CrossRef]
- Wolf, C.A.; Tonsor, G.T.; McKendree, M.G.S.; Thomson, D.U.; Swanson, J.C. Public and farmer perceptions of dairy cattle welfare in the United States. J. Dairy Sci. 2016, 99, 5892–5903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, K.; Makulska, J.; Jagusiak, W.; Węglarz, A. Associations between strain, herd size, age at first calving, culling reason and lifetime performance characteristics in Holstein-Friesian cows. Animal 2017, 11, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudeau, F.; Ohlson, A.; Emanuelson, U. Associations between bovine coronavirus and bovine respiratory syncytial virus infections and animal performance in Swedish dairy herds. J. Dairy Sci. 2010, 93, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Van der Fels-Klerx, H.J.; Martin, S.W.; Nielen, M.; Huirne, R.B.M. Effects on productivity and risk factors of bovine respiratory disease in dairy heifers; a review for the Netherlands. NJAS-Wag. J. Life Sci. 2002, 50, 27–45. [Google Scholar] [CrossRef] [Green Version]
- Inchaisri, C.; Hogeveen, H.; Vos, P.; Van Der Weijden, G.C.; Jorritsma, R. Effect of milk yield characteristics, breed, and parity on success of the first insemination in Dutch dairy cows. J. Dairy Sci. 2010, 93, 5179–5187. [Google Scholar] [CrossRef] [PubMed]
- Rearte, R.; LeBlanc, S.J.; Corva, S.G.; de la Sota, R.L.; Lacau-Mengido, I.M.; Giuliodori, M.J. Effect of milk production on reproductive performance in dairy herds. J. Dairy Sci. 2018, 101, 7575–7584. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Marques, E.C.; Gilbert, R.O.; Bicalho, R.C. The association of plasma glucose, BHBA, and NEFA with postpartum uterine diseases, fertility, and milk production of Holstein dairy cows. Theriogenology 2017, 88, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Martinez, N.; Lima, F.S.; Staples, C.R.; Thatcher, W.W.; Santos, J.E.P. Influences of nutrition and metabolism on fertility of dairy cows. Anim. Reprod. 2012, 9, 260–272. [Google Scholar]
- Zebeli, Q.; Ghareeb, K.; Humer, E.; Metzler-Zebeli, B.U.; Besenfelder, U. Nutrition, rumen health and inflammation in the transition period and their role on overall health and fertility in dairy cows. Res. Vet. Sci. 2015, 103, 126–136. [Google Scholar] [CrossRef]
- Bicalho, R.C.; Oikonomou, G. Control and prevention of lameness associated with claw lesions in dairy cows. Livest. Sci. 2013, 156, 96–105. [Google Scholar] [CrossRef]
- Charfeddine, N.; Pérez-Cabal, M.A. Effect of claw disorders on milk production, fertility, and longevity, and their economic impact in Spanish Holstein cows. J. Dairy Sci. 2017, 100, 653–665. [Google Scholar] [CrossRef]
