Animal Personality and Conservation: Basics for Inspiring New Research
Simple Summary
Abstract
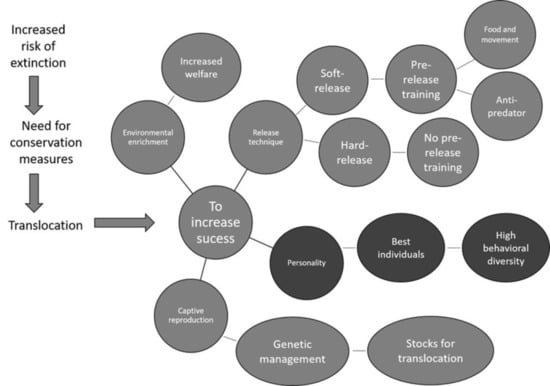
1. Introduction
2. Animal Personality and Animal Releases
2.1. Boldness, Exploration, and Activity: Influence on Survival after Release
2.2. Sociability
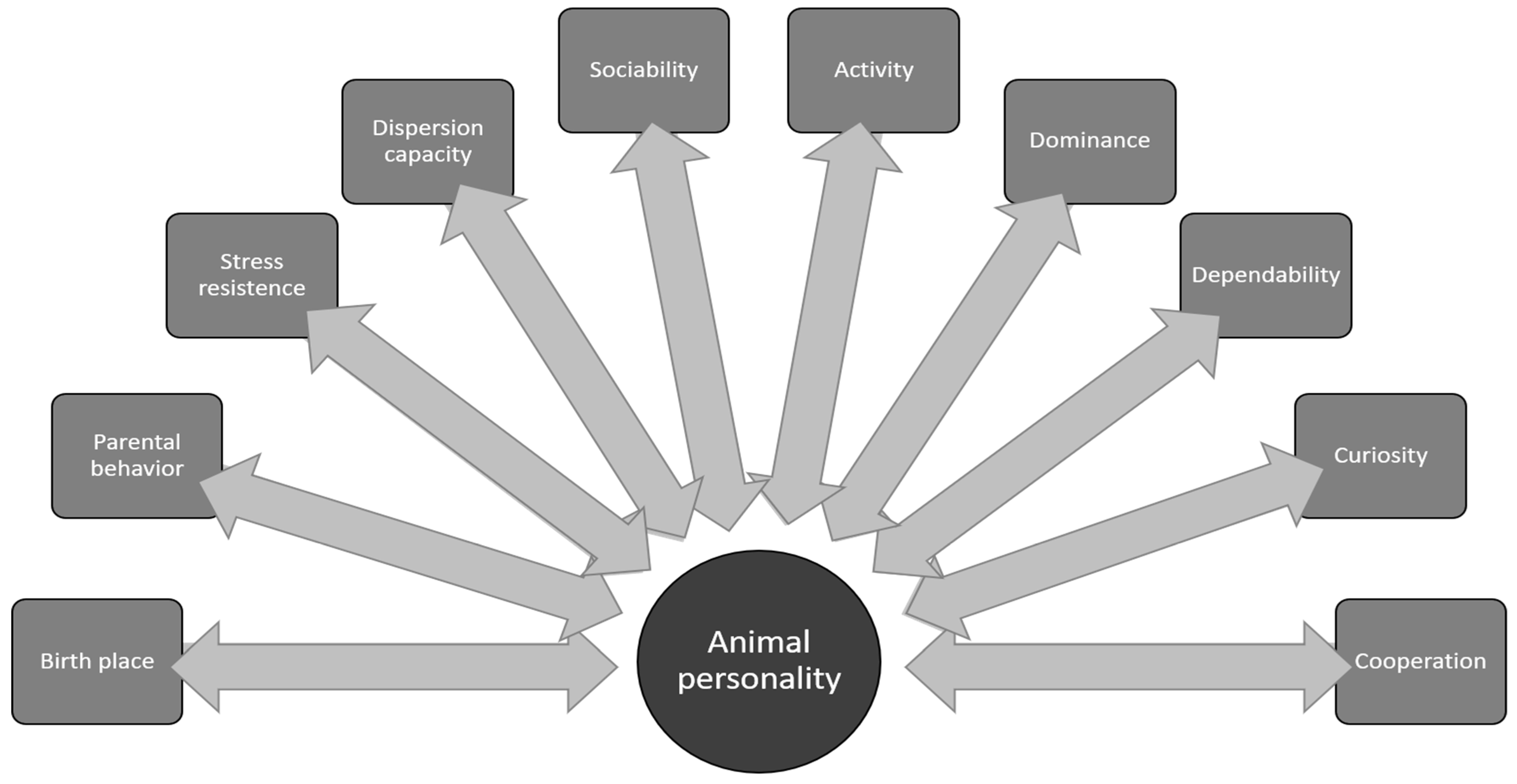
3. Other Links Between Personality and Species Conservation
3.1. Birth Environment
3.2. Stress
3.3. Dispersion
3.4. Reproduction
4. How to Evaluate Personality before Release
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Griffith, B.; Scott, J.M.; Carpenter, J.W.; Reed, C. Translocation as a Species Conservation Tool: Status and Strategy. Science 1989, 245, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Berger-Tal, O.; Blumstein, D.T.; Swaisgood, R.R. Conservation translocations: A review of common difficulties and promising directions. Anim. Conserv. 2020, 23, 121–131. [Google Scholar] [CrossRef]
- Benjamin-Fink, N.; Reilly, B.K. Conservation implications of wildlife translocations; The state’s ability to act as conservation units for wildebeest populations in South Africa. Glob. Ecol. Conserv. 2017, 12, 46–58. [Google Scholar] [CrossRef]
- Seddon, P.J.; Strauss, W.M.; Innes, J. Animal Translocations: What are they and why do we do them. In Reintroduction Biology: Integrating Science and Management; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 1–32. [Google Scholar] [CrossRef]
- Minterr, B.A.; Collins, J.P. Guidelines for Reintroductions and Other Conservation Translocations; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2010; Volume 20, ISBN 9782831716091. [Google Scholar]
- Resende, P.S.; Viana–Junior, A.B.; Young, R.J.; De Azevedo, C.S. A global review of animal translocation programs. Anim. Biodivers. Conserv. 2020, 2, 221–232. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D. An assessment of the published results of animal relocations. Biol. Conserv. 2000, 96, 1–11. [Google Scholar] [CrossRef]
- Wolf, C.M.; Garland, T.; Griffith, B. Predictors of avian and mammalian translocation success: Reanalysis with phylogenetically independent contrasts. Biol. Conserv. 1998, 86, 243–255. [Google Scholar] [CrossRef]
- Oro, D.; Martínez-Abraín, A.; Villuendas, E.; Sarzo, B.; Mínguez, E.; Carda, J.; Genovart, M. Lessons from a failed translocation program with a seabird species: Determinants of success and conservation value. Biol. Conserv. 2011, 144, 851–858. [Google Scholar] [CrossRef]
- Rummel, L.; Martínez-Abraín, A.; Mayol, J.; Ruiz-Olmo, J.; Mañas, F.; Jiménez, J.; Gómez, J.A.; Oro, D. Use of wild–caught individuals as a key factor for success in vertebrate translocations. Anim. Biodivers. Conserv. 2016, 39, 207–219. [Google Scholar] [CrossRef]
- Goldenberg, S.Z.; Owen, M.A.; Brown, J.L.; Wittemyer, G.; Oo, Z.M.; Leimgruber, P. Increasing conservation translocation success by building social functionality in released populations. Glob. Ecol. Conserv. 2019, 18, e00604. [Google Scholar] [CrossRef]
- Greggor, A.L.; Berger-Tal, O.; Blumstein, D.T.; Angeloni, L.; Bessa-Gomes, C.; Blackwell, B.F.; Clair, C.C.S.; Crooks, K.; De Silva, S.; Fernández-Juricic, E.; et al. Research Priorities from Animal Behaviour for Maximising Conservation Progress. Trends Ecol. Evol. 2016, 31, 953–964. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Polak, T.; Oron, A.; Lubin, Y.; Kotler, B.P.; Saltz, D. Integrating animal behavior and conservation biology: A conceptual framework. Behav. Ecol. 2011, 22, 236–239. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Blumstein, D.T.; Carroll, S.; Fisher, R.N.; Mesnick, S.L.; Owen, M.A.; Saltz, D.; Claire, C.C.S.; Swaisgood, R.R. A systematic survey of the integration of animal behavior into conservation. Conserv. Biol. 2016, 30, 744–753. [Google Scholar] [CrossRef]
