Heat Stress and Goat Welfare: Adaptation and Production Considerations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Goat as the Future Animal from Food Security Perspectives
3. Heat Stress and Goat Production
4. Physiological Mechanisms of Goats towards Adaptation or Reaction to Heat Stress
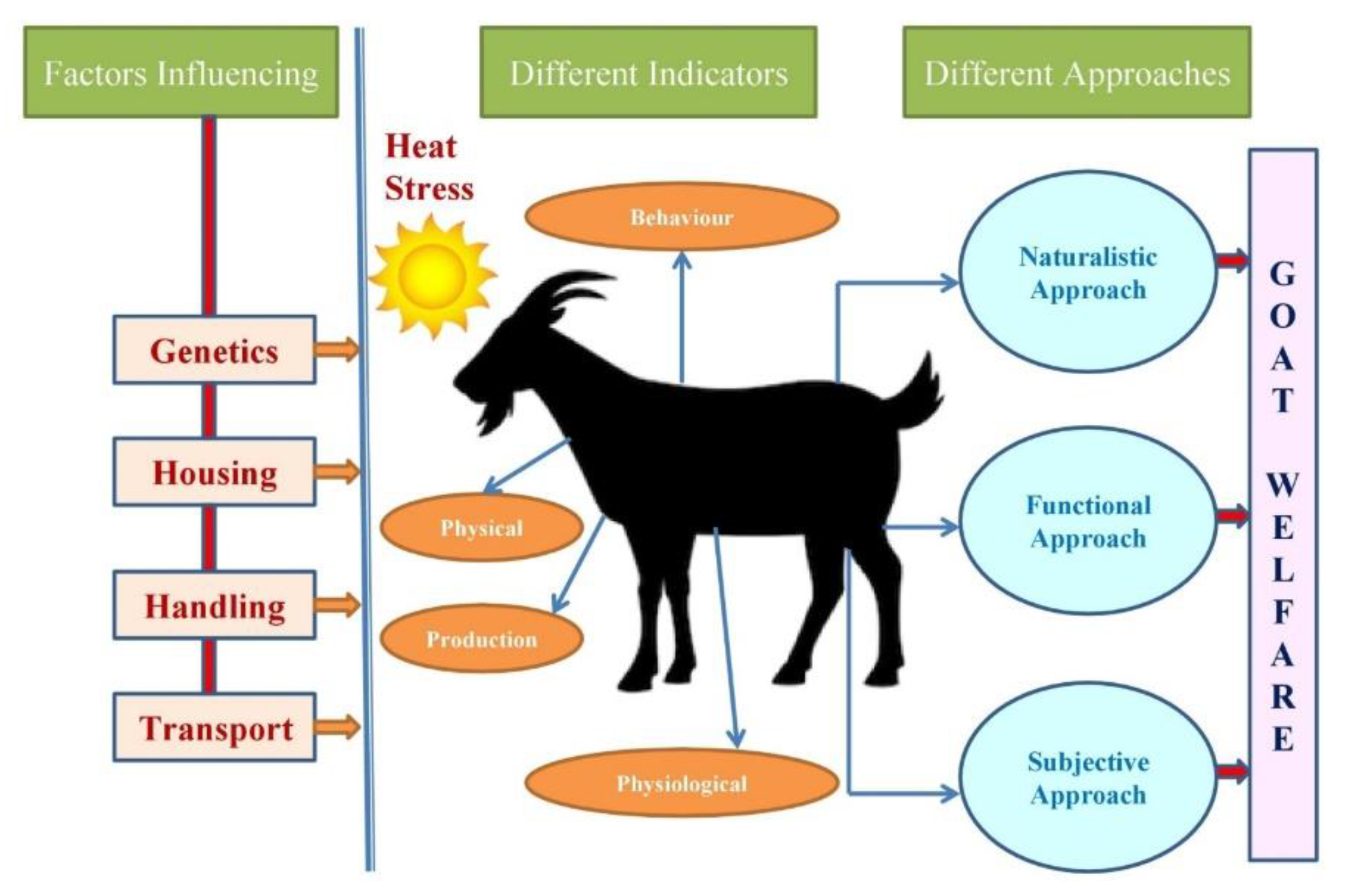
5. Welfare Considerations in Goat Production
5.1. Housing and Environment
5.2. Breeding and Genetics
5.3. Handling and Transport
5.3.1. Handling of Goats after Transport
5.3.2. Preloading Precautions
5.3.3. Break Journey during Transport
5.3.4. Transport during Cool Hours of the Day
5.3.5. Ensuring Clean Drinking Water Periodically during Transport
5.3.6. Supplementation of Electrolyte during Transport
6. Effect of the Production Systems on Welfare of Goats
7. Different Approaches and Methodologies to Assess Welfare in Goats
7.1. Different Approaches to Assess Welfare in Goats
7.1.1. Naturalistic Approach
7.1.2. Functional Approach
7.1.3. Subjective Approach
7.2. Different Methodologies to Assess Goat Welfare
8. Different Indicators of Goat Welfare
8.1. Behavioral Indicators
8.2. Physical Indicators
8.3. Physiological Indicators
8.4. Productive Indicators
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, P.K.; Gerber, P. Climate change and the growth of the livestock sector in developing countries. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 169–184. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Adaptation of animals to heat stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef] [Green Version]
- Thornton, P.K.; van de Steeg, J.; Notenbaert, A.; Herrero, M. The impacts of climate change on livestock and livestock systems in developing countries: A review of what we know and what we need to know. Agric. Syst. 2009, 101, 113–127. [Google Scholar] [CrossRef]
- Serradilla, J.M.; Carabaño, M.J.; Ramón, M.; Molina, A.; Diaz, C.; Menéndez-Buxadera, A. Characterisation of Goats’ Response to Heat Stress: Tools to Improve Heat Tolerance. Goat Sci. 2018, 15, 329–347. [Google Scholar]
- Stone, T.F.; Francis, C.A.; Eik, L.O. A survey of dairy-goat keeping in Zanzibar. Afr. J. Food Agric. Nutri. Dev. 2020, 20, 16220–16235. [Google Scholar] [CrossRef]
- Chebli, Y.; El Otmani, S.; Chentouf, M.; Hornick, J.L.; Bindelle, J.; Cabaraux, J.F. Foraging behavior of goats browsing in Southern Mediterranean forest rangeland. Animals 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, A.; Chakravarty, A.K. Disease resistance for different livestock species. Genet. Breed. Dis. Resist. Livest. 2020, 271. [Google Scholar]
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas. J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef]
- Kim, E.S.; Elbeltagy, A.R.; Aboul-Naga, A.M.; Rischkowsky, B.; Sayre, B.; Rothcschild, M.F. Multiple genomic signatures of selection in goats and sheep indigenous to a hot arid environment. Heridity 2016, 116, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.M.C.; Paiva, S.R.; Araújo, A.M.; Mariante, A.; Blackburn, H.D. Genetic structure of goat breeds from Brazil and the United States: Implications for conservation and breeding programs. J. Anim. Sci. 2015, 93, 4629–4636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megersa, B.; Markemann, A.; Angassa, A.; Ogutu, J.O.; Piepho, H.P.; Zárate, A.V. Livestock diversification: An adaptive strategy to climate and rangeland ecosystem changes in southern Ethiopia. Hum. Ecol. 2014, 42, 509–520. [Google Scholar] [CrossRef]