- Mottram, T. Animal board invited review: Precision livestock farming for dairy cows with a focus on oestrus detection. Animal 2016, 10, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Albaaj, A.; Foucras, G.; Raboisson, D. High somatic cell counts and changes in milk fat and protein contents around insemination are negatively associated with conception in dairy cows. Theriogenology 2017, 88, 18–27. [Google Scholar] [CrossRef]
- Hudson, C.D.; Bradley, A.J.; Breen, J.E.; Green, M.J. Associations between udder health and reproductive performance in United Kingdom dairy cows. J. Dairy Sci. 2012, 95, 3683–3697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, K.; Jagusiak, W.; Makulska, J. Analysis of lifetime performance and culling reasons in Black-and-White Holstein-Friesian cows compared with crossbreds. Ann. Anim. Sci. 2018, 18, 1061–1079. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Hooda, B.K. Prediction of milk production using artificial neural network. Curr. Adv. Agric. Sci. 2014, 6, 173–175. [Google Scholar] [CrossRef]
- Bhosale, M.D.; Singh, T.P. Development of lifetime milk yield equation using artificial neural network in Holstein Friesian crossbred dairy cattle and comparison with multiple linear regression model. Curr. Sci. 2017, 113, 951–955. [Google Scholar] [CrossRef]
- Ehret, A.; Hochstuhl, D.; Gianola, D.; Thaller, G. Application of neural networks with back-propagation to genome-enabled prediction of complex traits in Holstein-Friesian and German Fleckvieh cattle. Genet. Sel. Evol. 2015, 47, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Age Group | AFC | Culling Reason (R) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 (866) | R2 (2548) | R3 (16,923) | R4 (22,867) | R5 (42,566) | R6 (57,701) | R7 (379,582) | R8 (323,523) | R9 (51,520) | R10 (49,914) | Total (948,010) | ||
| 1 | 17–18 | 0 | 0 | 4 | 4 | 7 | 14 | 38 | 9 | 10 | 6 | 92 |
| 2 | 19 | 0 | 0 | 8 | 4 | 12 | 17 | 64 | 14 | 11 | 18 | 148 |
| 3 | 20 | 0 | 2 | 16 | 11 | 23 | 39 | 163 | 22 | 33 | 31 | 340 |
| 4 | 21 | 3 | 4 | 39 | 55 | 54 | 104 | 359 | 77 | 73 | 68 | 836 |
| 5 | 22 | 4 | 13 | 95 | 150 | 208 | 320 | 985 | 169 | 227 | 200 | 2371 |
| 6 | 23 | 10 | 37 | 204 | 313 | 475 | 674 | 2046 | 421 | 573 | 480 | 5233 |
| 7 | 24 | 12 | 49 | 267 | 443 | 697 | 907 | 2956 | 639 | 742 | 647 | 7359 |
| 8 | 25 | 13 | 49 | 256 | 416 | 698 | 892 | 2945 | 710 | 772 | 665 | 7416 |
| 9 | 26 | 7 | 56 | 226 | 369 | 619 | 758 | 2529 | 549 | 600 | 614 | 6327 |
