Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry, and Experimental Design
2.2. Skeletal Muscle Sampling
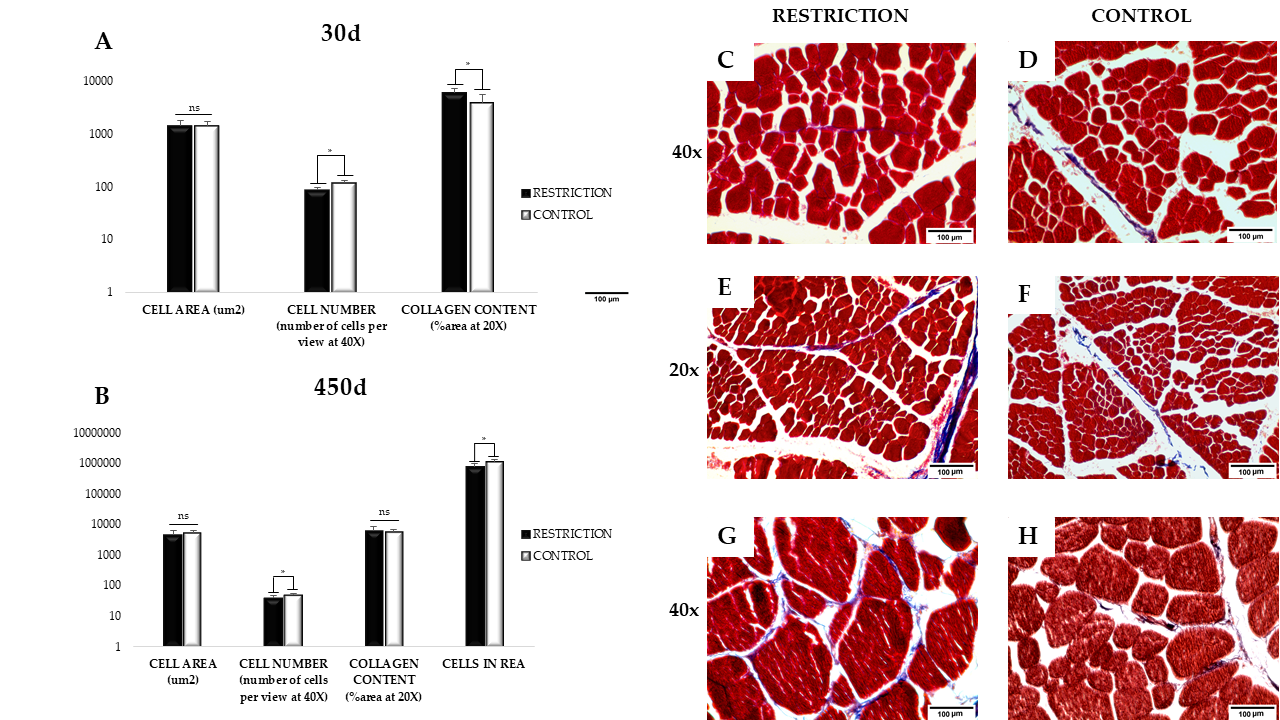
2.3. Morphometric Evaluation by Histochemical and Image Analysis
2.4. Total RNA Extraction and mRNA Expression Analysis
2.5. Statistical Analyses
3. Results
3.1. Maternal Intake and Performance
3.2. Muscle Cell Area, Number, and Collagen Content
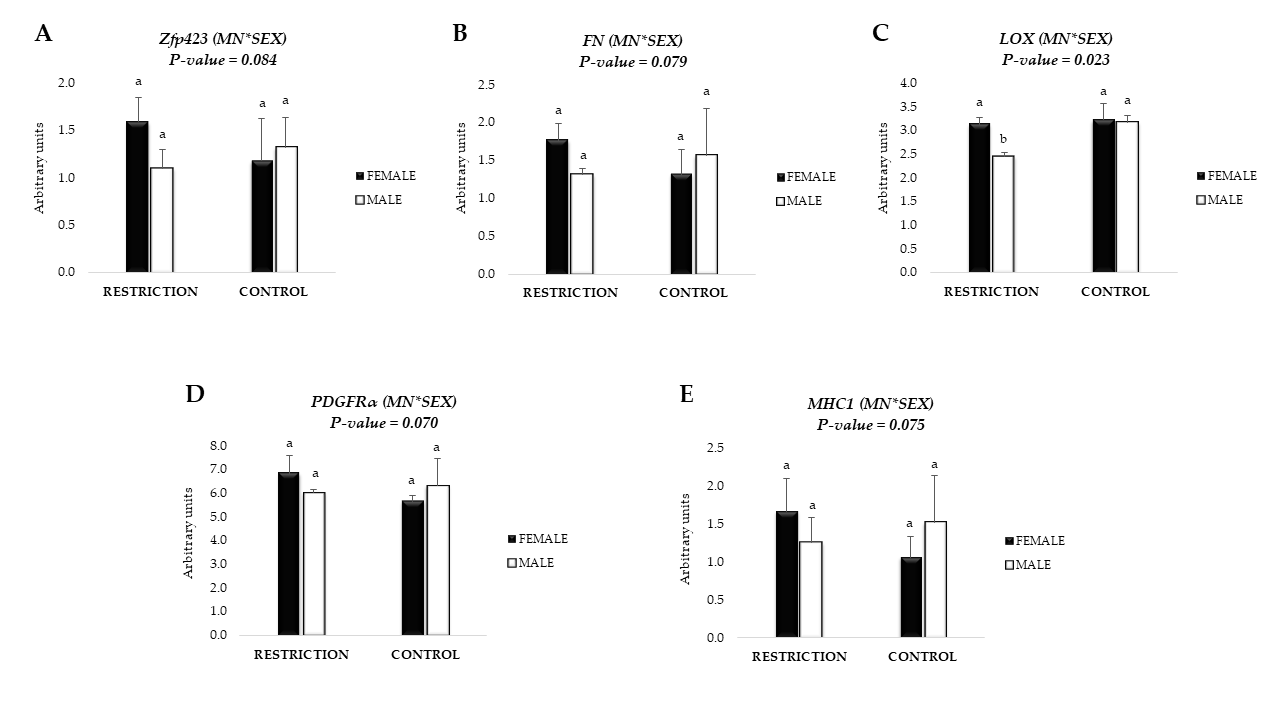
3.3. mRNA Expression of Adipogenic Markers
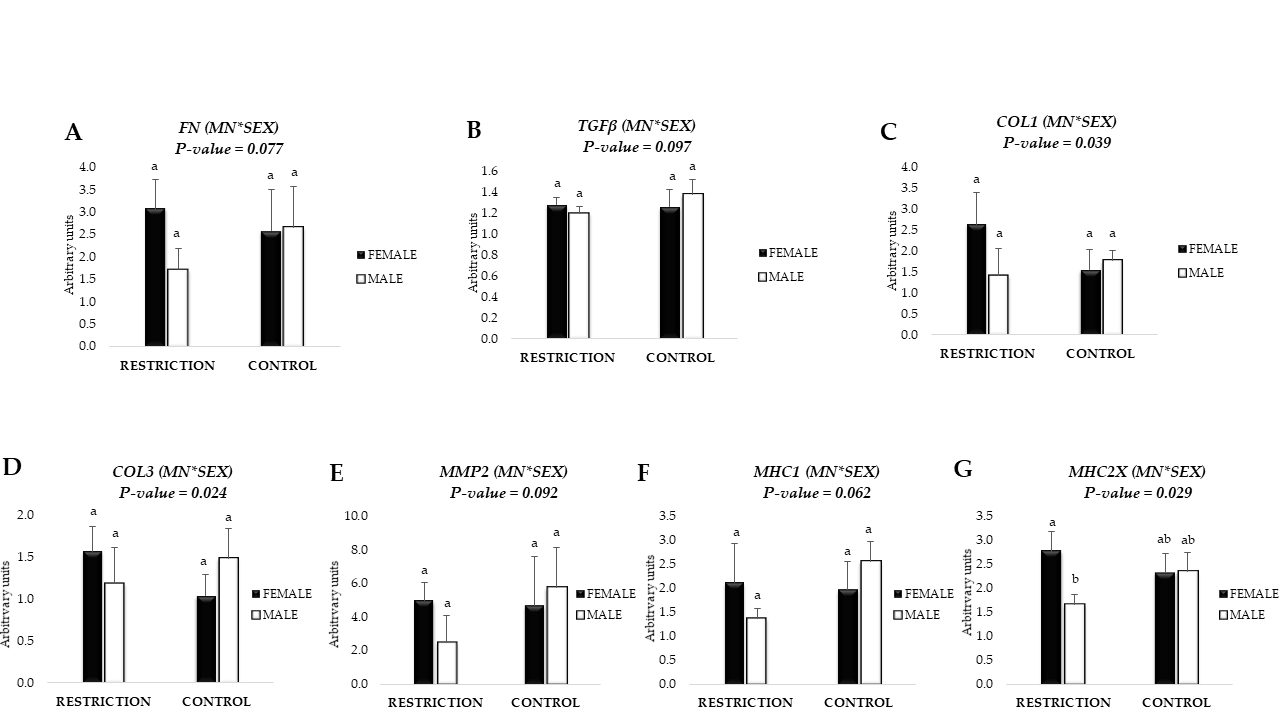
3.4. mRNA Expression of Fibrogenic Markers and Collagen Content
3.5. mRNA Expression of Markers related to Collagen Crosslinking and Remodeling
3.6. mRNA Expression of Markers Related with Satellite Cell and Fibro-Adipogenic Progenitor (FAP) Cells
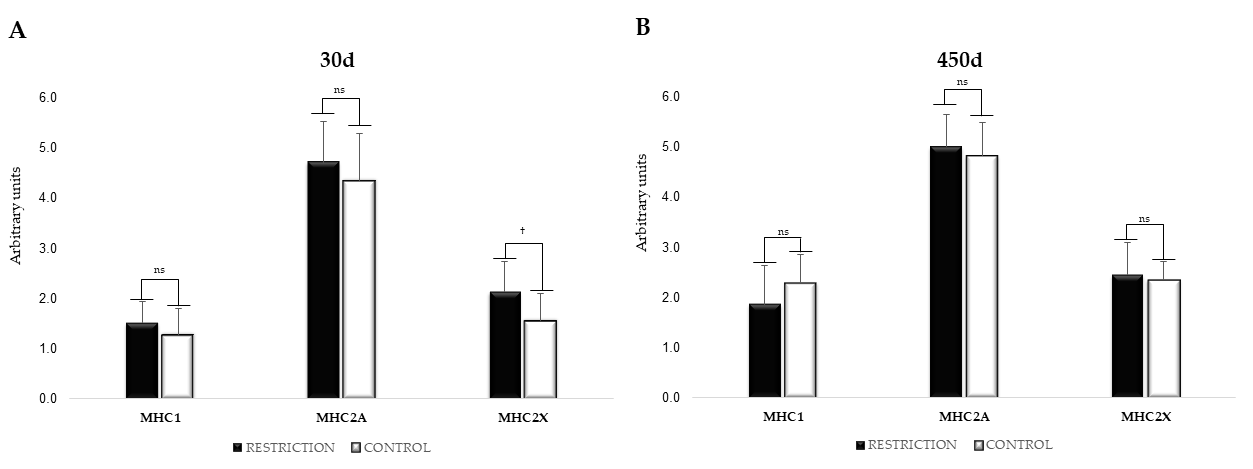
3.7. mRNA Expression of Myosin Heavy Chain Isoforms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, M.S.; Gionbelli, M.P.; Paulino, P.V.R.; Serão, N.V.L.; Martins, T.S.; Totaro, P.I.S.; Neves, C.A.; Valadares Filho, S.C.; Dodson, M.V.; Zhu, M.; et al. Effects of Maternal Nutrition on Development of Gastrointestinal Tract of Bovine Fetus at Different Stages of Gestation. Livest. Sci. 2013, 153, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Detmann, E.; Paulino, M.F.; Mantovani, H.C.; Filho, S.d.C.V.; Sampaio, C.B.; De Souza, M.A.; Lazzarini, Í.; Detmann, K.S.C. Parameterization of Ruminal Fibre Degradation in Low-Quality Tropical Forage Using Michaelis–Menten kinetics. Livest. Sci. 2009, 126, 136–146. [Google Scholar] [CrossRef]
- Bell, A.W.; Greenwood, P.L.; Ehrhardt, R.A. Regulation of Metabolism and Growth during Prenatal Life. In Biology of Growing Animals; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3, pp. 3–34. [Google Scholar]
- Bell, A.W.; Ehrhardt, R.A. Regulation of Macronutrient Partitioning Between Maternal and Conceptus Tissues in the Pregnant Ruminant. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Cronjé, P., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 275–293. [Google Scholar]