- Araújo, G.G.L.D.; Voltolini, T.V.; Chizzotti, M.L.; Turco, S.H.N.; Carvalho, F.F.R.D. Water and small ruminant production. Rev. Bras. Zootec. 2010, 39, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Fuller, A.; Mitchell, D.; Maloney, S.K.; Hetem, R.S. Towards a mechanistic understanding of the responses of large terrestrial mammals to heat and aridity associated with climate change. Clim. Chang. Responses 2016, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sanon, H.O.; Kaboré-Zoungrana, C.; Ledin, I. Behaviour of goats, sheep and cattle and their selection of browse species on natural pasture in a Sahelian area. Small Rumin. Res. 2007, 67, 64–74. [Google Scholar] [CrossRef]
- Formiga, L.D.A.D.S.; Paulo, P.F.M.D.; Cassuce, M.R.; Andrade, A.P.D.; Silva, D.S.D.; Saraiva, E.P. Ingestive behavior and feeding preference of goats reared in degraded caatinga. Ciência Anim. Bras. 2020, 21, e-5243. [Google Scholar] [CrossRef]
- Domingue, B.F.; Dellow, D.W.; Wilson, P.R.; Barry, T.N. Comparative digestion in deer, goats, and sheep. N. Z. J. Agric. Res. 1991, 34, 45–53. [Google Scholar] [CrossRef]
- Stilwell, G. Small ruminants’ welfare assessment—Dairy goat as an example. Small Rumin. Res. 2016, 142, 51–54. [Google Scholar] [CrossRef]
- Farias Machado, N.A.; Filho, J.A.D.B.; de Oliveira, K.P.L.; Parente, M.D.O.M.; de Siqueira, J.C.; Pereira, A.M.; Santos, A.R.D.; Sousa, J.M.S.; Rocha, K.S.; Viveiros, K.K.D.S.; et al. Biological rhythm of goats and sheep in response to heat stress. Bio. Rhythm Res. 2020, 51, 1044–1052. [Google Scholar] [CrossRef]
- Archana, P.R.; Sejian, V.; Ruban, W.; Bagath, M.; Krishnan, G.; Aleena, J.; Manjunathareddy, G.B.; Beena, V.; Bhatta, R. Comparative assessment of heat stress induced changes in carcass traits, plasma leptin profile and skeletal muscle myostatin and HSP70 gene expression patterns between indigenous Osmanabadi and Salem Black goat breeds. Meat Sci. 2018, 141, 66–80. [Google Scholar] [CrossRef]
- Pragna, P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Soren, N.M.; Beena, V.; Bhatta, R. Comparative assessment of growth performance of three different indigenous goat breeds exposed to summer heat stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, 825–836. [Google Scholar] [CrossRef]
- Aleena, J.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Resilience of three indigenous goat breeds to heat stress based on phenotypic traits and PBMC HSP70 expression. Int. J. Biometeorol. 2018, 62, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N. Effect of heat stress on the welfare of extensively managed domestic Ruminants. Review article. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Peacock, C.; Sherman, D.M. Sustainable goat production—Some global perspectives. Small Rumin. Res. 2010, 89, 70–80. [Google Scholar] [CrossRef]
- Alam, M.; Siwar, C.; Islam, R.; Toriman, M.E.; Basri, T. Climate change and vulnerability of paddy cultivation in north-west Selangor, Malaysia: A survey of farmers’ assessment/Md. Acad. Ser. Univ. Teknol. MARA Kedah 2011, 6, 45–56. [Google Scholar]
- Darcan, N.K.; Silanikove, N. The advantages of goats for future adaptation to climate change: A conceptual overview. Small Rumin. Res. 2018, 163, 34–38. [Google Scholar] [CrossRef]
- Rokonuzzaman, M.; Islam, M. Participation of rural women in goat rearing in a selected area of Bangladesh. J. Bangladesh Agric. Uni. 2009, 7, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Rao, C.A.; Kareemulla, K.; Venkateswarlu, B. Role of goats in Livelihood security of rural poor in the less favoured environments. Indian J. Agric. Econ. 2010, 65, 760–781. [Google Scholar]
- Ngambi, J.W.; Alabi, O.J.; Alabi, D.N.J.; Norris, D. Role of goats in food security, poverty alleviation and prosperity withspecial reference to Sub-Saharan Africa: A review. Indian J. Anim. Res. 2013, 47, 1–8. [Google Scholar]
- Raut, M.S.; Kurpatwar, L.C. Commercial Goat Farming in India: An Emerging Agri-Business Opportunity. Stud. Indian Place Names 2020, 40, 1034–1039. [Google Scholar]