- Shier, D. Manipulating animal behavior to ensure reintroduction success. Conservation Behavior 2016, 275–304. [Google Scholar] [CrossRef]
- Greggor, A.L.; Blumstein, D.T.; Wong, B.B.M.; Berger-Tal, O. Using animal behavior in conservation management: A series of systematic reviews and maps. Environ. Evid. 2019, 8, 23. [Google Scholar] [CrossRef]
- Angeloni, L.; Schlaepfer, M.A.; Lawler, J.J.; Crooks, K.R. A reassessment of the interface between conservation and behaviour. Anim. Behav. 2008, 75, 731–737. [Google Scholar] [CrossRef]
- Campbell, C.O.; Cheyne, S.M.; Rawson, B.M. Best Practice Guidelines for the Rehabilitation and Translocation of Gibbons; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2015; ISBN 9782831717203. [Google Scholar]
- Moseby, K.E.; Hill, B.M.; Lavery, T.H. Tailoring Release Protocols to Individual Species and Sites: One Size Does Not Fit All. PLoS ONE 2014, 9, e99753. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Sperry, J.H.; DeGregorio, B.A. Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biol. Conserv. 2019, 236, 324–331. [Google Scholar] [CrossRef]
- Alonso, R.; Orejas, P.; Lopes, F.; Sanz, C. Pre-release training of juvenile little owls Athene noctua to avoid predation. Anim. Biodivers. Conserv. 2011, 34, 389–393. [Google Scholar]
- Ebrahimi, M.; Bull, C.; Bull, M. Food supplementation reduces post-release dispersal during simulated translocation of the Endangered pygmy bluetongue lizard Tiliqua adelaidensis. Endanger. Species Res. 2012, 18, 169–178. [Google Scholar] [CrossRef][Green Version]
- Lopes, A.R.S.; Rocha, M.S.; Mesquita, W.U.; Drumond, T.; Ferreira, N.F.; Camargos, R.A.L.; Vilela, D.A.R.; Azevedo, C.S. Translocation and Post-Release Monitoring of Captive-Raised Blue-fronted Amazons Amazona aestiva. Acta Ornithol. 2018, 53, 37–48. [Google Scholar] [CrossRef]
- Vieira, B.P.; Fonseca, C.; Rocha, R.G. Critical steps to ensure the successful reintroduction of the Eurasian red squirrel. Anim. Biodivers. Conserv. 2015, 38, 49–58. [Google Scholar] [CrossRef]
- Jule, K.R.; Leaver, L.A.; Lea, S.E.G. The effects of captive experience on reintroduction survival in carnivores: A review and analysis. Biol. Conserv. 2008, 141, 355–363. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Weatherhead, P.J.; Tuberville, T.D.; Sperry, J.H. Time in captivity affects foraging behavior of ratsnakes: Implications for translocation. Herpetol. Conserv. Biol. 2013, 8, 581–590. [Google Scholar]
- Burk, K. How the Rehabilitation of Animals in Captivity Affects Survival Rates Upon Release: Focus on a Reintroduction Project with Amazonas vinacea; University of British Columbia Library: Vancouver, BC, Canada, 2012. [Google Scholar]
- Tenhumberg, B.; Tyre, A.J.; Shea, K.; Possingham, H.P. Linking Wild and Captive Populations to Maximize Species Persistence: Optimal Translocation Strategies. Conserv. Biol. 2004, 18, 1304–1314. [Google Scholar] [CrossRef]
- Scott, J.M.; Carpenter, J.W. Release of Captive-Reared or Translocated Endangered Birds: What Do We Need to Know? Auk 1987, 104, 544–545. [Google Scholar] [CrossRef]
- Brichieri-Colombi, T.A.; Lloyd, N.A.; McPherson, J.M.; Moehrenschlager, A. Limited contributions of released animals from zoos to North American conservation translocations. Conserv. Biol. 2018, 33, 33–39. [Google Scholar] [CrossRef]
- Robert, A. Captive breeding genetics and reintroduction success. Biol. Conserv. 2009, 142, 2915–2922. [Google Scholar] [CrossRef]
- Waples, K.A.; Stagoll, C.S. Ethical Issues in the Release of Animals from Captivity. Bioscience 1997, 47, 115–121. [Google Scholar] [CrossRef]