- LeMaster, C.T.; Taylor, R.K.; Ricks, R.E.; Long, N.M. The Effects of Late Gestation Maternal Nutrient Restriction with or without Protein Supplementation on Endocrine Regulation of Newborn and Postnatal Beef Calves. Theriogenology 2017, 87, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulino, M.F.; Detmann, E.; Valadares Filho, S.C. Simpósio Internacional de Produção de Gado de Corte, 6th ed.; SIMCORTE: Viçosa, MG, Brazil, 2008. [Google Scholar]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal Programming of Skeletal Muscle Development in Ruminant Animals. J. Anim. Sci. 2010, 88, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.; Huang, Y.; Das, A.; Yang, Q.; Duarte, M.S.; Dodson, M.V.; Zhu, M.J. Manipulating Mesenchymal Progenitor Cell Differentiation to Optimize Performance and Carcass Value of Beef Cattle. J. Anim. Sci. 2013, 91, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Mitsuhashi, T.; Mitsumoto, M.; Matsumoto, S.; Itoh, N.; Itagaki, K.; Kohno, Y.; Dohgo, T. The Characteristics of Muscle Fiber Types of Longissimus Thoracis Muscle and Their Influences on the Quantity and Quality of Meat from Japanese Black Steers. Meat Sci. 2000, 54, 65–70. [Google Scholar] [CrossRef]
- Hudson, N.J.; Reverter, A.; Greenwood, P.L.; Guo, B.; Cafe, L.M.; Dalrymple, B.P. Longitudinal Muscle Gene Expression Patterns Associated with Differential Intramuscular Fat in Cattle. Animal 2014, 39, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purslow, P.P. New Developments on the Role of Intramuscular Connective Tissue in Meat Toughness. Annu. Rev. Food Sci. Technol. 2014, 5, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.K.; Lemaster, C.T.; Mangrum, K.S.; Ricks, R.E.; Long, N.M. Effects of Maternal Nutrient Restriction During Early or Mid-Gestation without Realimentation on Maternal Physiology and Foetal Growth and Development in Beef Cattle. Animal 2018, 12, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Peñagaricano, F.; Wang, X.; Rosa, G.J.M.; Radunz, A.E.; Khatib, H. Maternal Nutrition induces Gene Expression Changes in Fetal Muscle and Adipose Tissues in Sheep. BMC Genom. 2014, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, T.C.; Moura, F.H.; Souza, R.O.; Lopes, M.M.; Fontes, M.M.S.; Serão, N.V.L.; Sanglard, L.P.; Du, M.; Gionbelli, M.P.; Duarte, M.S. Effect of Maternal Feed Restriction in Dairy Goats at Different Stages of Gestation on Skeletal Muscle Development and Energy Metabolism of Kids at the Time of Births. Anim. Reprod. Sci. 2019, 206, 46–59. [Google Scholar] [CrossRef]
