Reproductive Seasonality Affects In Vitro Embryo Production Outcomes in Adult Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Oocyte Recovery
2.3. In Vitro Maturation (IVM) of Oocytes
2.4. Sperm Preparation and IVF of Oocytes
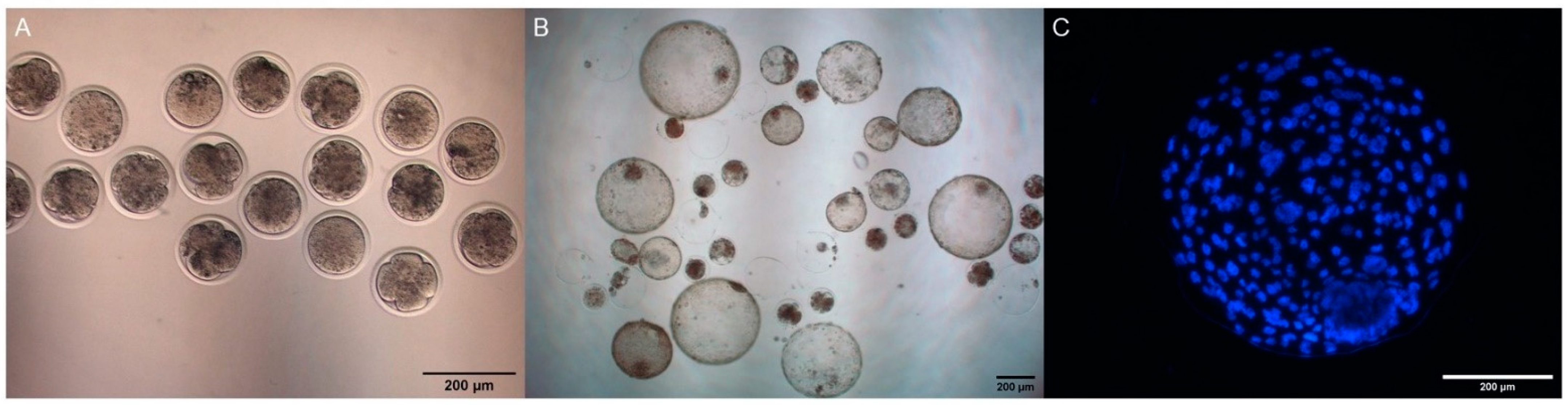
2.5. In Vitro Development (IVD) and Embryo Quality
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasantha, I. Physiology of seasonal breeding: A review. J. Vet. Sci. Technol. 2016, 7, 331. [Google Scholar] [CrossRef] [Green Version]
- Bronson, F.H. Climate change and seasonal reproduction in mammals. Philos. Trans. R. Soc. B 2009, 364, 3331–3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, M.A. The brain’s calendar: Neural mechanisms of seasonal timing. Biol. Rev. Camb. Philos. Soc. 2004, 79, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Mastromonaco, G.F.; Gonzalez-Grajales, A.L. Reproduction in female wild cattle: Influence of seasonality on ARTs. Theriogenology 2020, 150, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Guillame, D.; Migaud, M.; Thiery, J.C.; Pellicer-Rubio, M.T.; Maplaux, B. Seasonality of reproduction in mammals: Intimate regulatory mechanism and practical implications. Reprod. Domest. Anim. 2008, 43, 40–47. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Beard, A.P.; Rawlings, N.C. An ultrasound-aided study of temporal relationships between the patterns of LH/FSH secretion, development of ovulatory sized antral follicles and formation of corpora lutea in ewes. Theriogenology 2000, 54, 229–245. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, J.; Anzar, M. Effect of cryopreservation technique and season on the survival of in vitro produced cattle embryos. Anim. Reprod. Sci. 2016, 164, 162–168. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Waters, T.H.B.; Chung, J.P.W.; Li, T.C.; Chan, D.Y.L. The effects of daily meteorological perturbation on pregnancy outcome: Follow-up of a cohort of young women undergoing IVF treatment. Environ. Health 2019, 18, 103. [Google Scholar] [CrossRef] [Green Version]
- Abdoon, A.S.; Gabler, C.; Holder, C.; Kandil, O.M.; Einspanier, R. Seasonal variations in developmental competence and relative abundance of gene transcripts in buffalo (Bubalus bubalis) oocytes. Theriogenology 2014, 82, 1055–1067. [Google Scholar] [CrossRef]
- Ahmad, E.; Nazari, H.; Hossini-Fahraji, H. Low developmental competence and high tolerance to thermal stress of ovine oocytes in the warm compared with the cold season. Trop. Anim. Health Prod. 2019, 51, 1611–1618. [Google Scholar] [CrossRef]
- Davashi, N.D.; Shahneh, A.Z.; Kohram, H.; Zhandi, M.; Dashti, S.; Shamsi, H.; Moghadam, R. In vitro ovine embryo production: The study of seasonal and oocyte recovery method effects. Iran. Red. Crescent Med. J. 2014, 16, e20749. [Google Scholar] [CrossRef] [Green Version]
- Freistedt, P.; Stojkovic, M.; Stojkovic, E. Efficient in vitro production of cat embryos in modified synthetic oviduct fluid medium: Effects of season and ovarian status. Biol. Reprod. 2001, 65, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Stenbak, T.K.; Redmer, D.A.; Berginski, H.R.; Erickson, A.S.; Navanukraw, C.; Toutges, M.J.; Bilski, J.J.; Kirsch, J.D.; Kraft, K.C.; Reynolds, L.P.; et al. Effects of follicle stimulating hormone (FSH) on follicular development, oocyte retrieval, and in vitro fertilization (IVF) in ewes during breeding season and seasonal anestrus. Theriogenology 2001, 56, 51–64. [Google Scholar] [CrossRef]
