Tibetan Macaques with Higher Social Centrality and More Relatives Emit More Frequent Visual Communication in Collective Decision-Making
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Subjects
2.2. Data Collection and Behavioral Definition
2.3. Data Analysis
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Distribution of Visual Signals during Collective Movements
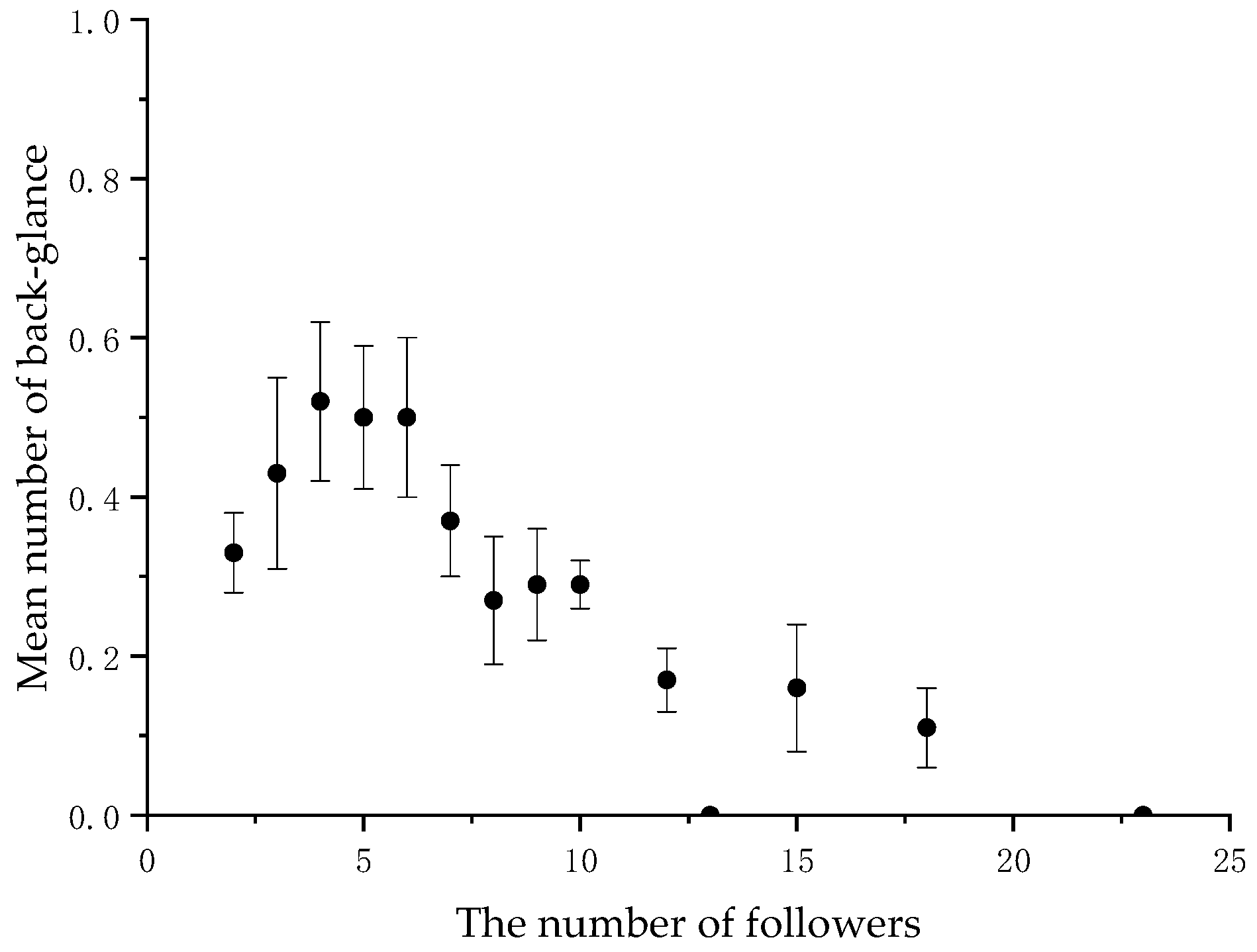
3.2. The Initiator’s Visual Signals Change with the Number of Followers
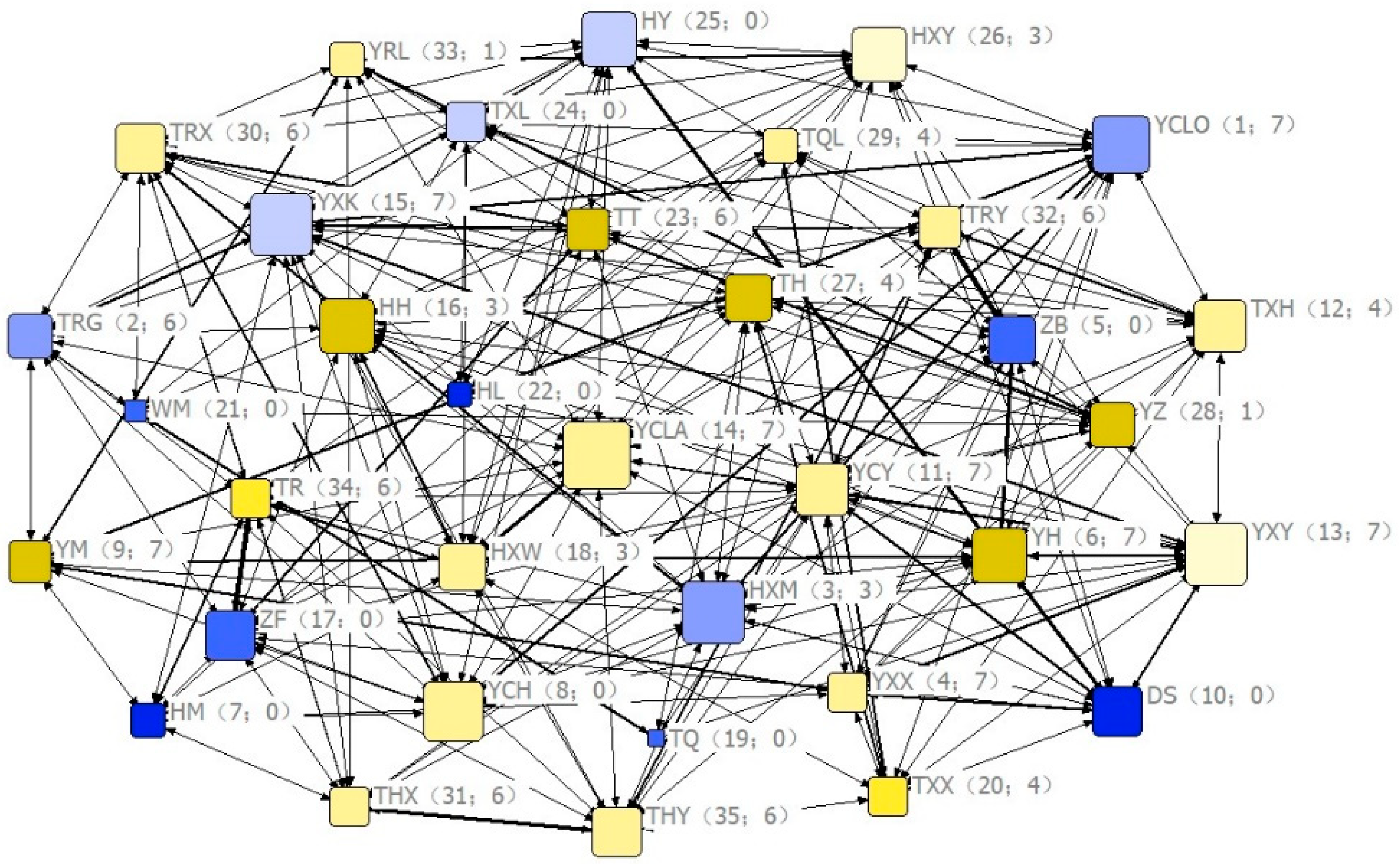
3.3. Factors Affecting Visual Signals
4. Discussion
4.1. Influence of Sex, Age, and Rank on Visual Communication (Hypothesis 2, Predictions 3–5)
4.2. Influence of Social Centrality on Visual Communication (Hypothesis 2, Predictions 6)
4.3. Influence of the Number of Relatives on Visual Communication (Hypothesis 2, Predictions 7)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Curley, E.A.; Rowley, H.E.; Speed, M.P. A field demonstration of the costs and benefits of group living to edible and defended prey. Biol. Lett. 2015, 11, 150–152. [Google Scholar] [CrossRef] [Green Version]
- Farine, D.R.; Strandburg-Peshkin, A.; Couzin, I.D.; Berger-Wolf, T.Y.; Crofoot, M.C. Individual variation in local interaction rules can explain emergent patterns of spatial organization in wild baboons. Proc. R. Soc. Lond. Ser. B 2017, 284, 2016–2243. [Google Scholar] [CrossRef]
- Miller, N.; Garnier, S.; Hartnett, A.T.; Couzin, I.D. Both information and social cohesion determine collective decisions in animal groups. Proc. Natl. Acad. Sci. USA 2013, 110, 5263–5268. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.E. Hamilton’s legacy: Kinship, cooperation and social tolerance inmammalian groups. Anim. Behav. 2014, 92, 291–304. [Google Scholar] [CrossRef]
- Strandburg-Peshkin, A.; Farine, D.R.; Couzin, I.D.; Crofoot, M.C. Shared decision-making drives collective movement in wild baboons. Science 2015, 348, 1358–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolles, J.W.; Boogert, N.J.; Sridhar, V.H.; Couzin, I.D.; Manica, A. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 2017, 27, 2862–2868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alma, A.M.; Farji-Brener, A.G.; Elizalde, L. Collective response of leaf-cutting ants to the effects of wind on foraging activity. Am. Nat. 2016, 188, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loretto, M.C.; Reimann, S.; Schuster, R.; Graulich, D.M.; Bugnyar, T. Shared space, individually used: Spatial behaviour of non-breeding ravens (Corvus corax) close to a permanent anthropogenic food source. J. Ornith. 