- McPhee, M.E. Effects of captivity on response to a novel environment in the oldfield mouse (Peromyscus polionotus subgriseus). Int. J. Comp. Psychol. 2003, 16, 85–94. [Google Scholar]
- Swaisgood, R.R. The conservation-welfare nexus in reintroduction programmes: A role for sensory ecology. Anim. Welf. 2010, 19, 125–137. [Google Scholar]
- De Faria, C.M.; de Souza Sá, F.; Costa, D.D.L.; Da Silva, M.M.; Da Silva, B.C.; Young, R.J.; De Azevedo, C.S. Captive-born collared peccary (Pecari tajacu, Tayassuidae) fails to discriminate between predator and non-predator models. Acta Ethologica 2018, 21, 175–184. [Google Scholar] [CrossRef]
- De Azevedo, C.S.; Young, R.J.; Rodrigues, M. Failure of captive-born greater rheas (Rhea americana, Rheidae, Aves) to discriminate between predator and nonpredator models. Acta Ethologica 2012, 15, 179–185. [Google Scholar] [CrossRef]
- Martin, M.S.; Owen, M.; Wintle, N.J.P.; Zhang, G.; Zhang, H.; Swaisgood, R.R. Stereotypic behaviour predicts reproductive performance and litter sex ratio in giant pandas. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Vickery, S.S.; Mason, G.J. Behavioural persistence in captive bears and its implication for reintroduction. Ursus 2003, 14, 35–43. [Google Scholar]
- Chudeau, K.R.; Johnson, S.P.; Caine, N.G. Enrichment reduces stereotypical behaviors and improves foraging development in rehabilitating Eastern Pacific Harbor Seals (Phoca vitulina richardii). Appl. Anim. Behav. Sci. 2019, 219, 104830. [Google Scholar] [CrossRef]
- McDougall, P.T.; Reale, D.; Sol, D.; Reader, S.M. Wildlife conservation and animal temperament: Causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim. Conserv. 2006, 9, 39–48. [Google Scholar] [CrossRef]
- Melfi, V. Is training zoo animals enriching? Appl. Anim. Behav. Sci. 2013, 147, 299–305. [Google Scholar] [CrossRef]
- Griffin, A.S.; Blumstein, D.T.; Evans, C.S. Training Captive-Bred or Translocated Animals to Avoid Predators. Conserv. Biol. 2000, 14, 1317–1326. [Google Scholar] [CrossRef]
- De Faria, C.M.; de Souza Sá, F.; Lovenstain Costa, D.D.; da Silva, M.M.; da Silva, B.C.; Young, R.J.; de Azevedo, C.S. Captive-born collared peccaries learning about their predators: Lessons learnt but not remembered. Behav. Process. 2020, 171, 104031. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, C.S.; Young, R.J. Behavioural responses of captive-born greater rheas Rhea americana Linnaeus (Rheiformes, Rheidae) submitted to antipredator training. Rev. Bras. Zool. 2006, 23, 186–193. [Google Scholar] [CrossRef]
- Akhund-Zade, J.; Ho, S.; O’Leary, C.; De Bivort, B. The effect of environmental enrichment on behavioral variability depends on genotype, behavior, and type of enrichment. J. Exp. Biol. 2019, 222, jeb202234. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral Diversity as a Potential Indicator of Positive Animal Welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Amaya-Villarreal, Á.M.; Estrada, A.; Vargas-Ramírez, N. Use of Wild Foods During the Rainy Season by a Reintroduced Population of Scarlet Macaws (Ara macao cyanoptera) in Palenque, Mexico. Trop. Conserv. Sci. 2015, 8, 455–478. [Google Scholar] [CrossRef]
- Haage, M.; Maran, T.; Bergvall, U.A.; Elmhagen, B.; Angerbjörn, A. The influence of spatiotemporal conditions and personality on survival in reintroductions–evolutionary implications. Oecologia 2017, 183, 45–56. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, C.S.; Young, R.J. Shyness and boldness in greater rheas Rhea americana Linnaeus (Rheiformes, Rheidae): The effects of antipredator training on the personality of the birds. Rev. Bras. Zool. 2006, 23, 202–210. [Google Scholar] [CrossRef]