- Di Francesco, S.; Novo, M.V.S.; Vecchio, D.; Neglia, G.; Boccia, L.; Campanile, G.; Zicarelli, L.; Gasparrini, B. Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian buffalo performed in different seasons. Theriogenology 2012, 77, 148–154. [Google Scholar] [CrossRef]
- Mara, L.; Sanna, D.; Casu, S.; Dattena, M.; Mayorga-Muñoz, I.M. Blastocyst rate of in vitro embryo production in sheep is affected by season. Zygote 2013, 22, 1–6. [Google Scholar] [CrossRef]
- Catala, M.G.; Roura, M.; Soto-Heras, S.; Menéndez, I.; Contreras-Solis, I.; Paramio, M.T.; Izquierdo, D. Effect of season on intrafollicular fatty acid concentrations and embryo production after in vitro fertilization and parthenogenic activation of prepubertal goat oocytes. Small Rumin. Res. 2018, 168, 82–86. [Google Scholar] [CrossRef]
- Souza-Fabjan, J.M.G.; Panneau, B.; Duffard, N.; Locatelli, Y.; de Figueiredo, J.R.; Freitas, V.J.F.; Mermillod, P. In vitro production of small ruminant embryos: Late improvements and further research. Theriogenology 2014, 81, 1149–1162. [Google Scholar] [CrossRef]
- Souza-Fabjan, J.M.G.; Locatelli, Y.; Duffard, N.; Corbin, E.; Batista, R.I.T.P.; Freitas, V.J.F.; Beckers, J.F.; Mermillod, P. Intrinsic quality of goat oocytes already found denuded at collection for in vitro embryo production. Theriogenology 2016, 86, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.G.; Duffard, N.; Bertoldo, M.J.; Locatelli, Y.; Corbin, E.; Fatet, A.; Freitas, V.J.F.; Mermillod, P. Influence of heparin or the presence of cumulus cells during fertilization on the in vitro production of goat embryos. Anim. Reprod. Sci. 2013, 138, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fabjan, J.M.G.; Batista, R.I.T.P.; Correia, L.F.L.; Paramio, M.T.; Fonseca, J.F.; Freitas, V.J.F.; Mermillod, P. In vitro production of small ruminant embryos: Latest improvements and further research. Reprod. Fertil. Dev. 2021, 33, 31–54. [Google Scholar] [CrossRef]
- Souza-Fabjan, J.M.G.; Batista, R.I.T.P.; Freitas, V.J.F.; Mermillod, P. In vitro culture of embryos from LOPU-derived goat oocytes. In Comparative Embryo Culture: Methods and Protocols; Herrick, J.R., Ed.; Humana Press: New York, NY, USA; Springer Nature: Basingstoke, UK, 2019; pp. 141–153. [Google Scholar] [CrossRef]
- Krishnakuma, S.; Whiteside, D.P.; Elkin, B.; Thundathil, J.C. Effect of reproductive seasonality on gamete quality in the North American Bison (Bison bison bison). Reprod. Dom. Anim. 2015, 50, 206–213. [Google Scholar] [CrossRef]
- Uccheddu, S.; Pintus, E.; Garde, J.J.; Fleba, L.; Muzzeddu, M.; Pudda, F.; Bogliolo, L.; Strina, A.; Nieddu, S.; Ledda, S. Post-mortem recovery, in vitro maturation and fertilization of fallow deer (Dama dama, Linnaeus 1758) oocytes collected during reproductive and no reproductive season. Reprod. Domest. Anim. 2020, 55, 1294–1302. [Google Scholar] [CrossRef]