2016, 157, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Seltmann, A.; Franz, M.; Majolo, B.; Qarro, M.; Ostner, J.; Schülke, O. Recruitment and monitoring behaviors by leaders predict following in wild Barbary macaques (Macaca sylvanus). Primate Biol. 2016, 3, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Lusseau, D.; Conradt, L. The emergence of unshared consensus decisions in bottlenose dolphins. Behav. Ecol. Sociobiol. 2009, 63, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Ramseyer, A.; Petit, O.; Thierry, B. Decision-making in group departures of female domestic geese. Behaviour 2009, 146, 351–371. [Google Scholar] [CrossRef]
- Ramos, A.; Petit, O.; Longour, P.; Pasquaretta, C.; Sueur, C. Collective decision making during group movements in European bison, Bison bonasus. Anim. Behav. 2015, 109, 149–160. [Google Scholar] [CrossRef]
- Smith, J.E.; Estrada, J.R.; Richards, H.R.; Dawes, S.E.; Mitsos, K.; Holekamp, K.E. Collective movements, leadership and consensus costs at reunions in spotted hyaenas. Anim. Behav. 2015, 105, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Benítez, M.E.; Roux, A.L.; Fischer, J.; Beehner, J.C.; Bergman, T.J. Acoustic and temporal variation in gelada (Theropithecus gelada) loud calls advertise male quality. Int. J. Primatol. 2016, 37, 1–18. [Google Scholar] [CrossRef]
- Petit, O.; Bon, R. Decision-making processes: The case of collective movements. Behav. Process. 2010, 84, 635–647. [Google Scholar] [CrossRef]
- Fichtel, C.; Hilgartner, R. Noises in the dark: Vocal communication in Lepilemur ruficaudatus and other nocturnal pair-living primates. In Leaping Ahead; Masters, J., Gamba, M., Génin, F., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zuberbühler, K. Experimental field studies with non-human primates. Curr. Opin. Neurobiol. 2014, 28, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Coye, C.; Ouattara, K.; Zuberbühler, K.; Lemasson, A. Suffixation influences receivers’ behaviour in non-human primates. Proc. R. Soc. Lond. B Biol. Sci. 2015, 282, 20150265. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, L.; Sheeran, L.K.; Sun, B.H.; Zhang, Q.X.; Zhang, D.; Xia, D.P.; Li, J.H. Social rank versus affiliation: Which is more closely related to leadership of group movements in Tibetan macaques (Macaca thibetana)? Am. J. Primatol. 