- Valadares Filhos, S.; Costa e Silva, L.; Gionbelli, M.; Rotta, P.; Marcondes, M.; Chizzotti, M.; Prados, L. BR-CORTE 3.0. Nutrient Requirements of Zebu and Crossbred Cattle, 3rd ed.; Suprema Gráfica Ltda: Vicosa (MG), Brazil, 2016; ISBN 9788581791111. [Google Scholar]
- Foidart, M.; Foidart, J.M.; Engel, W.K. Collagen Localization in Normal and Fibrotic Human Skeletal Muscle. Arch. Neurol. 1981, 38, 152–157. [Google Scholar] [CrossRef]
- Duarte, M.S.; Paulino, P.V.R.; Das, A.K.; Wei, S.; Serao, N.V.L.; Fu, X.; Harris, S.M.; Dodson, M.V.; Du, M. Enhancement of Adipogenesis and Fibrogenesis in Skeletal Muscle of Wagyu Compared with Angus Cattle. J. Anim. Sci. 2013, 91, 2938–2946. [Google Scholar] [CrossRef]
- Voge, J.L.; Santiago, C.A.T.; Aad, P.Y.; Goad, D.W.; Malayer, J.R.; Spicer, L.J. Quantification of Insulin-Like Growth Factor Binding Protein mRNA Using Real-Time PCR in Bovine Granulosa and Theca Cells: Effect of Estradiol, Insulin, and Gonadotropins. Domest. Anim. Endocrinol. 2004, 26, 241–258. [Google Scholar] [CrossRef]
- Gionbelli, M.P.; Valadares Filho, S.C.; Detmann, E. Adjusting Cattle Body Weight to Physiological and Feeding Conditions. In Nutrient Requirements of Zebu and Nellore Cattle, 3rd ed.; Suprema Gráfica Ltda: Viçosa (MG), Brazil, 2016; pp. 1–4. [Google Scholar]
- Paradis, F.; Wood, K.M.; Swanson, K.C.; Miller, S.P.; McBride, B.W.; Fitzsimmons, C. Maternal Nutrient Restriction in Mid-to-Late Gestation Influences Fetal mRNA Expression in Muscle Tissues in Beef Cattle. BMC Genom. 2017, 18, 632. [Google Scholar] [CrossRef]
- Sanglard, L.P.; Nascimento, M.; Moriel, P.; Sommer, J.; Ashwell, M.; Poore, M.H.; Duarte, M.d.S.; Serão, N.V.L. Impact of Energy Restriction During Late Gestation on the Muscle and Blood Transcriptome of Beef Calves after Preconditioning. BMC Genom. 2018, 19, 702. [Google Scholar] [CrossRef]
- Picard, B.; Barboiron, C.; Kaspric, N.; Vonnahme, K.; Bonnet, M.; Picard, B.; Barboiron, C.; Kaspric, N.; Vonnahme, K.; Bonnet, M. Impact on Muscle Fiber Properties of Maternal Nutrient Restriction Followed by Realimentation from Early to Mid-Gestation in Beef Cows. In Proceedings of the 61st International Congress of Meat Science and Technology (ICoMST), Clermont-Ferrand, France, 28 August 2015. [Google Scholar]