- Merrick, M.J.; Koprowski, J.L. Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 2017, 209, 34–44. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Stamps, J.; Groothuis, T.G.G. The development of animal personality: Relevance, concepts and perspectives. Biol. Rev. 2010, 85, 301–325. [Google Scholar] [CrossRef]
- Carere, C.; Maestripieri, D. Animal Personalities: Behavior, Physiology, and Evolution, 1st ed.; The University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Gosling, S.D.; John, O.P. Personality Dimensions in Nonhuman Animals. Curr. Dir. Psychol. Sci. 1999, 8, 69–75. [Google Scholar] [CrossRef]
- Weiss, A. Personality Traits: A View from the Animal Kingdom. J. Personal. 2018, 86, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Mooney, A.; Conde, D.A.; Healy, K.; Buckley, Y.M. A system wide approach to managing zoo collections for visitor attendance and in situ conservation. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Sampaio, M.B.; Schiel, N.; Souto, A. From exploitation to conservation: A historical analysis of zoos and their functions in human societies. Ethnobiol. Conserv. 2020, 9, 1–32. [Google Scholar] [CrossRef]
- Cuarón, A.D. Further role of zoos in conservation: Monitoring wildlife use and the dilemma of receiving donated and confiscated animals. Zoo Biol. 2005, 24, 115–124. [Google Scholar] [CrossRef]
- Kierulff, M.C.M.; Ruiz-Miranda, C.R.; de Oliveira, P.P.; Beck, B.B.; Martins, A.; Dietz, J.M.; Rambaldi, D.M.; Baker, A.J. The Golden lion tamarin Leontopithecus rosalia: A conservation success story. Int. Zoo Yearb. 2012, 46, 36–45. [Google Scholar] [CrossRef]
- Jachowski, D.S.; Lockhart, J.M. Reintroducing the Black—Footed Ferret Mustela nigripes to the Great Plains of North America. Small Carniv. Conserv. 2009, 41, 58–64. [Google Scholar]
- Harding, L.E.; Abu-Eid, O.F.; Hamidan, N.; Al Sha’Lan, A. Reintroduction of the Arabian oryx Oryx leucoryx in Jordan: War and redemption. Oryx 2007, 41, 478–487. [Google Scholar] [CrossRef]
- Sorenson, K.J.; Burnett, L.J.; Stake, M.M. Restoring a Bald Eagle Breeding Population in Central California and Monitoring 25 Years of Regional Population Growth. J. Raptor Res. 2017, 51, 145–152. [Google Scholar] [CrossRef]
- Bajomi, B.; Pullin, A.S.; Stewart, G.B.; Takács-Sánta, A. Bias and dispersal in the animal reintroduction literature. Oryx 2010, 44, 358–365. [Google Scholar] [CrossRef]
- Seddon, P.J.; Soorae, P.S.; Launay, F. Taxonomic bias in reintroduction projects. Anim. Conserv. 2005, 8, 51–58. [Google Scholar] [CrossRef]
- Bremner-Harrison, S.; Prodohl, P.A.; Elwood, R.W. Behavioural trait assessment as a release criterion: Boldness predicts early death in a reintroduction programme of captive-bred swift fox (Vulpes velox). Anim. Conserv. 2004, 7, 313–320. [Google Scholar] [CrossRef]
- De Azevedo, C.S.; Rodrigues, L.S.F.; Fontenelle, J.C.R. Important tools for Amazon Parrot reintroduction programs. Rev. Bras. Ornitol. 2017, 25, 1–11. [Google Scholar] [CrossRef]
- Allard, S.; Fuller, G.; Torgerson-White, L.; Starking, M.D.; Yoder-Nowak, T. Personality in Zoo-Hatched Blanding’s Turtles Affects Behavior and Survival After Reintroduction into the Wild. Front. Psychol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.M.; Parlato, E.H.; Walker, L.K.; Parker, K.A.; Ewen, J.G.; Armstrong, D.P. Links between personality, early natal nutrition and survival of a threatened bird. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190373. [Google Scholar] [CrossRef]
- May, T.M.; Page, M.J.; Fleming, P.A. Predicting survivors: Animal temperament and translocation. Behav. Ecol. 2016, 27, 969–977. [Google Scholar] [CrossRef]