2016, 78, 816–824. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Li, J.; Xia, D.; Sun, B.; Zhang, D. Collective movement in the Tibetan macaques (Macaca thibetana): Early joiners write the rule of the game. PLoS ONE 2015, 10, e0127459. [Google Scholar] [CrossRef]
- Fratellone, G.P.; Li, J.H.; Sheeran, L.K.; Wagner, R.S.; Wang, X.; Sun, L. Social connectivity among female Tibetan macaques (Macaca thibetana) increases the speed of collective movements. Primates 2018, 60, 183–189. [Google Scholar] [CrossRef]
- Berman, C.M.; Li, J.H. Impact of translocation, provisioning and range restriction on a group of Macaca thibetana. Int. J. Primatol. 2002, 23, 383–397. [Google Scholar] [CrossRef]
- Xia, D.P.; Li, J.H.; Garber, P.A.; Sun, L.X.; Zhu, Y.; Sun, B.H. Grooming reciprocity in female Tibetan macaques (Macaca thibetana). Am. J. Primatol. 2012, 74, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H. The Tibetan Macaque Society: A Field Study; Anhui University Press: Hefei, China, 1999. [Google Scholar]
- Rowe, A.K.; Li, J.H.; Sun, L.; Sheeran, L.K.; Wagner, R.S.; Xia, D.P.; Uheyf, D.A.; Chen, R. Collective decision making in Tibetan macaques: How followers affect the rules and speed of group movement. Anim. Behav. 2018, 146, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Sueur, C.; Petit, O. Signals used by leaders in Macaca tonkeana and Macaca mulatta: Group-mate recruitment and behaviour monitoring. Anim. Cogn. 2010, 13, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [Green Version]
- Sueur, C.; Petit, O. Shared or unshared consensus decision in macaques? Behav. Processes 2008, 78, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Pyritz, L.W.; King, A.J.; Sueur, C.; Fichtel, C. Reaching a consensus: Terminology and concepts used in coordination and decision-making research. Int. J. Primatol. 2011, 32, 1268–1278. [Google Scholar] [CrossRef]
- Sueur, C.; Petit, O.; De Marco, A.; Jacobs, A.T.; Watanabe, K.; Thierry, B. A comparative network analysis of social style in macaques. Anim. Behav. 2011, 82, 845–852. [Google Scholar] [CrossRef]
- Jacobs, A.; Watanabe, K.; Petit, O. Social structure affects initiations of group movements but not recruitment success in Japanese macaques (Macaca fuscata). Int. J. Primatol. 2011, 32, 1311–1324. [Google Scholar] [CrossRef]
