Laterality in Responses to Acoustic Stimuli in Giant Pandas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
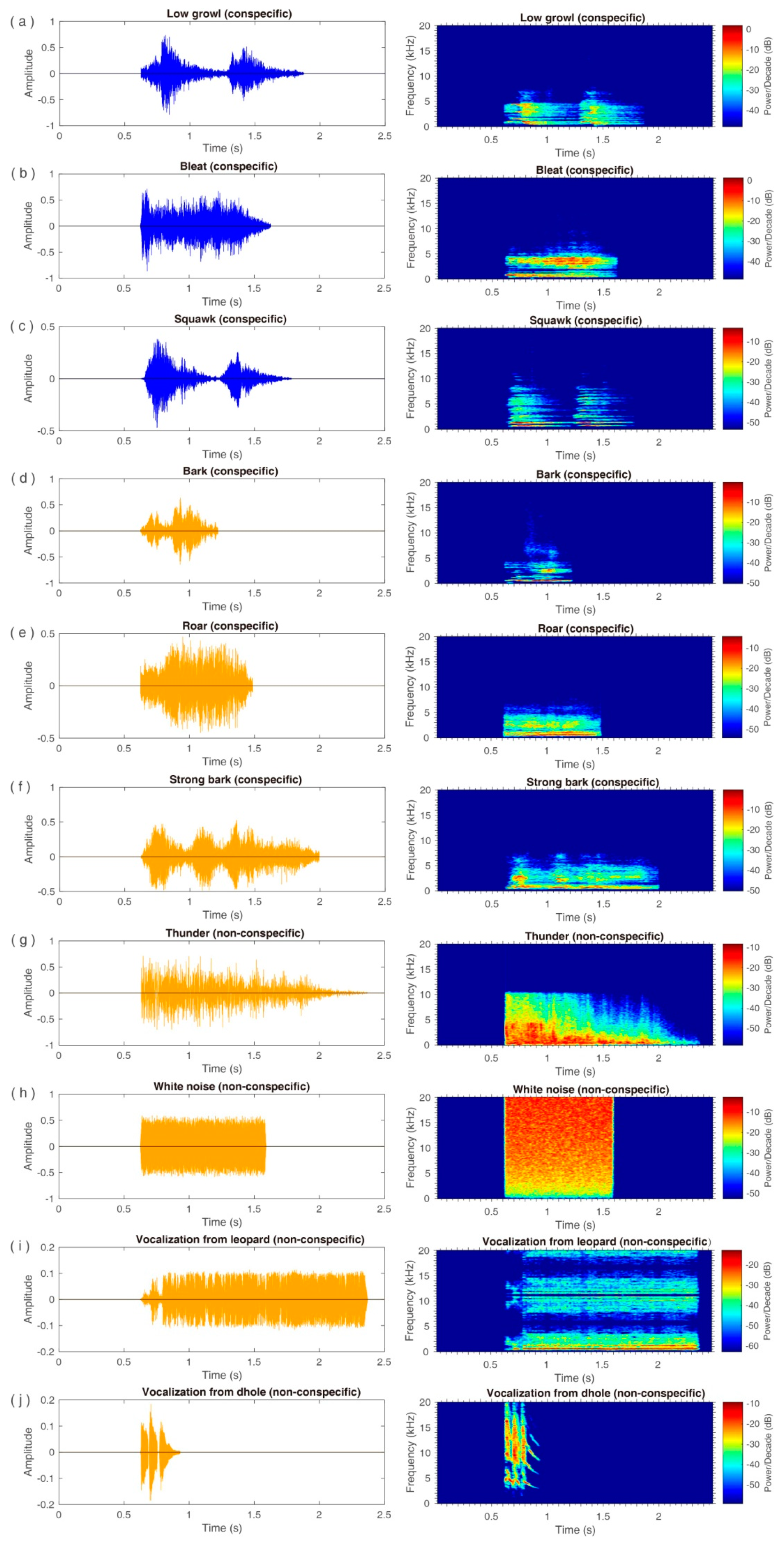
2.2. Auditory Stimuli
2.3. Experimental Procedures
2.4. Statistical Analyses
2.5. Ethics Approval
3. Results
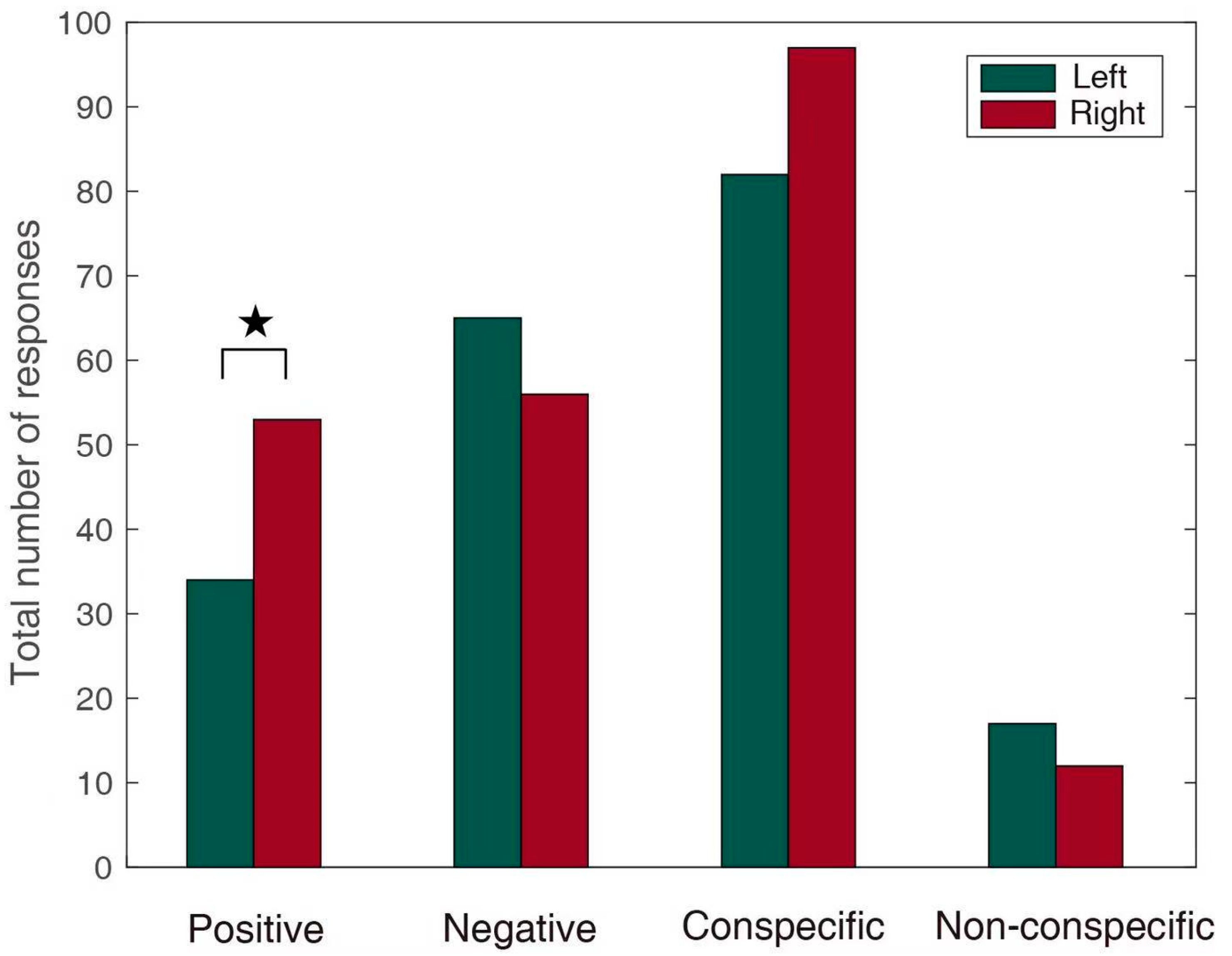
3.1. Emotional Effect
3.2. Conspecific and Non-Conspecific Effects
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugdahl, K. Hemispheric asymmetry: Contributions from brain imaging. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 2, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Vallortigara, G. When and Why Did Brains Break Symmetry? Symmetry 2015, 7, 2181–2194. [Google Scholar] [CrossRef] [Green Version]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain Lateralization: A Comparative Perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Padalino, B.; Aube, L.; Quaranta, A. Right-nostril use during sniffing at arousing stimuli produces higher cardiac activity in jumper horses. Laterality 2015, 20, 483–500. [Google Scholar] [CrossRef]
- Frasnelli, E.; Vallortigara, G.; Rogers, L.J. Left–right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef]
- Hausberger, M.; Cousillas, H.; Meter, A.; Karino, G.; George, I.; Lemasson, A.; Blois-Heulin, C. A Crucial Role of Attention in Lateralisation of Sound Processing? Symmetry 2019, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Bisazza, A.; Rogers, L.J.; Vallortigara, G. The Origins of Cerebral Asymmetry: A Review of Evidence of Behavioural and Brain Lateralization in Fishes, Reptiles and Amphibians. Neurosci. Biobehav. Rev. 1998, 22, 411–426. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nat. Cell Biol. 1987, 325, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.D.; Andersson, K. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: Field experiments. Proc. Natl. Acad. Sci. USA 1994, 91, 3946–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heffner, H.E.; Heffner, R.S. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science 1984, 236, 75–76. [Google Scholar] [CrossRef]
- Szymańska, J.; Trojan, M.; Jakucińska, A.; Wejchert, K.; Kapusta, M.; Sikorska, J. Brain Functional Asymmetry of Chimpanzees (Pan troglodytes): The Example of Auditory Laterality. Pol. Psychol. Bull. 2017, 48, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Gil-da-Costa, R.; Hauser, M.D. Vervet monkeys and humans show brain asymmetries for processing conspecific vocaliza-tions, but with opposite patterns of laterality. Proc. R. Soc. B 2006, 273, 