- Greenwood, P.L.; Slepetis, R.M.; Hermanson, J.W.; Bell, A.W. Intrauterine Growth Retardation is Associated with Reduced Cell Cycle Activity, but not Myofibre Number, in Ovine Fetal Muscle. Reprod. Fertil. Dev. 1999, 11, 281–291. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Effect of Maternal Nutrient Restriction in Sheep on the Development of Fetal Skeletal Muscle. Biol. Reprod. 2004, 71, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, S.M.; Price, F.D.; Castiglioni, A.; Gee, A.W.; Schneider, J.; Matyas, M.N.; Hayhurst, M.; Tabebordbar, M.; Wagers, A.J.; Rubin, L.L. Pro-Myogenic Small Molecules Revealed by a Chemical Screen on Primary Muscle Stem Cells. Skelet. Muscle 2020, 10, 28. [Google Scholar] [CrossRef]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and Myogenic Progression in Skeletal Muscle Satellite Cells. J. Cell Sci. 2006, 119, 1824–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.E.; Partridge, T.A. Muscle Satellite Cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Raja, J.S.; Hoffman, M.L.; Govoni, K.E.; Zinn, S.A.; Reed, S.A. Restricted Maternal Nutrition Alters Myogenic Regulatory Factor Expression in Satellite Cells of Ovine Offspring. Animal 2016, 10, 1200–1203. [Google Scholar] [CrossRef]
- De Melo, J.F.; Aloulou, N.; Duval, J.L.; Vigneron, P.; Bourgoin, L.; Leandro, C.G.; De Castro, C.M.M.B.; Nagel, M.D. Effect of a Neonatal Low-Protein Diet on the Morphology of Myotubes in Culture and the Expression of Key Proteins that Regulate Myogenesis in Young and Adult rats. Eur. J. Nutr. 2011, 50, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ithurralde, J.; Genovese, P.; Abud, M.J.; López-Pérez, Á.; Pérez-Clariget, R.; Bielli, A. Maternal Undernutrition Affects Secondary Myogenesis in a Muscle-Dependent Way Across the Major Muscles of 70-day Old Ovine Fetuses. Small Rumin. Res. 2020, 191, 106174. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Du, M. Farm Animals for Studying Muscle Development and Metabolism: Dual Purposes for Animal Production and Human Health. Anim. Front. 2019, 9, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Soto, S.M.; Blake, A.C.; Wesolowski, S.R.; Rozance, P.J.; Barthel, K.B.; Gao, B.; Hetrick, B.; McCurdy, C.E.; Garza, N.G.; Hay, W.W.; et al. Myoblast Replication is Reduced in the IUGR Fetus Despite Maintained Proliferative Capacity in Vitro. J. Endocrinol. 