- Herdegen-Radwan, M. Bolder guppies do not have more mating partners, yet sire more offspring. BMC Evol. Biol. 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schuett, W.; Tregenza, T.; Dall, S.R.X. Sexual selection and animal personality. Biol. Rev. 2010, 85, 217–246. [Google Scholar] [CrossRef]
- Wilson, A.D.M.; Binder, T.R.; McGrath, K.P.; Cooke, S.J.; Godin, J.J. Capture technique and fish personality: Angling targets timid bluegill sunfish, Lepomis macrochirus. Can. J. Fish. Aquat. Sci. 2011, 68, 749–757. [Google Scholar] [CrossRef]
- Kelleher, S.R.; Silla, A.J.; Byrne, P.G. Animal personality and behavioral syndromes in amphibians: A review of the evidence, experimental approaches, and implications for conservation. Behav. Ecol. Sociobiol. 2018, 72, 79. [Google Scholar] [CrossRef]
- Smith, B.R.; Blumstein, D.T. Animal personalities and conservation biology: The importance of behavioral diversity. In Animal Personalities: Behavior, Physiology, and Evolution; Carere, C., Maestripieri, D., Eds.; University of Chicago Press: Chicago, IL, USA, 2013; pp. 379–411. [Google Scholar]
- Sinn, D.L.; Cawthen, L.; Jones, S.M.; Pukk, C.; Jones, M.E. Boldness towards novelty and translocation success in captive-raised, orphaned Tasmanian devils. Zoo Biol. 2013, 33, 36–48. [Google Scholar] [CrossRef]
- Sinn, D.L.; Moltschaniwskyj, N.A.; Wapstra, E.; Dall, S.R.X. Are behavioral syndromes invariant? Spatiotemporal variation in shy/bold behavior in squid. Behav. Ecol. Sociobiol. 2009, 64, 693–702. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Both, C.; Drent, P.J.; Tinbergen, J.M. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. B Biol. Sci. 2004, 271, 847–852. [Google Scholar] [CrossRef]
- Mathot, K.J.; Wright, J.; Kempenaers, B.; Dingemanse, N.J. Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos 2012, 121, 1009–1020. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Réale, D. Natural selection and animal personality. Behaviour 2005, 142, 1159–1184. [Google Scholar] [CrossRef]
- Lopes, A.R.S.; Rocha, M.S.; Junior, M.G.J.; Mesquita, W.U.; Silva, G.G.G.R.; Vilela, D.A.R.; Azevedo, C.S. The influence of anti-predator training, personality and sex in the behavior, dispersion and survival rates of translocated captive-raised parrots. Glob. Ecol. Conserv. 2017, 11, 146–157. [Google Scholar] [CrossRef]
- van den Berg, P.; Weissing, F.J. William von Hippel 12. In Evolutionary Perspectives on Social Psychology; Zeigler-Hill, V., Welling, L.L.M., Shackelford, T.K., Eds.; Springer: Cham, Switzerland, 2015; pp. 149–158. ISBN 9783319126975. [Google Scholar]
- Ericsson, R.J.; Langevin, C.N.; Nishino, M. Isolation of Fractions rich in Human Y Sperm. Nat. Cell Biol. 1973, 246, 421–424. [Google Scholar] [CrossRef]
- Watters, J.V.; Sih, A. The mix matters: Behavioural types and group dynamics in water striders. Behaviour 2005, 142, 1417–1431. [Google Scholar] [CrossRef]
- Wolf, M.; Weissing, F.J. Animal personalities: Consequences for ecology and evolution. Trends Ecol. Evol. 2012, 27, 452–461. [Google Scholar] [CrossRef]
- Van Oers, K.; Mueller, J.C. Evolutionary genomics of animal personality. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3991–4000. [Google Scholar] [CrossRef]
- Van Oers, K.; Sinn, D.L. Quantitative and molecular genetics of animal personality. In Animal Personalities: Behavior, Physiology, and Evolution; Carere, C., Maestripieri, D., Eds.; University of Chicago Press: Chicago, IL, USA, 2013; pp. 149–200. [Google Scholar]
- Petelle, M.B.; Martin, J.G.A.; Blumstein, D.T. Heritability and genetic correlations of personality traits in a wild population of yellow-bellied marmots (Marmota flaviventris). J. Evol. Biol. 2015, 28, 1840–1848. [Google Scholar] [CrossRef]