- Gammell, M.P.; de Vries, H.; Jennings, D.M.J.; Carlin, C.M.; Hayden, T.J. David’s score: A more appropriate dominance ranking method than Clutton-Brock et al.’s index. Anim. Behav. 2003, 66, 601–605. [Google Scholar] [CrossRef] [Green Version]
- David, H.A. The Method of Paired Comparisons; Charles Griffin: London, UK, 1988. [Google Scholar]
- de Vries, H. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim. Behav. 1998, 55, 827–843. [Google Scholar] [CrossRef] [Green Version]
- Kasper, C.; Voelkl, B. A social network analysis of primate groups. Primates 2009, 50, 343. [Google Scholar] [CrossRef]
- Hanneman, R.A.; Riddle, M. Introduction to Social Network Methods; University of California: Riverside, CA, USA, 2005; Available online: http://www.faculty.ucr.edu/~hanneman/nettext/ (accessed on 16 March 2020).
- Zhou, X.B. Semiparametric and Nonparametric Estimation of Tobit Models; Science Press: Beijing, China, 2015. [Google Scholar]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS; Allen & Uwin: Sydney, Australia, 2007. [Google Scholar]
- Meunier, H.; Deneubourg, J.L.; Petit, O. How many for dinner? Recruitment and monitoring by glances in capuchins. Primates 2008, 49, 26–31. [Google Scholar] [CrossRef]
- Barclli, C.; Reichard, U.H. Female white-handed gibbons (Hylobates lar) lead group movements and have priority of access to food resources. Behaviour 2008, 145, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Kappeler, P.M.; Schaik, C.P.V. Grouping and movement patterns in Malagasy primates. In On the Move; Boinski, S., Garber, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Boinski, S. Social manipulation within and between troops mediates primate group movement. In On the Move; Boinski, S., Garber, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Fennessy, J. Home range and seasonal movements of Giraffa camelopardalis angolensis in the northern Namib Desert. Afr. J. Ecol. 2009, 47, 318–327. [Google Scholar] [CrossRef]
- Chapman, C.A. Association patterns of spider monkeys: The influence of ecology and sex on social organization. Behav. Ecol. Sociobiol. 1990, 26, 409–414. [Google Scholar] [CrossRef]
- Sueur, C.; King, A.J.; Pelé, M.; Petit, O. Fast and accurate decisions as a result of scale-free network properties in two primate species. In Proceedings of the 9th European Conference on Complex Systems, Bruxelles, Belgium, 2–7 September 2012; pp. 579–584. [Google Scholar]