| 10 | 27 | 10 | 30 | 171 | 282 | 537 | 637 | 2128 | 382 | 495 | 436 | 5108 |
| 11 | 28 | 2 | 29 | 147 | 214 | 405 | 538 | 1658 | 305 | 401 | 371 | 4070 |
| 12 | 29 | 4 | 15 | 98 | 190 | 348 | 462 | 1345 | 231 | 314 | 297 | 3304 |
| 13 | 30 | 2 | 14 | 78 | 129 | 274 | 369 | 1025 | 182 | 249 | 226 | 2548 |
| 14 | 31 | 4 | 17 | 63 | 106 | 201 | 270 | 787 | 152 | 193 | 177 | 1970 |
| 15 | 32 | 1 | 11 | 43 | 84 | 160 | 205 | 634 | 103 | 155 | 139 | 1535 |
| 16 | 33 | 0 | 5 | 56 | 66 | 131 | 138 | 507 | 82 | 126 | 118 | 1229 |
| 17 | 34 | 5 | 8 | 26 | 53 | 104 | 131 | 432 | 73 | 88 | 73 | 993 |
| Total | - | 77 | 339 | 1797 | 2889 | 4953 | 6475 | 20,601 | 4120 | 5062 | 4566 | 50,879 |
| Variable | Training Set (n = 33,071) | Validation Set (n = 7632) | Test Set (n = 10,176) | Total (n = 50,879) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| HERD (number of animals) | 192.68 | 290.44 | 195.97 | 295.83 | 191.88 | 265.77 | 193.02 | 291.07 |
| TD | 9.54 | 3.60 | 9.63 | 3.62 | 9.64 | 3.35 | 9.87 | 3.54 |
| AFC (months) | 26.28 | 3.09 | 26.26 | 3.06 | 26.24 | 3.32 | 26.27 | 3.09 |
| DIM (days) | 286.86 | 119.65 | 287.42 | 120.27 | 286.23 | 110.77 | 286.81 | 119.78 |
| a | 28.56 | 1.22 | 28.56 | 1.21 | 28.55 | 1.29 | 28.56 | 1.22 |
| b | 0.13 | 0.04 | 0.13 | 0.04 | 0.13 | 0.04 | 0.13 | 0.04 |
| c | 0.06 | 0.02 | 0.06 | 0.02 | 0.06 | 0.03 | 0.06 | 0.02 |
| MILK (kg) | 24.33 | 6.37 | 24.34 | 6.39 | 24.21 | 6.00 | 24.31 | 6.36 |
| MILKMIN (kg) | 17.76 | 6.63 | 17.64 | 6.65 | 17.65 | 5.96 | 17.72 | 6.61 |
| MILKMAX (kg) | 30.48 | 7.52 | 30.56 | 7.60 | 30.36 | 7.37 | 30.47 | 7.53 |
| MILKSD (kg) | 4.52 | 2.27 | 4.57 | 2.34 | 4.51 | 2.02 | 4.52 | 2.28 |
| FAT (%) | 4.12 | 0.59 | 4.12 | 0.59 | 4.12 | 0.54 | 4.12 | 0.58 |
| FATMIN (%) | 3.35 | 0.62 | 3.34 | 0.62 | 3.36 | 0.59 | 3.35 | 0.62 |
| FATMAX (%) | 5.09 | 1.00 | 5.09 | 0.99 | 5.07 | 0.97 | 5.08 | 0.99 |
| FATSD (%) | 0.61 | 0.36 | 0.61 | 0.35 | 0.60 | 0.28 | 0.61 | 0.35 |
| PROT (%) | 3.34 | 0.29 | 3.33 | 0.29 | 3.33 | 0.28 | 3.34 | 0.29 |
| PROTMIN (%) | 2.93 | 0.26 | 2.92 | 0.27 | 2.93 | 0.25 | 2.93 | 0.27 |
| PROTMAX (%) | 3.76 | 0.47 | 3.76 | 0.48 | 3.76 | 0.46 | 3.76 | 0.47 |
| PROTSD (%) | 0.30 | 0.15 | 0.30 | 0.15 | 0.30 | 0.14 | 0.30 | 0.15 |
| LACT (%) | 4.84 | 0.16 | 4.84 | 0.16 | 4.84 | 0.15 | 4.84 | 0.16 |
| LACTMIN (%) | 4.61 | 0.27 | 4.61 | 0.28 | 4.61 | 0.26 | 4.61 | 0.27 |
| LACTMAX (%) | 5.02 | 0.16 | 5.02 | 0.16 | 5.02 | 0.15 | 5.02 | 0.16 |
| LACTSD (%) | 0.14 | 0.09 | 0.14 | 0.09 | 0.14 | 0.07 | 0.14 | 0.09 |