- Mangwai, T.; Fahim, A.; Singh, R.; Ali, N.; Kumar, A.; Sahu, D.S. Feeding efficiency of improved feeder in stall fed kids. Indian J. Small Rumin. 2020, 26, 67–70. [Google Scholar] [CrossRef]
- Salama, A.A.K.; Caja, G.; Hamzaoui, S.; Badaoui, B.; Castro-Costa, A.; Façanha, D.A.E.; Guilhermino, M.M.; Bozzi, R. Different levels of response to heat stress in dairy goats. Small Rumin. Res. 2014, 121, 73–79. [Google Scholar] [CrossRef]
- Hashem, M.A.; Hossain, M.M.; Rana, M.S.; Islam, M.S.; Saha, N.G. Effect of heat stress on blood parameter, carcass and meat quality of Black Bengal goat. Bangladesh J. Anim. Sci. 2013, 42, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Dangi, S.S.; Gupta, M.; Dangi, S.K.; Chouhan, V.S.; Maurya, V.P.; Kumar, P.; Singh, G.; Sarkar, M. Expression of HSPs: An adaptive mechanism during long-term heat stress in goats (Capra hircus). Int. J. Biometeorol. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Katoh, K. Effects of heat exposure and restricted feeding on behavior, digestibility and growth hormone secretion in goats. Asian-Australas. J. Anim. Sci. 2004, 17, 655–658. [Google Scholar] [CrossRef]
- Popoola, M.A.; Bolarinwa, M.O.; Yahaya, M.O.; Adebisi, G.L.; Saka, A.A. Thermal comfort effects on physiological adaptations and growth performance of West African dwarf goats raised in Nigeria. Eur. Sci. J. 2014, 3, 275–382. [Google Scholar]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Silanikove, N.; Koluman, N. Impact of climate change on the dairy industry in temperate zones: Predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Rumin. Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- Wood, P.D.P. A note on the estimation of total lactation yield from production on a single day. Anim. Prod. 1974, 19, 393–396. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P.; Sahlu, T.; Satter, L.D. The effect of diets on milk production and composition, and on lactation curves in pastured dairy goats. J. Dairy Sci. 2005, 88, 2604–2615. [Google Scholar] [CrossRef] [Green Version]
- Goetsch, A.L.; Zeng, S.S.; Gipson, T.A. Factors affecting goat milk production and quality. Small Rumin. Res. 2011, 101, 55–63. [Google Scholar] [CrossRef]
- Granado, R.J.; Rodríguez, M.S.; Arce, C.; Estévez, V.R. Factors affecting somatic cell count in dairy goats: A review. Span. J. Agric. Res. 2014, 1, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Sophia, I.; Sejian, V.; Bagath, M.; Bhatta, R. Quantitative expression of hepatic toll-like receptors 1–10 mRNA in Osmanabadi goats during different climatic stresses. Small Rumin. Res. 2016, 141, 11–16. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Upadhyay, R. Heat stress and immune function. In Heat Stress and Animal Productivity; Springer: New Delhi, India, 2013; pp. 113–136. [Google Scholar]
- Carroll, J.A.; Burdick, N.C.; Chase, C.C., Jr.; Coleman, S.W.; Spiers, D.E. Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to a provocative immune challenge. Domest. Anim. Endocrinol. 2012, 43, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.P.; Dangi, S.S.; Chouhan, V.S.; Gupta, M.; Dangi, S.K.; Singh, G.; Maurya, V.P.; Kumar, P.; Sarkar, M. Expression analysis of NOS family and HSP genes during thermal stress in goat (Capra hircus). Int. J. Biometeorol. 2016, 60, 381–389. [Google Scholar] [CrossRef]
- Hamzaoui, S.; Salama, A.A.K.; Caja, G.; Albanell, E.; Flores, C.; Such, X. Milk production losses in early lactating dairy goats under heat stress. J. Dairy Sci. 2012, 95, 672–673. [Google Scholar]
- Okoruwa, M.I. Effect of heat stress on thermoregulatory, live body weight and physiological responses of dwarf goats in southern Nigeria. Eur. Sci. J. 2014, 10, 255–264. [Google Scholar]