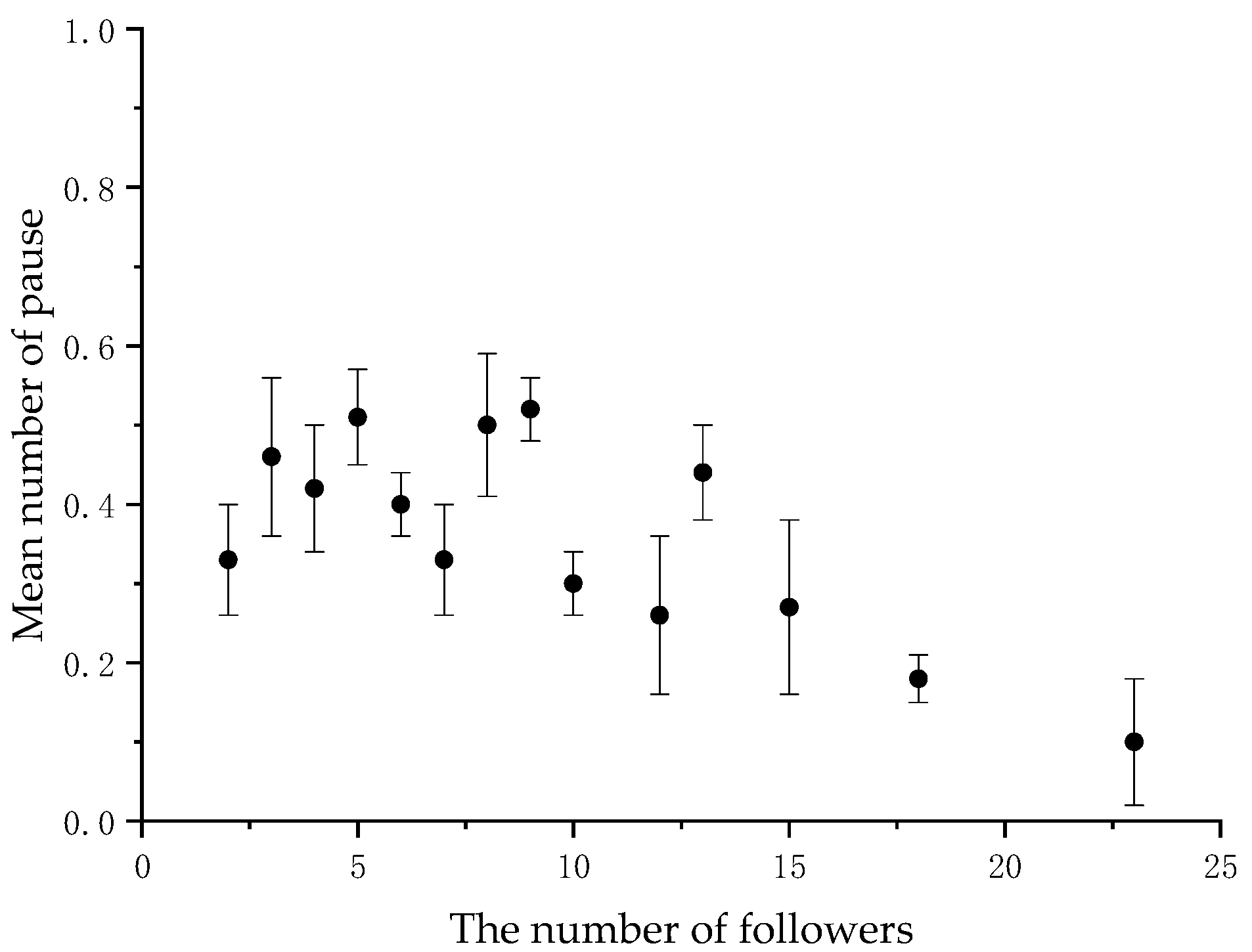
| Catalog | Definition |
|---|---|
| Initiator | The first individual that walks more than 10 m in less than 30 s. |
| Follower | Any individual that walks more than 5 m within 45° in the direction to which the initiator departs before the joining is terminated. |
| Successful movement | A successful collective movement is recorded when the number of all participants, including the initiator, is equal to or greater than 3. |
| Termination of joining | When no more individual joins the movement within five minutes after the joining of the last individual. |
| Back-glance | The individual looks in the direction of other group members, measured as a frequency throughout the movement (i.e., if the individual moves). In the cases, where eyes of animals could not be observed, we used the direction of the head (with an angle wider than 135° with the direction of the movement) to determine a back-glance. |
| Pause | The individual stops moving for at least 2 s. The frequency of pauses throughout the movement was recorded. The interval of two distinct pauses was more than 2 s. |
| Proximity | Two or more individuals keep a sitting or lying posture within a certain distance. The distance in this study was 1m. |
| Aggression | An individual stared, hit on the ground, chased, or bit another individual. |
| Submission | An individual was attacked by another, but away quickly or fled in opposite direction. |
| Hypothesis | Prediction | Supported by Analysis? |
|---|---|---|
| (1) Back-glance and pause are indeed used as communication signals. | (1) The signals will decrease significantly as the number of followers increases. | YES |
| (2) Discrimination in sex, age class, rank, number of relatives within the group, social network centrality, along with location in the movement queue would influence visual signal emissions. | (2) The further back the position, the weaker the visual signal. | Not supported: the back-glance and pause signals emitted by the participating individuals were stronger as the position moves further back. |
| (3) The emission of visual signals differs between females and males. | Not supported: Sex had no effect on visual signals. | |
| (4) There are variations in the emission of visual signals at different age class. | Not supported: Age class had no effect on visual signals. | |