2017, 232, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Comai, G.; Tajbakhsh, S. Molecular and Cellular Regulation of Skeletal Myogenesis. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 110, pp. 1–73. [Google Scholar]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal Progenitors Distinct from Satellite Cells Contribute to Ectopic Fat Cell Formation in Skeletal Muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle Injury Activates Resident Fibro/Adipogenic Progenitors that Facilitate Myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα Induces Adipogenesis through PPARγ: A Unified Pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Madsen, M.S.; Siersbaek, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome Proliferator-Activated Receptor and C/EBP Synergistically Activate Key Metabolic Adipocyte Genes by Assisted Loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef] [Green Version]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and Adipogenesis Originate from a Common Mesenchymal Progenitor in Skeletal Muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef] [Green Version]
- Vallecillo-García, P.; Orgeur, M.; Vom Hofe-Schneider, S.; Stumm, J.; Kappert, V.; Ibrahim, D.M.; Börno, S.T.; Hayashi, S.; Relaix, F.; Hildebrandt, K.; et al. Odd Skipped-Related 1 Identifies a Population of Embryonic Fibro-Adipogenic Progenitors Regulating Myogenesis During Limb Development. Nat. Commun. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Miao, Z.G.; Zhang, L.P.; Fu, X.; Yang, Q.Y.; Zhu, M.J.; Dodson, M.V.; Du, M. Invited Review: Mesenchymal Progenitor Cells in Intramuscular Connective Tissue Development. Animal 2016, 10, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Ceco, E.; McNally, E.M. Modifying Muscular Dystrophy through Transforming Growth Factor-β. FEBS J. 2013, 280, 4198–4209. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.R.; Barton, E.R. Regulation of Fibrosis in Muscular Dystrophy. Matrix Biol. 2018, 68–69, 602–615. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Regnault, T.R.H.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental Adaptations in Growth Restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef] [Green Version]