- Santos, F.C.B.; Souza, B.B.; Alfaro, C.E.P.; Cézar, M.F.; Pimenta Filho, E.C.; Acosta, A.A.A.; Santos, J.R.S. Adaptabilidade de caprinos exóticos e naturalizados ao clima semi-árido do nordeste brasileiro. Ciência Agrotecnologia 2005, 29, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.C.C.; Costa, A.P.R.; Azevedo, D.M.M.R.; Nascimento, H.T.S.; Cardoso, F.S.; Muratori, M.C.S.; Lopes, J.B. Adaptabilidade climática de caprinos Saanen e Azul no Meio-Norte do Brasil. Arq. Bras. Med. Vet. Zootec. 2009, 61, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, L.F.D.; Vieris, D.H.; Oliveira, C.A.; Fonseca, C.E.M.; Pedrosa, I.A.; Guerson, D.F.; Pereira, V.V.; Madeiro, A.S. Physiologic parameters in black and white nondescript goats of different ages, under shade, at Rio de Janeiro, RJ, Brazil. Boletim da Indústria Animal 2007, 64, 277–287. [Google Scholar]
- Leite, L.O.; Stamm, F.D.O.; Garcia, R.D.C.M. Indicators to assess goat welfare on-farm in the semiarid region of Brazilian Northeast. Ciência Rural 2017, 47, e20161073. [Google Scholar] [CrossRef] [Green Version]
- Shilja, S.; Sejian, V.; Bagath, M.; Mech, A.; David, I.C.G.; Kurien, E.K.; Varma, G.; Bhatta, R. Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. Int. J. Biometeorol. 2016, 60, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M., Jr.; Costa, A.P.R.; Ribeiro, D.M.M.; Turco, S.H.N.N.; Muratori, M.C.S. Physiologic parameters of boer and anglonubiana’ goats in climatic conditions of Braziĺmeio-norte. Rev. Caatinga 2008, 20, 01–07. [Google Scholar]
- Silva, E.M.N.; Souza, B.B.; Sousa, O.B.; Silva, G.A.; Freitas, M.M.S. Evaluation of adaptability of goats to semiarid through physiologic parameters and structures of the tegument. Rev. Caatinga 2010, 2, 142–148. [Google Scholar]
- Souza, P.T.; Salles, M.G.F.; Costa, A.N.L.; Carneiro, H.A.V.; Souza, L.P.; Rondina, D.; Araújo, A.A. Physiological and production response of dairy goats bred in a tropical climate. Int. J. Biometeorol. 2014, 58, 1559–1567. [Google Scholar] [CrossRef]
- Ribeiro, N.L.; Costa, R.G.; Pimenta Filho, E.C.; Ribeiro, M.N.; Bozzi, R. Effects of the dry and the rainy season on endocrine and physiologic profiles of goats in the Brazilian semi-arid region. Ital. J. Anim. Sci. 2018, 17, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Al-Tamimi, H.J. Thermoregulatory response of goat kids subjected to heat stress. Small Rumin. Res. 2007, 71, 280–285. [Google Scholar] [CrossRef]
- Baker, M.A. Effects of dehydration and rehydration on thermoregulatory sweating in goats. J. Physiol. 1989, 417, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Nijland, M.J.; Baker, M.A. Effects of hydration state on exercise thermoregulation in goats. Am. J. Physiol. Reg. Int. Comp. Physiol. 1992, 263, R201R205. [Google Scholar] [CrossRef]
- Nordquist, R.E.; van der Staay, F.J.; van Eerdenburg, F.J.; Velkers, F.C.; Fijn, L.; Arndt, S.S. Mutilating Procedures, Management Practices, and Housing Conditions That May Affect theWelfare of Farm Animals: Implications for Welfare Research. Animals 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Battini, M.; Vieira, A.; Barbieri, S.; Ajuda, I.; Stilwell, G.; Mattiello, S. Invited review: Animal-based indicators for on-farm welfare assessment for dairy goats. J. Dairy Sci. 2014, 97, 6625–6648. [Google Scholar] [CrossRef] [Green Version]
- Patt, A.; Gygax, L.; Wechsler, B.; Hillmann, E.; Palme, R.; Keil, N.M. The introduction of individual goats into small established groups has serious negative effects on the introduced goat but not on resident goats. Appl. Anim. Behav. Sci. 2012, 138, 47–59. [Google Scholar] [CrossRef]
- AWIN. Animal Welfare Indicators for Goats. Available online: http://www.animal-welfare-indicators.net/site/flash/pdf/AWINProtocolGoats.pdf (accessed on 26 February 2021).