2313–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, M.R.; Beecher, M.D.; Zoloth, S.R.; Moody, D.B.; Stebbins, W.C. Neural Lateralization of Species-Specific Vocaliza-tions by Japanese Macaques (Macaca fuscata). Science 1978, 202, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.; Boivin, S.; Boutin, A.; Blois-Heulin, C.; Hausberger, M.; Lemasson, A. Socially dependent auditory laterality in domestic horses (Equus caballus). Anim. Cogn. 2009, 12, 611–619. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Quaranta, A.; Rogers, L.J. Hemispheric Specialization in Dogs for Processing Different Acoustic Stimuli. PLoS ONE 2008, 3, e3349. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Laddago, S.; Quaranta, A. Auditory lateralization of conspecific and heterospecific vocalizations in cats. Laterality 2015, 21, 1–13. [Google Scholar] [CrossRef]
- Gainotti, G. Emotional Behavior and Hemispheric Side of the Lesion. Cortex 1972, 8, 41–55. [Google Scholar] [CrossRef]
- Gainotti, G. Unconscious processing of emotions and the right hemisphere. Neuropsychology 2012, 50, 205–218. [Google Scholar] [CrossRef]
- Calvo, M.G.; Rodríguez-Chinea, S.; Fernández-Martín, A. Lateralized discrimination of emotional scenes in peripheral vision. Exp. Brain Res. 2015, 233, 997–1006. [Google Scholar] [CrossRef]
- Scheumann, M.; Zimmermann, E. Sex-specific asymmetries in communication sound perception are not related to hand preference in an early primate. BMC Biol. 2008, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leliveld, L.M.; Langbein, J.; Puppe, B. The emergence of emotional lateralization: Evidence in non-human vertebrates and implications for farm animals. Appl. Anim. Behav. Sci. 2013, 145, 1–14. [Google Scholar] [CrossRef]
- Najt, P.; Bayer, U.; Hausmann, M. Models of hemispheric specialization in facial emotion perception—A reevaluation. Emotion 2013, 13, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Fourie, B.; Berezina, E.; Giljov, A.; Karenina, K. Visual lateralization in artiodactyls: A brief summary of research and new evidence on saiga antelope. Laterality 2020, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bryden, M.P.; Ley, R.G. Right hemisphere involvement in imagery and affect. In Cognitive Processing in the Right Hemisphere; Perecman, E., Ed.; Academic Press: New York, NY, USA, 1983; pp. 111–123. [Google Scholar]
- Hagemann, D.; Hewig, J.; Naumann, E.; Seifert, J.; Bartussek, D. Resting brain asymmetry and affective reactivity: Aggregated data support the right-hemisphere hypothesis. J. Individ. Differ. 2005, 26, 139–154. [Google Scholar] [CrossRef]