- Cordero-Rivera, A. Behavioral Diversity (Ethodiversity): A Neglected Level in the Study of Biodiversity. Front. Ecol. Evol. 2017, 5, 7. [Google Scholar] [CrossRef]
- Sih, A.; Mathot, K.J.; Moirón, M.; Montiglio, P.-O.; Wolf, M.; Dingemanse, N.J. Animal personality and state–behaviour feedbacks: A review and guide for empiricists. Trends Ecol. Evol. 2015, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Moiron, M.; Laskowski, K.L.; Niemelä, P.T. Individual differences in behaviour explain variation in survival: A meta-analysis. Ecol. Lett. 2020, 23, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Bergmüller, R.; Taborsky, M. Animal personality due to social niche specialisation. Trends Ecol. Evol. 2010, 25, 504–511. [Google Scholar] [CrossRef]
- Von Merten, S.; Zwolak, R.; Rychlik, L. Social personality: A more social shrew species exhibits stronger differences in personality types. Anim. Behav. 2017, 127, 125–134. [Google Scholar] [CrossRef]
- Laskowski, K.L.; Pruitt, J.N. Evidence of social niche construction: Persistent and repeated social interactions generate stronger personalities in a social spider. Philos. Trans. R. Soc. B Biol. Sci. 2014, 281, 20133166. [Google Scholar] [CrossRef]
- Castanheira, M.F.; Cerqueira, M.; Millot, S.; Gonçalves, R.A.; Oliveira, C.C.V.; Conceição, L.E.C.; Martins, C.I.M. Are personality traits consistent in fish?—The influence of social context. Appl. Anim. Behav. Sci. 2016, 178, 96–101. [Google Scholar] [CrossRef]
- Jolles, J.W.; Taylor, B.A.; Manica, A. Recent social conditions affect boldness repeatability in individual sticklebacks. Anim. Behav. 2016, 112, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Laplaza, L.M.; Morgan, E. Effects of short-term isolation on the locomotor activity of the angelfish (Pterophyllum scalare). J. Comp. Psychol. 1991, 105, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.C.; Pinaud, D.; Weimerskirch, H. Boldness predicts an individual’s position along an exploration-exploitation foraging trade-off. J. Anim. Ecol. 2017, 86, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Stumpe, M.C.; Manica, A.; Johnstone, R.A. Experience overrides personality differences in the tendency to follow but not in the tendency to lead. Philos. Trans. R. Soc. B Biol. Sci. 2013, 280, 20131724. [Google Scholar] [CrossRef]
- Duboscq, J.; Romano, V.; MacIntosh, A.; Sueur, C. Social Information Transmission in Animals: Lessons from Studies of Diffusion. Front. Psychol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pouca, C.V.; Heinrich, D.; Huveneers, C.; Brown, C. Social learning in solitary juvenile sharks. Anim. Behav. 2020, 159, 21–27. [Google Scholar] [CrossRef]
- Snijders, L.; Naguib, M. Chapter Eight—Communication in Animal Social Networks: A Missing Link. Adv. Study Behav. 2017, 49, 297–359. [Google Scholar] [CrossRef]
- Bratko, D.; Butković, A.; Hlupić, T.V. Heritability of Personality. Psihol. teme 2017, 26, 1–24. [Google Scholar] [CrossRef]
- Huang, P.; St. Mary, C.M.; Kimball, R.T. Habitat urbanization and stress response are primary predictors of personality variation in northern cardinals (Cardinalis cardinalis). J. Urban Ecol. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Herborn, K.A.; Macleod, R.; Miles, W.T.S.; Schofield, A.N.B.; Alexander, L.; Arnold, K.E. Personality in captivity reflects personality in the wild. Anim. Behav. 2010, 79, 835–843. [Google Scholar] [CrossRef]
- Reddon, A.R. Parental effects on animal personality. Behav. Ecol. 2012, 23, 242–245. [Google Scholar] [CrossRef]
- Hertler, S.C. Beyond birth order: The biological logic of personality variation among siblings. Cogent Psychol. 2017, 4, 1325570. [Google Scholar] [CrossRef]