- Madhusoodan, A.P.; Sejian, V.; Afsal, A.; Bagath, M.; Krishnan, G.; Savitha, S.T.; Rashamol, V.P.; Devaraj, C.; Bhatta, R. Differential expression patterns of candidate genes pertaining to productive and immune functions in hepatic tissue of heat-stressed Salem Black goats. Biol. Rhythm Res. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Afsal, A.; Sejian, V.; Bagath, M.; Krishnan, G.; Devaraj, C.; Bhatta, R. Heat Stress and Livestock Adaptation: Neuro-endocrine Regulation. Int J. Vet. Anim. Med. 2018, 1, 108. [Google Scholar]
- Carabaño, M.J.; Ramón, M.; Menéndez-Buxadera, A.; Molina, A.; Díaz, C. Selecting for heat tolerance. Anim. Front. 2019, 9, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Yakubu, A.; Salako, A.E.; De Donato, M.; Peters, S.O.; Takeet, M.I.; Wheto, M.; Okpeku, M.; Imumorin, I.G. Association of SNP variants of MHC Class II DRB gene with thermo-physiological traits in tropical goats. Trop. Anim. Health Prod. 2017, 49, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Mishra, C.; Dige, M. Association of novel polymorphisms in caprine SOD3 gene with physiological and biochemical parameters. Biol. Rhythm Res. 2019, 52, 759–773. [Google Scholar] [CrossRef]
- Angel, S.P.; Bagath, M.; Sejian, V.; Krishnan, G.; Bhatta, R. Expression patterns of candidate genes reflecting the growth performance of goats subjected to heat stress. Mol. Biol. Rep. 2018, 45, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Effect of heat stress on the quantitative expression patterns of different cytokine genes in Malabari goats. Int. J. Biometeorol. 2019, 63, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.W.; Pugh, D.G. Handling and examining sheep and goats. In Sheep and Goat Medicine, 2nd ed.; Elsevier Saunders: Maryland Heights, MO, USA, 2012; pp. 1–17. [Google Scholar]
- Ayo, J.O.; Minka, N.S.; Sackey, A.K.B.; Adelaiye, A.B. Response of serum electrolyte of goats to twelve hours of road transportation during the hot-dry season in Nigiria, and the effect of pre-treatment with ascorbic acid. J. Vet. Res. 2009, 76, 408–419. [Google Scholar]
- Gupta, D.; Ashutosh, M.; Kashyap, G.; Punetha, M.; Patel, B.; Para, I.; Ahirwar, M. Seasonal effect of vitamin C, electrolyte and jaggery supplementation on body weight of goats transported at different flocking density. Int. J. Curr. Microbiol. A Sci. 2018, 7, 1761–1768. [Google Scholar] [CrossRef]
- Kannan, G.; Terrill, T.H.; Kouakou, B.; Gelaye, S.; Amoah, E.A. Simulated preslaughter holding and isolation effects on stress responses and live weight shrinkage in meat goats. J. Anim. Sci. 2002, 80, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Warriss, P.D. Stress physiology of animals during transport. In Livestock Handling and Transport, 3rd ed.; Grandin, T., Ed.; CAB International Walling Ford: Oxon, UK, 2007; pp. 312–328. [Google Scholar]
- Chambers, P.G.; Grandin, T.; Heinz, G.; Srisuvan, T. Guidelines for Humane Handling, Transport and Slaughter of Livestock; Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific (RAP): Bangkok, Thailand, 2001. [Google Scholar]
- European Food Safety Authority (EFSA). Panel on animal health and welfare, 2011. Scientific opinion concerning the welfare of animals during transport. EFSA J. 2011, 9, 1966. [Google Scholar]
- Richardson, C. Lowering Stress in Transported Goats; Ontario Ministry of Agriculture and Food—Livestock Technology Branch, Queen’s Printer: Ottawa, ON, Canada, 2002; ISSN 1198-712X.
- Minka, N.S.; Ayo, J.O. Physiological and behavioural responses of goats to 12-hour road transportation, lairage and grazing periods, and the modulatory role of ascorbic acid. J. Vet. Behavior. 2013, 8, 349–356. [Google Scholar] [CrossRef]
- Ambore, B.N.; Maini, S.; Patodkar, V.R.; Borikar, S.T. Effect of transportation on body weight and hematology in goats. Int. J. Curr. Microbiol. A Sci. 2019, 8, 947–952. [Google Scholar] [CrossRef]
- Polycarp, T.N.; Obukowho, E.B.; Yusoff, S.M. Changes in haematological parameters and oxidative stress response of goats subjected to road transport stress in a hot humid tropical environment. Comp. Clin. Pathol. 2016, 25, 285–293. [Google Scholar] [CrossRef]
- Minka, N.S.; Ayo, J.O. Physiological responses of transported goats treated with ascorbic acid during the hot-dry season. Anim. Sci. J. 2007, 78, 164–172. [Google Scholar] [CrossRef]
- Alamer, M.; Al-Hozab, A. Effect of water deprivation and season on feed intake, body weight and thermoregulation in Awassi and Najdi sheep breeds in Saudi Arabia. J. Arid. Environ. 2004, 59, 71–84. [Google Scholar] [CrossRef]
- Montane, J.; Marco, I.; Lopez-Olvera, J.; Manteca, X.; Lavin, S. Transport stress in roe deer (Capreolus capreolus): Effect of the short acting antipsychotic. Anim. Welf. 2002, 11, 295–303. [Google Scholar]
- Schaefer, A.L.; Jones, S.D.M.; Stanley, R.W. The use of electrolyte solutions for reducing transport stress. J. Anim. Sci. 1997, 75, 258–265. [Google Scholar] [CrossRef]
- Minka, N.S.; Ayo, J.O. Hydration state of goats transported by road for 12 hours during the hot-dry conditions and the modulating role of ascorbic acid. J. Appl. Anim. Welf. Sci. 2012, 15, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Akerfeldt, M.P.; Gunnarsson, S.; Bernes, G.; Blanco-Penedo, I. Health and welfare in organic livestock production systems-a systematic mapping of current knowledge. Org. Agr. 2020. [Google Scholar] [CrossRef]
- Temple, D.; Manteca, X. Animal welfare in extensive production systems is still an area of concern. Front. Sustain. Food Syst. 2020, 4, 545902. [Google Scholar] [CrossRef]
- Minka, N.S.; Ayo, J.O. Physiological responses of erythrocytes of goats to transportation and the mondulatory role of ascorbic acid. J. Vet. Med. Sci. 2010, 72, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Sevi, A.; Casamassima, D.; Pulina, G.; Pazzona, A. Factors of welfare reduction in dairy sheep and goats. Ital. J. Anim. Sci. 2009, 8, 81–101. [Google Scholar] [CrossRef]
- Codevasf. Manual de Criação de Caprinos e Ovinos; Instituto Ambiental Brasil Sustentável (IABS): Brasília, Brasil, 2011; p. 142.