- Kumfor, F.; Landinromero, R.; Devenney, E.; Hutchings, R.; Grasso, R.; Hodges, J.R.; Piguet, O. On the right side? A longitu-dinal study of left-versus right-lateralized semantic dementia. Brain 2016, 139, 986–998. [Google Scholar] [CrossRef] [Green Version]
- Baciadonna, L.; Nawroth, C.; Briefer, E.F.; McElligott, A.G. Perceptual lateralization of vocal stimuli in goats. Curr. Zool. 2018, 65, 67–74. [Google Scholar] [CrossRef]
- Davidson, R.J. Cerebral asymmetry and emotion: Conceptual and methodological conundrums. Cogn. Emot. 1993, 7, 115–138. [Google Scholar] [CrossRef]
- Rodway, P.; Wright, L.; Hardie, S. The valence-specific laterality effect in free viewing conditions: The influence of sex, handedness, and response bias. Brain Cogn. 2003, 53, 452–463. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Ross, E.D.; Homan, R.W.; Buck, R. Differential hemispheric lateralization of primary social emotions: Implications for de-veloping a comprehensive neurology for emotions, repression, and the subconscious. Cogn. Behav. Neurol. 1994, 7, 1–19. [Google Scholar]
- George, I.; Cousillas, H.; Richard, J.-P.; Hausberger, M. State-dependent hemispheric specialization in the songbird brain. J. Comp. Neurol. 2005, 488, 48–60. [Google Scholar] [CrossRef]
- Lemasson, A.; Koda, H.; Kato, A.; Oyakawa, C.; Blois-Heulin, C.; Masataka, N. Influence of sound specificity and familiarity on Japanese macaques’ (Macaca fuscata) auditory laterality. Behav. Brain Res. 2010, 208, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Charlton, B.D.; Owen, M.A.; Keating, J.L.; Martin-Wintle, M.S.; Zhang, H.; Swaisgood, R.R. Sound transmission in a bamboo forest and its implications for information transfer in giant panda (Ailuropoda melanoleuca) bleats. Sci. Rep. 2018, 8, 12754. [Google Scholar] [CrossRef]
- Charlton, B.D.; Zhihe, Z.; Snyder, R.J. Vocal cues to identity and relatedness in giant pandas (Ailuropoda melanoleuca). J. Acoust. Soc. Am. 2009, 126, 2721–2732. [Google Scholar] [CrossRef]
- Charlton, B.D.; Keating, J.L.; Rengui, L.; Huang, Y.; Swaisgood, R.R. Female giant panda (Ailuropoda melanoleuca) chirps advertise the caller’s fertile phase. Proc. R. Soc. B Boil. Sci. 2009, 277, 1101–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlton, B.D.; Keating, J.L.; Kersey, D.; Rengui, L.; Huang, Y.; Swaisgood, R.R. Vocal cues to male androgen levels in giant pandas. Biol. Lett. 2010, 7, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlton, B.D.; Swaisgood, R.R.; Zhihe, Z.; Snyder, R.J. Giant pandas attend to androgen-related variation in male bleats. Behav. Ecol. Sociobiol. 2012, 66, 969–974. [Google Scholar] [CrossRef]