| UREA (mg/L) | 223.64 | 60.62 | 222.63 | 60.79 | 223.66 | 60.81 | 223.49 | 61.39 |
| UREAMIN (mg/L) | 145.70 | 60.76 | 144.53 | 59.66 | 145.22 | 58.95 | 145.43 | 60.76 |
| UREAMAX (mg/L) | 312.24 | 89.55 | 310.87 | 94.24 | 313.17 | 90.49 | 312.22 | 91.89 |
| UREASD (mg/L) | 58.15 | 28.49 | 57.95 | 29.67 | 58.87 | 26.27 | 58.27 | 29.20 |
| SCC (thousands/mL) | 532.26 | 913.81 | 528.85 | 878.79 | 554.89 | 738.16 | 536.27 | 925.37 |
| SCCMIN (thousands/mL) | 89.94 | 262.47 | 92.64 | 250.65 | 98.53 | 152.69 | 92.06 | 277.86 |
| SCCMAX (thousands/mL) | 1836.30 | 3046.97 | 1824.38 | 2969.94 | 1890.08 | 2974.92 | 1845.27 | 3053.84 |
| SCCSD (thousands/mL) | 640.55 | 1161.20 | 632.64 | 1117.94 | 661.23 | 999.18 | 643.50 | 1160.49 |
| DMSR (%) | 13.01 | 0.73 | 13.00 | 0.74 | 13.00 | 0.71 | 13.01 | 0.73 |
| DMMIN (%) | 12.03 | 0.73 | 12.01 | 0.73 | 12.04 | 0.71 | 12.03 | 0.73 |
| DMMAX (%) | 14.14 | 1.17 | 14.14 | 1.16 | 14.14 | 1.14 | 14.14 | 1.16 |
| DMSD (%) | 0.75 | 0.39 | 0.75 | 0.38 | 0.74 | 0.33 | 0.74 | 0.39 |
| Variant | Training Set | Validation Set | Test Set | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Calving season | ||||||||
| Spring | 8593 | 26.0 | 1947 | 25.5 | 2634 | 25.9 | 13,174 | 25.9 |
| Summer | 7608 | 23.0 | 1730 | 22.7 | 2285 | 22.5 | 11,623 | 22.8 |
| Autumn | 8008 | 24.2 | 1873 | 24.5 | 2466 | 24.2 | 12,347 | 24.3 |
| Winter | 8862 | 26.8 | 2082 | 27.3 | 2791 | 27.4 | 13,735 | 27.0 |
| Calving difficulty | ||||||||
| Unassisted | 12,541 | 37.9 | 2885 | 37.8 | 3835 | 37.7 | 19,261 | 37.9 |
| Easy | 18,608 | 56.3 | 4293 | 56.3 | 5718 | 56.2 | 28,619 | 56.3 |
| Moderate | 1491 | 4.5 | 355 | 4.7 | 468 | 4.6 | 2314 | 4.6 |
| Difficult | 148 | 0.5 | 35 | 0.5 | 53 | 0.5 | 236 | 0.5 |
| Abortions | 251 | 0.8 | 58 | 0.8 | 87 | 0.9 | 396 | 0.8 |
| Caesarean | 32 | 0.1 | 6 | 0.1 | 15 | 0.2 | 53 | 0.1 |
| Culling reason (output variable) | ||||||||
| R1 | 51 | 0.2 | 14 | 0.2 | 12 | 0.1 | 77 | 0.2 |
| R2 | 217 | 0.7 | 47 | 0.6 | 75 | 0.7 | 339 | 0.7 |
| R3 | 1131 | 3.4 | 303 | 4.0 | 363 | 3.6 | 1797 | 3.5 |
| R4 | 1861 | 5.6 | 445 | 5.8 | 583 | 5.7 | 2889 | 5.7 |
| R5 | 3206 | 9.7 | 722 | 9.5 | 1025 | 10.1 | 4953 | 9.7 |
| R6 | 4221 | 12.8 | 940 | 12.3 | 1314 | 12.9 | 6475 | 12.7 |
| R7 | 13,460 | 40.7 | 3086 | 40.4 | 4055 | 39.9 | 20,601 | 40.5 |
| R8 | 2686 | 8.1 | 604 | 7.9 | 830 | 8.2 | 4120 | 8.1 |
| R9 | 3283 | 9.9 | 732 | 9.6 | 1047 | 10.3 | 5062 | 10.0 |
| R10 | 2955 | 8.9 | 739 | 9.7 | 872 | 8.6 | 4566 | 9.0 |
| Variable | a | b | AFC | c | CALV | DM | FAT | SEASON |
| Ratio | 188.901 | 113.527 | 45.863 | 34.940 | 8.870 | 1.316 | 1.284 | 1.112 |