- Tiezzi, F.; Tomassone, L.; Mancin, G.; Cornale, P.; Tarantola, M. The Assessment of Housing Conditions, Management, Animal-Based Measure of Dairy Goats’ Welfare and Its Association with Productive and Reproductive Traits. Animals 2019, 9, 893. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.D.; Miller, B.A. Current status, challenges and prospects for dairy goat production in the Americas. Asian-Australas. J. Anim. Sci. 2019, 32, 1244–1255. [Google Scholar] [CrossRef]
- Albenzio, M.; Taibi, L.; Caroprese, M.; De Rosa, G.; Muscio, A.; Sevi, A. Immune response, udder health and productive traits of machine milked and suckling ewes. Small Rumin. Res. 2003, 48, 189–200. [Google Scholar] [CrossRef]
- FAO. Impact of Animal Nutrition on Animal Welfare—Expert Consultation 26−30 September 2011—FAO Headquarters, Rome, Italy; Animal Production and Health Report. No. 1; FAO: Rome, Italy, 2012. [Google Scholar]
- Hamadeh, S.K.; Rawda, N.; Jaber, L.S.; Habre, A.; Abi Said, M.; Barbour, E.K. Physiological response to water restriction in dry and lactating Awassi ewes. Livest. Prod. Sci. 2006, 101, 101–109. [Google Scholar] [CrossRef]
- Lia, R.; Pantone, N. Influenza delle infestazioni da strongilidi-gastrointestinali sulla produzione quanti qualitativa del latte negli allevamenti degli ovini condotti con sistemi tradizionali. In Proceedings of the Standing Seminar on Sheep Milk Quality, Foggia, Italy, 2001; pp. 81–91. [Google Scholar]
- Sevi, A.; Albenzio, M.; Annicchiarico, G.; Caroprese, M.; Marino, R.; Taibi, L. Effects of ventilation regimen on the welfare and performance of lactating ewes in summer. J. Anim. Sci. 2002, 80, 2349–2361. [Google Scholar] [CrossRef]
- Andersen, I.L.; Roussel, S.; Ropstad, E.; Braastad, B.O.; Steinheim, G.; Janczak, A.M.; Jørgensen, G.M.; Bøe, K.E. Social instability increases aggression in groups of dairy goats, but with minor consequences for the goats’ growth, kid production and development. Appl. Anim. Behav. Sci. 2008, 144, 132–148. [Google Scholar] [CrossRef]
- De la Lama, M.G.C.; Mattiello, S. The importance of social behaviour for goat welfare in livestock farming. Small Rumin. Res. 2010, 90, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Haenlein, G.F.W. Goat milk and human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Villalba, J.J.; Manteca, X.; Vercoe, P.E.; Maloney, S.K.; Blache, D. Integrating nutrition and animal welfare in extensive systems. In Nutrition and the Welfare of Farm Animals; Phillips, C.J.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 135–163. [Google Scholar] [CrossRef]
- Veissier, I.; Butterworth, A.; Bock, B.; Roe, E. European approaches to ensure good animal welfare. Appl. Anim. Behav. Sci. 2008, 113, 279–297. [Google Scholar] [CrossRef] [Green Version]
- Lusk, J.L.; Norwood, F.B. Animal welfare economics. Appl. Econ. Perspect. Policy 2011, 33, 463–483. [Google Scholar] [CrossRef]
- Barroso, F.G.; Alados, C.L.; Boza, J. Social hierarchy in the domestic goat: Effect on food habits and production. Appl. Anim. Behav. Sci. 2000, 69, 35–53. [Google Scholar] [CrossRef]
- Rassu, S.P.G.; Enne, G.; Ligios, S.; Molle, G. Nutrition and Reproduction. In Dairy Sheep Nutrition; Pulina, G., Ed.; CABI Publishing: Wallingford, UK, 2004; pp. 109–128. [Google Scholar]
- Goddard, P.; Waterhouse, T.; Dwyer, C.; Stott, A. The perception of the welfare of sheep in extensive systems. Small Rumin. Res. 2006, 62, 215–225. [Google Scholar] [CrossRef]
- Duncan, I.J.H.; Fraser, D. Understanding animal welfare. In Animal Welfare; Appleby, M.C., Hughes, B.O., Eds.; CAB International: Wallingford, UK, 1997; pp. 19–31. [Google Scholar]
- Gupta, M.; Kumar, S.; Dangi, S.S.; Jangir, B.L. Physiological, biochemical and molecular responses to thermal stress in goats. Int. J. Livest. Res. 2013, 3, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Anzuino, K.; Bell, N.J.; Bazeley, K.J.; Nicol, C.J. Assessment of welfare on 24 commercial UK dairy goat farms based on direct observations. Vet. Record 2010, 167, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Eze, C.A. Lameness and reproductive performance in small ruminants in Nsukka area of Enugu State, Nigeria. Small Rumin. Res. 2002, 44, 263–267. [Google Scholar] [CrossRef]
- Mazurek, M.; Marie, M.; Desor, D. Potential animal-centred indicators of dairy goat welfare. Anim. Welf. 2007, 16, 161–163. [Google Scholar]
- Christodoulopoulos, G. Foot lameness in dairy goats. Res. Vet. Sci. 2009, 86, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Sherman, D.M. Musculoskeletal system. In Goat Medicine; Cann, C., Hunsberger, S., Lukens, R., Denardo., M., Lippincott, W., Eds.; Wilkins: Des Moines, IA, USA, 1994; pp. 63–113. [Google Scholar]