- Wei, F.; Hu, Y.; Yan, L.; Nie, Y.; Wu, Q.; Zhang, Z. Giant Pandas Are Not an Evolutionary culdesac: Evidence from Multi-disciplinary Research. Mol. Biol. Evol. 2014, 32, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.Y. The Vocal Communication of Captive Giant Panda (Ailuropoda melanoleuca) and Its Significance on Ecology. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2015. (In Chinese). [Google Scholar]
- McGregor, P.K. Playback experiments: Design and analysis. Acta Ethologica 2000, 3, 3–8. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS for Windows (Version 15); Open University Press: Maidenhead, UK, 2007. [Google Scholar]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the Left & Right Brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef]
- Xue, F.; Fang, G.; Yang, P.; Zhao, E.; Brauth, S.E.; Tang, Y. The biological significance of acoustic stimuli determines ear preference in the music frog. J. Exp. Biol. 2015, 218, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Frasnelli, E.; Vallortigara, G. Individual-Level and Population-Level Lateralization: Two Sides of the Same Coin. Symmetry 2018, 10, 739. [Google Scholar] [CrossRef]
- Ströckens, F.; Güntürkün, O.; Ocklenburg, S. Limb preferences in non-human vertebrates. Laterality 2012, 18, 536–575. [Google Scholar] [CrossRef]
- Reimchen, T.E.; Spoljaric, M.A. Right paw foraging bias in wild black bear (Ursus americanus kermodei). Laterality 2010, 16, 471–478. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wei, Z.F.; Tian, C.H.; Zhang, Y.; Wei, R.P.; Zhang, G.Q.; Liu, D.Z. A study on hand preference of foraging in giant pandas. Bull. Biol. 2012, 47, 51–55. (In Chinese) [Google Scholar]
- Rogers, L.J.; Anson, J.M. Lateralization of function in the chicken forebrain. Pharmacol. Biochem. Behav. 1979, 10, 679–686. [Google Scholar] [CrossRef]
- Sion, G.; Tal, R.; Meiri, S. Asymmetric Behavior in Ptyodactylus guttatus: Can a Digit Ratio Reflect Brain Laterality? Symmetry 2020, 12, 1490. [Google Scholar] [CrossRef]
- Gainotti, G. Emotions and the Right Hemisphere: Can New Data Clarify Old Models? Neuroscientist 2018, 25, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Yurgelun-Todd, D.A. The right-hemisphere and valence hypotheses: Could they both be right (and some-times left)? Soc. Cogn. Affect Neurosci. 2007, 2, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; D’Ingeo, S.; Fornelli, S.; Quaranta, A. Lateralized behavior and cardiac activity of dogs in response to human emotional vocalizations. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stancher, G.; Anna, S.V.; Vallortigara, G. Chapter 2—Motor Asymmetries in Fishes, Amphibians, and Reptiles Progress in Brain Research; Elsevier Academic Press: Amsterdam, The Netherlands, 2018; Volume 238, pp. 33–56. [Google Scholar]