| Rank | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Variable | LACT | SCCSD | PROT | DIM | TD | FATMAX | LACTSD | FATSD |
| Ratio | 1.112 | 1.093 | 1.069 | 1.056 | 1.052 | 1.049 | 1.049 | 1.043 |
| Rank | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Variable | SCC | MILK | UREAMAX | LACTMAX | PROTMAX | MILKMAX | DMMAX | DMSD |
| Ratio | 1.041 | 1.037 | 1.037 | 1.036 | 1.035 | 1.032 | 1.030 | 1.029 |
| Rank | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| Variable | PROTMIN | FATMIN | DMMIN | UREAMIN | UREASD | MILKMIN | PROTSD | LACTMIN |
| Ratio | 1.028 | 1.025 | 1.024 | 1.016 | 1.016 | 1.015 | 1.014 | 1.012 |
| Rank | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 |
| Variable | SCCMIN | MILKSD | SCCMAX | HERD | UREA | - | - | - |
| Ratio | 1.012 | 1.009 | 1.008 | 1.008 | 1.005 | - | - | - |
| Rank | 33 | 34 | 35 | 36 | 37 | - | - | - |
| Ranking | Number of Input Variables | Network Structure | Quality of the MLP [%] | ||
|---|---|---|---|---|---|
| Training Set | Validation Set | Test Set | |||
| 1 | 37 | 45-29-10 | 96.17 | 95.96 | 95.94 |
| 5 | 10-19-10 | 83.01 | 83.52 | 82.99 | |
| 2 | 37 | 45-27-10 | 90.42 | 89.70 | 89.74 |
| 5 | 10-20-10 | 79.07 | 79.42 | 78.46 | |
| 3 | 37 | 45-29-10 | 88.70 | 88.40 | 88.77 |
| 5 | 10-6-10 | 75.95 | 76.01 | 75.35 | |
| 4 | 37 | 45-24-10 | 86.96 | 86.87 | 86.56 |
| 5 | 10-20-10 | 74.39 | 74.38 | 74.14 | |
| 5 | 37 | 45-22-10 | 86.73 | 87.33 | 86.54 |
| 5 | 10-12-10 | 71.46 | 71.40 | 70.82 | |
| Predicted Culling Reason | No. of Input Variables | Observed Culling Reason | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | ||
| R1 | 37 | 11 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 5 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R2 | 37 | 1 | 68 | 2 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| 5 | 0 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R3 | 37 | 0 | 0 | 279 | 11 | 0 | 0 | 19 | 0 | 0 | 1 |
| 5 | 0 | 0 | 198 | 56 | 0 | 15 | 0 | 0 | 0 | 16 | |
| R4 | 37 | 0 | 3 | 50 | 567 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 68 | 515 | 0 | 0 | 0 | 2 | 0 | 0 | |
| R5 | 37 | 0 | 0 | 1 | 0 | 977 | 2 | 2 | 0 | 18 | 43 |
| 5 | 4 | 15 | 5 | 0 | 755 | 5 | 0 | 0 | 0 | 145 | |
| R6 | 37 | 0 | 1 | 18 | 0 | 0 | 1238 | 0 | 0 | 66 | 2 |
| 5 | 0 | 0 | 33 | 0 | 32 | 996 | 0 | 0 | 362 | 0 | |
| R7 | 37 | 0 | 0 | 8 | 4 | 14 | 26 | 4024 | 1 | 0 | 16 |
| 5 | 0 | 11 | 1 | 0 | 122 | 73 | 4054 | 0 | 86 | 214 | |
| R8 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 829 | 0 | 0 |