- Archile-Contreras, A.C.; Mandell, I.B.; Purslow, P.P. Disparity of Dietary Effects on Collagen Characteristics and Toughness Between Two Beef Muscles. Meat Sci. 2010, 86, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, H.S. Matrix Metalloproteinases in Skeletal Muscles: Friends or Foes? Neurobiol. Dis. 2012, 48, 508–518. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Hocquette, J.F. Endocrine and Metabolic Regulation of Muscle Growth and Body Composition in Cattle. Animal 2010, 4, 1797–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, Z.C.T.R.; Brameld, J.M.; Craigon, J.; Scollan, N.D.; Buttery, P.J. Effect of Maternal Dietary Restriction During Pregnancy on Lamb Carcass Characteristics and Muscle Fiber Composition. J. Anim. Sci. 2007, 85, 1565. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Means, W.J.; Hess, B.W.; Nathanielsz, P.W.; Du, M. Maternal Nutrient Restriction Affects Properties of Skeletal Muscle in Offspring. J. Physiol. 2006, 575, 241–250. [Google Scholar] [CrossRef]
- Aragão, R.S.; Guzmán, O.Q.; Pérez, G.G.; Toscano, A.E.; Gois, C.L.; Manhães, R.C.; Bolaños, F.J. Differential Developmental Programming by Early Protein Restriction of Rat Skeletal Muscle According to its Fibre-Type Composition. Acta Physiol. 2014, 210, 70–83. [Google Scholar] [CrossRef]

| Item | Mid-Gestation 1 | Offspring 2 | ||
|---|---|---|---|---|
| Basal Diet | Supplement | Corn Silage | Concentrate | |
| DM (g/Kg) | 418 | 881 | 458 | 907 |
| OM (g/Kg DM) | 951 | 957 | 960 | 885 |
| CP (g/Kg DM) | 53.3 | 400 | 99.1 | 351 |
| NDFap (g/Kg DM) 3 | 631 | 213 | 468 | 167 |
| NFC (g/Kg DM) | 242 | 342 | 367 | 340 |
| EE (g/Kg DM) | 24.1 | 41.2 | 25.8 | 25.5 |
| Gene Name | Gene Abbreviation | Primer |
|---|---|---|
| Paired box 7 | PAX7 | F: CGGGCATGTTTAGCTGGGAGA |
| R: TCTGAGCACTCGGCTAATCGAAC | ||
| Platelet-derived growth factor receptor A | PDGFRα | F: GCTCCCTTCCTTTGTCTCTTAC |
| R: CGATTTGTCCTCCCATCTAACC | ||
| Myosin heavy chain 1 | MHC1 | F: ATCGCTGAATCCCAGGTCAA |
| R: ACCAAGATGTGGCACGGCTA | ||
| Myosin heavy chain 2A | MHC 2A | F: CACCCTGGAGCAGACAGAGA |
| R: TCCCTGGATTTGCGTGATG | ||
| Myosin heavy chain 2X | MHC 2X | F: TTTCCAGACCGTGTCTGCTC |
| R: GGGATGATGCAGCGTACAAAG | ||
| Zinc finger protein 423 | ZFP423 | F: CCAGATGACCTTCGAGAATGAG |
| R: CACTAGCTGTAGCAGGACAATAA | ||
| CCAAT-enhancer-binding protein alpha | C/EBPα | F: GGCAACGACTTTGACTACC |
| R: CTCGTACAGAGGCTCCAG | ||