- Leitner, G.; Silanikove, N.; Merin, U. Estimate of milk and curd yield loss of sheep and goats with intramammary infection and its relation to somatic cell count. Small Rumin. Res. 2008, 74, 221–225. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.; Paul, E.S. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B Biolog. Sci. 2010, 277, 2895–2904. [Google Scholar] [CrossRef] [Green Version]
- Briefer, E.F.; McElligott, A.G. Social effects on vocal ontogeny in an ungulate, the goat, Capra hircus. Anim. Behav. 2012, 83, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Bergamasco, L.; Macchi, E.; Facello, C.; Badino, P.; Odore, R.; Pagliasso, S.; Bellino, C.; Osella, M.C.; Re, G. Effects of brief maternal separation in kids on neurohormonal and electroencephalographic parameters. Appl. Anim. Behav. Sci. 2005, 93, 39–52. [Google Scholar] [CrossRef]
- Atasoglu, C.; Yurtman, I.Y.; Savas, T.; Gültepe, M.; Özcan, Ö. Effect of weaning on behavior and serum parameters in dairy goat kids. Anim. Sci. J. 2008, 79, 435–442. [Google Scholar] [CrossRef]
- McMillan, F.D. Welfare, well being and pain: Psychological well-being. In Encyclopedia of Animal Behaviour; Bekoff, M., Ed.; Greenwood Press: Westport, CT, USA, 2004; pp. 1133–1144. [Google Scholar]
- Blokhuis, H.J.; Veissier, I.; Miele, M.; Jones, B. The Welfare Quality® project and beyond: Safeguarding farm animal well-being. Acta Agric. Scand. Sect. 2010, 60, 129–140. [Google Scholar] [CrossRef]
- EFSA. Panel on Animal Health and Welfare (AHAW). Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767. [Google Scholar]
- Stilwell, G. Why and How to Measure Goats’ Welfare. In Sustainable Goat Production in Adverse Environments; Springer: Cham, Switzerland, 2017; Volume 1, pp. 439–453. [Google Scholar]
- Battini, M.; Stilwell, G.; Vieira, A.; Barbieri, S.; Canali, E.; Mattiello, S. On-farmwelfare assessment protocol for adult dairy goats in intensive production systems. Animals 2015, 5, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Darcan, N.; Cedden, F.; Cankaya, S. Spraying effects on some physiological and behavioural traits of goats in a subtropical climate. Ital. J. Anim. Sci. 2007, 7, 77–85. [Google Scholar] [CrossRef]
- Sejian, V.; Srivastava, R.S. Effects of melatonin on adrenal cortical functions of Indian goats under thermal stress. Vet. Med. Int. 2010, 2010, 348919. [Google Scholar] [CrossRef] [Green Version]
- Ratnakaran, A.P.; Sejian, V.; Jose, V.S.; Vaswani, S.; Bagath, M.; Krishnan, G.; Beena, V.; Indira Devi, P.; Varma, G.; Bhatta, R. Behavioural responses to livestock adaptation to heat stress challenges. Asian J. Anim. Sci. 2017, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wemelsfelder, F. How animals communicate quality of life: The qualitative assessment of behaviour. Anim. Welf. 2007, 16, 25–31. [Google Scholar]
- El-Tarabany, M.S.; El-Tarabany, A.A.; Atta, M.A. Physiological and lactation responses of Egyptian dairy Baladi goats to natural thermal stress under subtropical environmental conditions. Int. J. Biometeorol. 2017, 61, 61–68. [Google Scholar] [CrossRef]
- Mason, G.; Rushen, J. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare, 2nd ed.; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Laister, S.; Brorkens, N.; Lolli, S.; Zucca, D.; Knierim, U.; Minero, M.; Canali, E.; Winckler, C. Reliability of measures of agonistic behaviour in dairy and beef cattle. In Welfare Quality® Report No. 11—Assessment of Animal Welfare Measures for Dairy Cattle, Beef Bulls and Veal Calves; Forkman, B., Keeling, L., Eds.; Cardiff University: Cardiff, UK, 2009; pp. 95–112. [Google Scholar]
- Van, D.T.T.; Mui, N.T.; Ledin, I. Effect of group size on feed intake, aggressive behaviour and growth rate in goat kids and lambs. Small Rum. Res. 2007, 72, 187–196. [Google Scholar] [CrossRef]
- Fournier, F.; Festa-Bianchet, M. Social dominance in adult female mountain goats. Anim. Behav. 1995, 49, 1449–1459. [Google Scholar] [CrossRef]
- Jørgensen, G.H.M.; Andersen, I.L.; Bøe, K.E. Feed intake and social interactions in dairy goats—The effects of feeding space and type of roughage. Appl. Anim. Behav. Sci. 2007, 107, 239–251. [Google Scholar] [CrossRef]
- Leite, L.O.; Stamm, F.O.; Souza, R.A.; Camarinha Filho, J.A.; Garcia, R.C.M. On-farm welfare assessment in dairy goats in the Brazilian Northeast. Arq. Bras. Med. Vet. Zootec. 2020, 72, 2308–2320. [Google Scholar] [CrossRef]