| 5 | 0 | 0 | 0 | 12 | 0 | 2 | 0 | 828 | 0 | 0 | |
| R9 | 37 | 0 | 3 | 4 | 0 | 2 | 26 | 2 | 0 | 961 | 1 |
| 5 | 0 | 0 | 6 | 0 | 5 | 215 | 1 | 0 | 599 | 53 | |
| R10 | 37 | 0 | 0 | 1 | 1 | 32 | 21 | 2 | 0 | 2 | 809 |
| 5 | 1 | 0 | 52 | 0 | 111 | 8 | 0 | 0 | 0 | 444 | |
| Culling Reason | n | MLP37 | MLP5 | GDA37 | GDA5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cor. | Incor. | PPV | Cor. | Incor. | PPV | Cor. | Incor. | PPV | Cor. | Incor. | PPV | |||
| R1 | 12 | 91.67 | 8.33 | 91.67 | 58.33 | 41.67 | 100.00 | 0.00 | 100.00 | 0.00 | 0.00 | 100.00 | 0.00 | |
| R2 | 75 | 90.67 | 9.33 | 88.31 | 65.33 | 34.67 | 100.00 | 6.67 | 93.33 | 83.33 | 1.33 | 98.67 | 100.00 | |
| R3 | 363 | 76.86 | 23.14 | 90.00 | 54.55 | 45.45 | 69.47 | 22.87 | 77.13 | 41.91 | 0.83 | 99.17 | 13.04 | |
| R4 | 583 | 97.26 | 2.74 | 91.45 | 88.34 | 11.66 | 88.03 | 88.16 | 11.84 | 70.99 | 90.05 | 9.95 | 66.37 | |
| R5 | 1025 | 95.32 | 4.68 | 93.67 | 73.66 | 26.34 | 81.27 | 32.20 | 67.80 | 46.61 | 3.22 | 96.78 | 76.74 | |
| R6 | 1314 | 94.22 | 5.78 | 93.43 | 75.80 | 24.20 | 69.99 | 37.98 | 62.02 | 47.89 | 4.19 | 95.81 | 20.44 | |
| R7 | 4055 | 99.24 | 0.76 | 98.31 | 99.98 | 0.02 | 88.88 | 91.39 | 8.61 | 58.91 | 97.63 | 2.37 | 49.81 | |
| R8 | 830 | 99.88 | 0.12 | 100.00 | 99.76 | 0.24 | 98.34 | 90.36 | 9.64 | 78.13 | 91.32 | 8.68 | 69.73 | |
| R9 | 1047 | 91.79 | 8.21 | 96.20 | 57.21 | 42.79 | 68.15 | 2.96 | 97.04 | 40.26 | 0.00 | 100.00 | 0.00 | |
| R10 | 872 | 92.78 | 7.22 | 93.20 | 50.92 | 49.08 | 72.08 | 4.82 | 95.18 | 28.00 | 0.00 | 100.00 | 0.00 | |
| Total | 10,176 | 95.94 | 4.06 | - | 82.99 | 17.01 | - | 58.57 | 41.43 | - | 52.41 | 47.59 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, K.; Grzesiak, W.; Zaborski, D. The Use of Artificial Neural Networks and a General Discriminant Analysis for Predicting Culling Reasons in Holstein-Friesian Cows Based on First-Lactation Performance Records. Animals 2021, 11, 721. https://doi.org/10.3390/ani11030721
Adamczyk K, Grzesiak W, Zaborski D. The Use of Artificial Neural Networks and a General Discriminant Analysis for Predicting Culling Reasons in Holstein-Friesian Cows Based on First-Lactation Performance Records. Animals. 2021; 11(3):721. https://doi.org/10.3390/ani11030721
Chicago/Turabian StyleAdamczyk, Krzysztof, Wilhelm Grzesiak, and Daniel Zaborski. 2021. "The Use of Artificial Neural Networks and a General Discriminant Analysis for Predicting Culling Reasons in Holstein-Friesian Cows Based on First-Lactation Performance Records" Animals 11, no. 3: 721. https://doi.org/10.3390/ani11030721