| (5) There would be a negative relationship between rank and the frequency of visual signals. | Not supported: Rank had no effect on visual signals. | |
| (6) Individuals at the core of the social network would emit higher frequency visual signals | Partial supported: Individuals with higher eigenvector centrality coefficient emitted higher frequency of pause signal, but no effect on the back-glance signal. | |
| (7) A positive relationship would exist between the number of relatives within the group and the frequency of visual signals. | Partial supported: Individuals with more maternal relatives in the group had higher frequency of back-glance signal, but no effect on the pause signal. |
| Factor | Coefficient | SE | Z | p |
|---|---|---|---|---|
| Position | 0.037 | 0.015 | 2.46 | <0.01 |
| Sex | −0.290 | 0.155 | −1.87 | 0.061 |
| Age | −0.038 | 0.058 | −0.65 | 0.513 |
| Rank | 0.009 | 0.006 | 1.33 | 0.183 |
| Centrality | −0.135 | 0.511 | −0.26 | 0.792 |
| Relatives | 0.021 | 0.013 | 2.15 | <0.05 |
| _cons | 0.677 | 0.261 | 2.59 | <0.01 |
| Factor | Coefficient | SE | Z | p |
|---|---|---|---|---|
| Position | 0.035 | 0.014 | 2.44 | <0.05 |
| Sex | −0.169 | 0.133 | −1.27 | 0.203 |
| Age | 0.009 | 0.049 | 0.18 | 0.855 |
| Rank | 0.005 | 0.005 | 0.88 | 0.377 |
| Centrality | 0.015 | 0.008 | 0.59 | <0.05 |
| Relatives | 0.002 | 0.022 | 0.11 | 0.912 |
| _cons | 0.423 | 0.226 | 1.87 | 0.062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Wang, X.; Wu, M.; Chen, S.; Li, J. Tibetan Macaques with Higher Social Centrality and More Relatives Emit More Frequent Visual Communication in Collective Decision-Making. Animals 2021, 11, 876. https://doi.org/10.3390/ani11030876
Tang Z, Wang X, Wu M, Chen S, Li J. Tibetan Macaques with Higher Social Centrality and More Relatives Emit More Frequent Visual Communication in Collective Decision-Making. Animals. 2021; 11(3):876. https://doi.org/10.3390/ani11030876
Chicago/Turabian StyleTang, Zifei, Xi Wang, Mingyang Wu, Shiwang Chen, and Jinhua Li. 2021. "Tibetan Macaques with Higher Social Centrality and More Relatives Emit More Frequent Visual Communication in Collective Decision-Making" Animals 11, no. 3: 876. https://doi.org/10.3390/ani11030876
APA StyleTang, Z., Wang, X., Wu, M., Chen, S., & Li, J. (2021). Tibetan Macaques with Higher Social Centrality and More Relatives Emit More Frequent Visual Communication in Collective Decision-Making. Animals, 11(3), 876. https://doi.org/10.3390/ani11030876