- Siebert, K.; Langbein, J.; Schön, P.C.; Tuchscherer, A.; Puppe, B. Degree of social isolation affects behavioural and vocal response patterns in dwarf goats (Capra hircus). Appl. Anim. Behav. Sci. 2011, 131, 53–62. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Moreno-Indias, I.; Morales-Delanuez, A.; Ruiz-Díaz, M.D.; Hernández-Castellano, L.E.; Castro, N.; Argüello, A. Effects of feeding management and time of day on the occurrence of self-suckling in dairy goats. Vet. Record 2011, 168, 378. [Google Scholar] [CrossRef]
- McGregor, B.A.; Butler, K.L. Relationship of body condition score, live weight, stocking rate and grazing system to the mortality of Angora goats from hypothermia and their use in the assessment of welfare risks. Aust. Vet. J. 2008, 86, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Caroprese, M.; Casamassima, D.; Rassu, S.P.G.; Napolitano, F.; Sevi, A. Monitoring the on-farm welfare of sheep and goats. Ital. J. Anim. Sci. 2009, 8, 343–354. [Google Scholar] [CrossRef]
- Bøe, K.E.; Ehrlenbruch, R. Thermoregulatory behavior of dairy goats at low temperatures and the use of outdoor yards. Can. J. Anim. Sci. 2013, 93, 35–41. [Google Scholar] [CrossRef]
- Battini, M.; Barbieri, S.; Fioni, L.; Mattiello, S. Feasibility and validity of animal-based indicators for on-farm welfare assessment of thermal stress in dairy goats. Int. J. Biometeorol. 2016, 60, 289–296. [Google Scholar] [CrossRef]
- Adedeji, T.A. Effect of some qualitative traits and non-genetic factors on heat tolerance attributes of extensively reared West African Dwarf goats. Int. J. Appl. Agric. Apicul. Res. 2012, 8, 68–81. [Google Scholar]
- Marai, I.F.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A. Physiological traits as affected by heat stress in sheep-a review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Sarangi, S. Adaptability of goats to heat stress: A review. Pharm. Innov. J. 2018, 7, 1114–1126. [Google Scholar]
- Robertshaw, D.; Dmiel, R. The effect of dehydration on the control of panting and sweating in the black Bedouin goat. Physiol. Zool. 1983, 56, 412–418. [Google Scholar] [CrossRef]
- Yousif, H.S. Some physiological responses in Nubian goats exposed to heat load. Int. J. Sci. Eng. Sci. 2019, 3, 6–9. [Google Scholar]
- Bello, J.M.; Arroyo, G.; Ruiz, S.; Gonzalez, G.; Marques, F. Welfare indicators of milking sheep and goats in commercial farms in Spain: Evaluation and differences among species, locations and performances. J. Anim. Nutr. 2016, 1, 4. [Google Scholar]
- Brasil, L.H.D.; Wechesler, F.S.; Junior, F.B.; Goncalves, H.C.; Bonassi, I.A. Thermal stress effects on milk yield and chemical composition and thermoregulatory responses of lactating alpines goats. Braz. Anim. Sci. 2000, 9, 1632–1641. [Google Scholar]
- Delgado-Pertinez, M.; Gutierrez-Pena, R.; Mena, Y.; Fernandez-Cabanas, V.M.; Laberye, D. Milk production, fatty acid composition and vitamin E content of Payoya goats according to grazing level in summer on Mediterranean shrublands. Small Rumin. Res. 2013, 114, 167–175. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptivemetabolism and energetics. Ann. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amitha, J.P.; Krishnan, G.; Bagath, M.; Sejian, V.; Bhatta, R. Heat stress impact on the expression patterns of different reproduction related genes in Malabari goats. Theriogenology 2019, 131, 169–176. [Google Scholar] [CrossRef]
- Habeeb, A.A.; Gad, A.E.; Atta, M.A. Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int. J. Biotechnol. Recent. Adv. 2018, 1, 35–50. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sejian, V.; Silpa, M.V.; Reshma Nair, M.R.; Devaraj, C.; Krishnan, G.; Bagath, M.; Chauhan, S.S.; Suganthi, R.U.; Fonseca, V.F.C.; König, S.; et al. Heat Stress and Goat Welfare: Adaptation and Production Considerations. Animals 2021, 11, 1021. https://doi.org/10.3390/ani11041021
Sejian V, Silpa MV, Reshma Nair MR, Devaraj C, Krishnan G, Bagath M, Chauhan SS, Suganthi RU, Fonseca VFC, König S, et al. Heat Stress and Goat Welfare: Adaptation and Production Considerations. Animals. 2021; 11(4):1021. https://doi.org/10.3390/ani11041021
Chicago/Turabian StyleSejian, Veerasamy, Mullakkalparambil V. Silpa, Mini R. Reshma Nair, Chinnasamy Devaraj, Govindan Krishnan, Madiajagan Bagath, Surinder S. Chauhan, Rajendran U. Suganthi, Vinicius F. C. Fonseca, Sven König, and et al. 2021. "Heat Stress and Goat Welfare: Adaptation and Production Considerations" Animals 11, no. 4: 1021. https://doi.org/10.3390/ani11041021
APA StyleSejian, V., Silpa, M. V., Reshma Nair, M. R., Devaraj, C., Krishnan, G., Bagath, M., Chauhan, S. S., Suganthi, R. U., Fonseca, V. F. C., König, S., Gaughan, J. B., Dunshea, F. R., & Bhatta, R. (2021). Heat Stress and Goat Welfare: Adaptation and Production Considerations. Animals, 11(4), 1021. https://doi.org/10.3390/ani11041021