- Zheng, P.; Si, W.; Wang, H.; Zou, R.; Bavister, B.D.; Ji, W. Effect of age and breeding season on the developmental capacity of oocytes from unstimulated and follicle-stimulating hormone-stimulated rhesus monkeys. Biol. Reprod. 2001, 64, 1417–1421. [Google Scholar] [CrossRef] [Green Version]
- Watson, A.J. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J. Anim. Sci. 2007, 85, E1–E3. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.H.; Zheng, Q.; Li, Y.S.; Sui, M.H.; Wu, H.; Zhang, Y.H.; Chu, M.X.; Ma, Y.H.; Fang, F.G.; Xu, L.N. Identification of lncRNAs by RNA sequencing analysis during in vivo pre-implantation developmental transformation in the goat. Front. Genet. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, M.; Forcada, F.; Casao, A.; Abecia, J.; Sosa, C.; Palacín, I. Undernutrition and exogenous melatonin can affect the in vitro developmental competence of ovine oocytes on a seasonal basis. Reprod. Domest. Anim. 2010, 45, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Koeman, J.; Keefer, C.L.; Baldassarre, H.; Downey, B.R. Developmental competence of prepubertal and adult goat oocytes cultured in semi-defined media following laparoscopic recovery. Theriogenology 2003, 60, 879–889. [Google Scholar] [CrossRef]
- Hammami, S.; Morato, R.; Romaguera, R.; Roura, M.; Catala, M.G.; Paramio, M.T.; Mogas, T.; Izquierdo, D. Developmental competence and embryo quality of small oocytes from pre-pubertal goats cultured in IVM medium supplemented with low level of hormones, insulin-transferrin-selenium and ascorbic acid. Reprod. Domest. Anim. 2013, 48, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef] [PubMed]

| Season | n | Replicates | Cleavage (%) | Bl/COC (%) | Bl/Cleaved (%) | Hbl/totBl (%) | Total Cells |
|---|---|---|---|---|---|---|---|
| Autumn | 811 | 17 | 72 ± 2.1 a | 52 ± 2.5 a | 73 ± 2.7 a | 68 ± 2.9 | 198 ± 4.6 |
| Spring | 404 | 7 | 51 ± 7.1 b | 28 ± 4.7 b | 55 ± 2.6 b | 65 ± 3.8 | 187 ± 3.6 |
| Summer | 639 | 15 | 71 ± 2.0 a | 45 ± 2.3 c | 63 ± 3.3 a,b | 76 ± 5.1 | 191 ± 3.3 |
| Winter | 494 | 10 | 66 ± 4.1 a,b | 42 ± 2.1 c | 63 ± 4.1 a,b | 67 ± 4.4 | 196 ± 4.2 |
| Total | 2348 | 49 | 67 ± 1.8 | 44 ± 1.7 | 65 ± 1.8 | 66 ± 2.0 | 193 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza-Fabjan, J.M.G.; Correia, L.F.L.; Batista, R.I.T.P.; Locatelli, Y.; Freitas, V.J.F.; Mermillod, P. Reproductive Seasonality Affects In Vitro Embryo Production Outcomes in Adult Goats. Animals 2021, 11, 873. https://doi.org/10.3390/ani11030873
Souza-Fabjan JMG, Correia LFL, Batista RITP, Locatelli Y, Freitas VJF, Mermillod P. Reproductive Seasonality Affects In Vitro Embryo Production Outcomes in Adult Goats. Animals. 2021; 11(3):873. https://doi.org/10.3390/ani11030873
Chicago/Turabian StyleSouza-Fabjan, Joanna M.G., Lucas F.L. Correia, Ribrio I.T.P. Batista, Yann Locatelli, Vicente J.F. Freitas, and Pascal Mermillod. 2021. "Reproductive Seasonality Affects In Vitro Embryo Production Outcomes in Adult Goats" Animals 11, no. 3: 873. https://doi.org/10.3390/ani11030873
APA StyleSouza-Fabjan, J. M. G., Correia, L. F. L., Batista, R. I. T. P., Locatelli, Y., Freitas, V. J. F., & Mermillod, P. (2021). Reproductive Seasonality Affects In Vitro Embryo Production Outcomes in Adult Goats. Animals, 11(3), 873. https://doi.org/10.3390/ani11030873