- Austin, N.; Rogers, L. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Lippolis, G.; Westman, W.; McAllan, B.M.; Rogers, L.J. Lateralisation of escape responses in the stripe-faced dunnart, Sminthopsis macroura (Dasyuridae: Marsupialia). Laterality 2005, 10, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Bonati, B.; Csermely, D.; López, P.; Martín, J. Lateralization in the escape behaviour of the common wall lizard (Podarcis muralis). Behav. Brain Res. 2010, 207, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teufel, C.; Hammerschmidt, K.; Fischer, J. Lack of orienting asymmetries in Barbary macaques: Implications for studies of lateralized auditory processing. Anim. Behav. 2007, 73, 249–255. [Google Scholar] [CrossRef]
- Böye, M.; Güntürkün, O.; Vauclair, J. Right ear advantage for conspecific calls in adults and subadults, but not infants, Cali-fornia sea lions (Zalophus californianus): Hemispheric specialization for communication? Eur. J. Neurosci. 2005, 21, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Heffner, E.H.; Heffner, R.S.; Hefner, H.E. Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. J. Neurophysiol. 1986, 56, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Cynx, J.; Williams, H.; Nottebohm, F. Hemispheric differences in avian song discrimination. Proc. Natl. Acad. Sci. USA 1992, 89, 1372–1375. [Google Scholar] [CrossRef] [Green Version]
- Giljov, A.; Malashichev, Y.; Karenina, K. What do wild saiga antelopes tell us about the relative roles of the two brain hemispheres in social interactions? Anim. Cogn. 2019, 22, 635–643. [Google Scholar] [CrossRef]


| Name | Sex | Age |
|---|---|---|
| DADI | ♂ | Adult |
| JINI | ♀ | Adult |
| GUGU | ♂ | Adult |
| FULU | ♀ | Juvenile |
| MENGDA | ♂ | Juvenile |
| MENGER | ♂ | Juvenile |
| DIANDIAN | ♀ | Juvenile |
| MENGLAN | ♂ | Juvenile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Tang, Y.; Ni, Y.; Fang, G. Laterality in Responses to Acoustic Stimuli in Giant Pandas. Animals 2021, 11, 774. https://doi.org/10.3390/ani11030774
Liu H, Tang Y, Ni Y, Fang G. Laterality in Responses to Acoustic Stimuli in Giant Pandas. Animals. 2021; 11(3):774. https://doi.org/10.3390/ani11030774
Chicago/Turabian StyleLiu, He, Yezhong Tang, Yanxia Ni, and Guangzhan Fang. 2021. "Laterality in Responses to Acoustic Stimuli in Giant Pandas" Animals 11, no. 3: 774. https://doi.org/10.3390/ani11030774