- Zajitschek, S.; Herbert-Read, J.E.; Abbasi, N.M.; Zajitschek, F.; Immler, S. Paternal personality and social status influence offspring activity in zebrafish. BMC Evol. Biol. 2017, 17, 157. [Google Scholar] [CrossRef]
- Chira, A. How does parental personality influence offspring quality in animals? Ann. For. Res. 2014, 57, 347–362. [Google Scholar] [CrossRef]
- Mutzel, A.; Dingemanse, N.J.; Araya-Ajoy, Y.G.; Kempenaers, B. Parental provisioning behaviour plays a key role in linking personality with reproductive success. Philos. Trans. R. Soc. B Biol. Sci. 2013, 280, 20131019. [Google Scholar] [CrossRef]
- Baugh, A.T.; Senft, R.A.; Firke, M.; Lauder, A.; Schroeder, J.; Meddle, S.L.; Van Oers, K.; Hau, M. Risk-averse personalities have a systemically potentiated neuroendocrine stress axis: A multilevel experiment in Parus major. Horm. Behav. 2017, 93, 99–108. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Teixeira, C.P.; de Azevedo, C.S.; Mendl, M.; Cipreste, C.F.; Young, R.J. Revisiting translocation and reintroduction programmes: The importance of considering stress. Anim. Behav. 2007, 73, 1–13. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Carere, C.; Van Oers, K. Shy and bold great tits (Parus major): Body temperature and breath rate in response to handling stress. Physiol. Behav. 2004, 82, 905–912. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Individual consistency in flight initiation distances in burrowing owls: A new hypothesis on disturbance-induced habitat selection. Biol. Lett. 2009, 6, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, B.; Santos, E.S.A.; Lara, C.E.; Nakagawa, S. Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance. Philos. Trans. R. Soc. B Biol. Sci. 2017, 284, 20170943. [Google Scholar] [CrossRef]
- Monceau, K.; Dechaume-Moncharmont, F.-X.; Moreau, J.; Lucas, C.; Capoduro, R.; Motreuil, S.; Moret, Y. Personality, immune response and reproductive success: An appraisal of the pace-of-life syndrome hypothesis. J. Anim. Ecol. 2017, 86, 932–942. [Google Scholar] [CrossRef]
- Carter, A.J.; Goldizen, A.W.; Tromp, S.A. Agamas exhibit behavioral syndromes: Bolder males bask and feed more but may suffer higher predation. Behav. Ecol. 2010, 21, 655–661. [Google Scholar] [CrossRef]
- Both, C.; Dingemanse, N.J.; Drent, P.J.; Tinbergen, J.M. Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 2005, 74, 667–674. [Google Scholar] [CrossRef]
- Razal, C.; Pisacane, C.; Miller, L. Multifaceted Approach to Personality Assessment in Cheetahs (Acinonyx jubatus). Anim. Behav. Cogn. 2016, 3, 22–31. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- Princée, F.P.G. Exploring Studbooks for Wildlife Management and Conservation; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-50031-7. [Google Scholar]
- Mellen, J.D. Factors influencing reproductive success in small captive exotic felids (Felis spp.): A multiple regression analysis. Zoo Biol. 1991, 10, 95–110. [Google Scholar] [CrossRef]
- Løvendahl, P.; Damgaard, L.H.; Nielsen, B.L.; Thodberg, K.; Su, G.; Rydhmer, L. Aggressive behaviour of sows at mixing and maternal behaviour are heritable and genetically correlated traits. Livest. Prod. Sci. 2005, 93, 73–85. [Google Scholar] [CrossRef]
- Watters, J.V.; Powell, D.M. Measuring Animal Personality for Use in Population Management in Zoos: Suggested Methods and Rationale. Zoo Biol. 2011, 31, 1–12. [Google Scholar] [CrossRef]
- Highfill, L.; Hanbury, D.; Kristiansen, R.; Kuczaj, S.; Watson, S. Rating vs. coding in animal personality research. Zoo Biol. 2009, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Murayama, M.; Kawamura, S.; Weiss, A. From Genes to Animal Behavior: Social Structures, Personalities, Communication by Color; Springer: Tokyo, Japan, 2011. [Google Scholar]