| Peroxissome proliferator activated-receptor gamma | PPARγ | F: TCCACTCCGCACTATGAG |
| R: GGGATACAGGCTCCACTT | ||
| Transforming growth factor beta | TGFβ | F: AACCTGTGTTGCTCTCTCGG |
| R: GAGGTAGCGCCAGGAATTGT | ||
| Fibronectin | FN1 | F: CTGAGACCACCATCACCATTAG |
| R: CTCGGAACTGTAAGGGTTCTTC | ||
| Collagen type I, alpha 1 | COL1A1 | F: CCACCCCAGCCGCAAAGAGT |
| R: ACGCAGGTGACTGGTGGGATGTC | ||
| Collagen type III, alpha 1 | COL3A1 | F: GGCCCCCTGGAAAGGACGGA |
| R: CCCCGCCAGCACCACAACAT | ||
| Lysyl oxidase | LOX | F: CAGAAGATCCAATGGGAGAACA |
| R: TGGCATCAAGCAGGTCATAG | ||
| Prolyl 4-Hydroxylase Subunit Alpha 1 | P4HA1 | F: GGATGAGTGGGACAAGCCTC |
| R: ACCGTCTCCAAGTCTCCTGT | ||
| Matrix metalloproteinase-2 | MMP2 | F: CGGCAAGTATGGCTTCTG |
| R: CTCCTCCTGTGGGTCTTC | ||
| TIMP metallopeptidase inhibitor 1 | TIMP1 | F: CAGAGAGGCTACACCAGAG |
| R: CACAACCAGCAGCATAGG | ||
| TIMP metallopeptidase inhibitor 2 | TIMP2 | F: GAAGGAGGTGGACTCTGG |
| R: CCGGAGAGGAGATGTAGC | ||
| 18 S ribosomal | 18S | F: CCTGCGGCTTAATTTGACTC |
| R: AACTAAGAACGGCCATGCAC |
| Gene | Maternal Nutrition 1 | Sex | p—Value 2 | ||||
|---|---|---|---|---|---|---|---|
| Restriction | Control | Female | Male | MN | S | MN×S | |
| mRNA expression of adipogenic markers (arbitrary units) | |||||||
| C/EBPα | 6.20 ± 1.52 | 5.95 ± 2.40 | 6.46 ± 1.36 | 5.57 ± 2.53 | 0.796 | 0.403 | 0.596 |
| PPARγ | 8.25 ± 1.04 | 7.43 ± 0.98 | 7.96 ± 1.13 | 7.69 ± 1.01 | 0.116 | 0.876 | 0.360 |
| mRNA expression of fibrogenic markers (arbitrary units) | |||||||
| TGFβ | 1.36 ± 0.13 | 0.99 ± 0.21 | 1.02 ± 0.18 | 1.00 ± 0.16 | 0.629 | 0.970 | 0.472 |
| COL1 | 0.92 ± 0.15 | 0.93 ± 0.20 | 0.97 ± 0.16 | 0.88 ± 0.20 | 0.920 | 0.358 | 0.120 |
| COL3 | 1.28 ± 0.16 | 1.13 ± 0.38 | 1.19 ± 0.28 | 1.23 ± 0.30 | 0.300 | 0.618 | 0.135 |
| mRNA expression of collagen crosslinking markers (arbitrary units) | |||||||
| P4Ha1 | 1.83 ± 0.52 | 1.62 ± 0.65 | 1.70 ± 0.62 | 1.76 ± 0.47 | 0.411 | 0.690 | 0.256 |
| mRNA expression of collagen remodeling markers (arbitrary units) | |||||||
| TIMP1 | 8.17 ± 0.60 | 8.30 ± 0.65 | 8.32 ± 0.68 | 8.13 ± 0.52 | 0.670 | 0.496 | 0.181 |
| TIMP2 | 3.05 ± 0.16 | 2.75 ± 0.53 | 3.02 ± 0.27 | 2.76 ± 0.52 | 0.132 | 0.278 | 0.405 |
| MMP2 | 2.32 ± 1.25 | 2.72 ± 1.33 | 2.61 ± 1.27 | 2.42 ± 1.34 | 0.537 | 0.667 | 0.330 |
| mRNA expression of satellite cells marker (arbitrary units) | |||||||
| PAX7 | 5.55 ± 0.26 a | 5.23 ± 0.37 b | 5.44 ± 0.39 | 5.33 ± 0.29 | 0.099 | 0.623 | 0.501 |
| Gene | Maternal Nutrition 1 | Sex | p–Value 2 | ||||
|---|---|---|---|---|---|---|---|
| Restriction | Control | Female | Male | MN | S | MN×S | |
| mRNA expression of adipogenic markers (arbitrary units) | |||||||
| ZFP423 | 2.52 ± 0.57 | 2.68 ± 0.62 | 2.54 ± 0.50 | 2.67 ± 0.71 | 0.598 | 0.718 | 0.139 |
| C/EBPα | 8.39 ± 1.91 | 8.82 ± 3.61 | 8.84 ± 3.27 | 8.31 ± 2.30 | 0.762 | 0.658 | 0.353 |
| PPARγ | 9.64 ± 1.64 | 8.74 ± 1.25 | 9.64 ± 1.47 | 8.49 ± 1.21 | 0.208 | 0.167 | 0.134 |
| mRNA expression of collagen crosslinking markers (arbitrary units) | |||||||
| LOX | 4.14 ± 0.84 | 4.25 ± 1.31 | 4.42 ± 1.17 | 3.92 ± 0.91 | 0.845 | 0.314 | 0.294 |
| P4Ha1 | 2.51 ± 0.26 | 2.69 ± 0.38 | 2.60 ± 0.34 | 2.58 ± 0.30 | 0.289 | 0.696 | 0.156 |
| mRNA expression of collagen remodeling markers (arbitrary units) | |||||||
| TIMP1 | 6.64 ± 2.97 | 5.93 ± 2.65 | 6.57 ± 2.66 | 6.01 ± 3.02 | 0.634 | 0.808 | 0.723 |
| TIMP2 | 3.33 ± 0.54 | 3.04 ± 0.64 | 3.41 ± 0.57 | 2.94 ± 0.55 | 0.310 | 0.162 | 0.205 |
| mRNA expression of satellite cells marker (arbitrary units) | |||||||
| PAX7 | 6.66 ± 1.02 | 6.46 ± 0.89 | 6.95 ± 0.85 a | 5.98 ± 0.76 b | 0.638 | 0.042 | 0.412 |
| mRNA expression of fibro-adipogenic progenitor cells marker (arbitrary units) | |||||||
| PDGFRα | 6.61 ± 2.47 | 7.08 ± 1.51 | 6.75 ± 2.48 | 6.98 ± 1.35 | 0.649 | 0.901 | 0.433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, T.C.; Du, M.; Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Schultz, E.B.; Gionbelli, M.P.; Duarte, M.d.S. Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation. Animals 2021, 11, 860. https://doi.org/10.3390/ani11030860
Costa TC, Du M, Nascimento KB, Galvão MC, Meneses JAM, Schultz EB, Gionbelli MP, Duarte MdS. Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation. Animals. 2021; 11(3):860. https://doi.org/10.3390/ani11030860
Chicago/Turabian StyleCosta, Thais Correia, Min Du, Karolina Batista Nascimento, Matheus Castilho Galvão, Javier Andrés Moreno Meneses, Erica Beatriz Schultz, Mateus Pies Gionbelli, and Marcio de Souza Duarte. 2021. "Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation" Animals 11, no. 3: 860. https://doi.org/10.3390/ani11030860