- Meagher, R.K. Observer ratings: Validity and value as a tool for animal welfare research. Appl. Anim. Behav. Sci. 2009, 119, 1–14. [Google Scholar] [CrossRef]
- Finkemeier, M.-A.; Langbein, J.; Puppe, B. Personality Research in Mammalian Farm Animals: Concepts, Measures, and Relationship to Welfare. Front. Veter. Sci. 2018, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Zoo, S.L. Applications of Personality to the Management and Conservation of Nonhuman Animals. In From Genes to Animal Behavior; Inoue-Murayama, M., Kawamura, S., Weiss, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 185–199. ISBN 9784431538929. [Google Scholar]
- Tetley, C.L.; O’Hara, S.J. Ratings of animal personality as a tool for improving the breeding, management and welfare of zoo mammals. Anim. Welf. 2012, 21, 463–476. [Google Scholar] [CrossRef]
- Minero, M.; Costa, E.D.; Dai, F.; Murray, L.A.M.; Canali, E.; Wemelsfelder, F. Use of Qualitative Behaviour Assessment as an indicator of welfare in donkeys. Appl. Anim. Behav. Sci. 2016, 174, 147–153. [Google Scholar] [CrossRef]
- Góis, K.C.R.; Ceballos, M.C.; Sant’Anna, A.C.; Da Costa, M.J.R.P. Using an observer rating method to assess the effects of rotational stocking method on beef cattle temperament over time. Rev. Bras. Zootec. 2016, 45, 501–508. [Google Scholar] [CrossRef]
- Kubinyi, E.; Gosling, S.D.; Miklósi, Á. A comparison of rating and coding behavioural traits in dogs. Acta Biol. Hung. 2015, 66, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Horback, K.M.; Miller, L.J.; Kuczaj, S.A. Personality assessment in African elephants (Loxodonta africana): Comparing the temporal stability of ethological coding versus trait rating. Appl. Anim. Behav. Sci. 2013, 149, 55–62. [Google Scholar] [CrossRef]
- Ijichi, C.; Collins, L.M.; Creighton, E.; Elwood, R.W. Harnessing the power of personality assessment: Subjective assessment predicts behaviour in horses. Behav. Process. 2013, 96, 47–52. [Google Scholar] [CrossRef]
- Silva, V.S.; Azevedo, C.S. Evaluating personality traits of captive maned wolves, Chrysocyon brachyurus (Illiger, 1815) (Mammalia: Canidae), for conservation purposes. Lundiana 2013, 11, 35–41. [Google Scholar]
- Carlstead, K.; Mellen, J.; Kleiman, D.G. Black rhinoceros (Diceros bicornis) in U.S. Zoos: I. Individual behavior profiles and their relationship to breeding success. Zoo Biol. 1999, 18, 17–34. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780511810893. [Google Scholar]
- Beckmann, C.; Biro, P.A. On the Validity of a Single (Boldness) Assay in Personality Research. Ethology 2013, 119, 937–947. [Google Scholar] [CrossRef]
- Bremner-Harrison, S.; Cypher, B.L.; Job, C.V.H.; Harrison, S.W.R. Assessing personality in San Joaquin kit fox in situ: Efficacy of field-based experimental methods and implications for conservation management. J. Ethol. 2018, 36, 23–33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Azevedo, C.S.; Young, R.J. Animal Personality and Conservation: Basics for Inspiring New Research. Animals 2021, 11, 1019. https://doi.org/10.3390/ani11041019
de Azevedo CS, Young RJ. Animal Personality and Conservation: Basics for Inspiring New Research. Animals. 2021; 11(4):1019. https://doi.org/10.3390/ani11041019
Chicago/Turabian Stylede Azevedo, Cristiano Schetini, and Robert John Young. 2021. "Animal Personality and Conservation: Basics for Inspiring New Research" Animals 11, no. 4: 1019. https://doi.org/10.3390/ani11041019
APA Stylede Azevedo, C. S., & Young, R. J. (2021). Animal Personality and Conservation: Basics for Inspiring New Research. Animals, 11(4), 1019. https://doi.org/10.3390/ani11041019