Role of Infection and Immunity in Bovine Perinatal Mortality: Part 1. Causes and Current Diagnostic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Infectious Causes of Perinatal Mortality
2.1. Principles of Perinatal Infections
2.1.1. Types of Infectious Agents
2.1.2. Co-Infection
2.1.3. Primary and Secondary Pathogens
2.1.4. Infectious Agents Linked to Perinatal Mortality
Bacillus licheniformis
Bovine Viral Diarrhoea Virus (BVDv)
Leptospira spp.
Neospora caninum
2.1.5. Infectious Agents Less Commonly Causally Linked to Perinatal Mortality
Bovine Herpes Viruses
Chlamydia and Chlamydia-Like Organisms
Coxiella burnetii
Escherichia coli
Fungi and Yeasts
Listeria monocytogenes
Mycoplasma Species
Salmonella Species
Schmallenberg Virus (SBV)
Trueperella pyogenes
2.1.6. Contaminant Bacteria
2.1.7. In Utero vs. Postnatal Infection
Intra-Uterine Infections
Postnatal Infections
2.1.8. Exposure, Infection and Causality
2.1.9. Proportion of Perinatal Mortality Caused by Infection
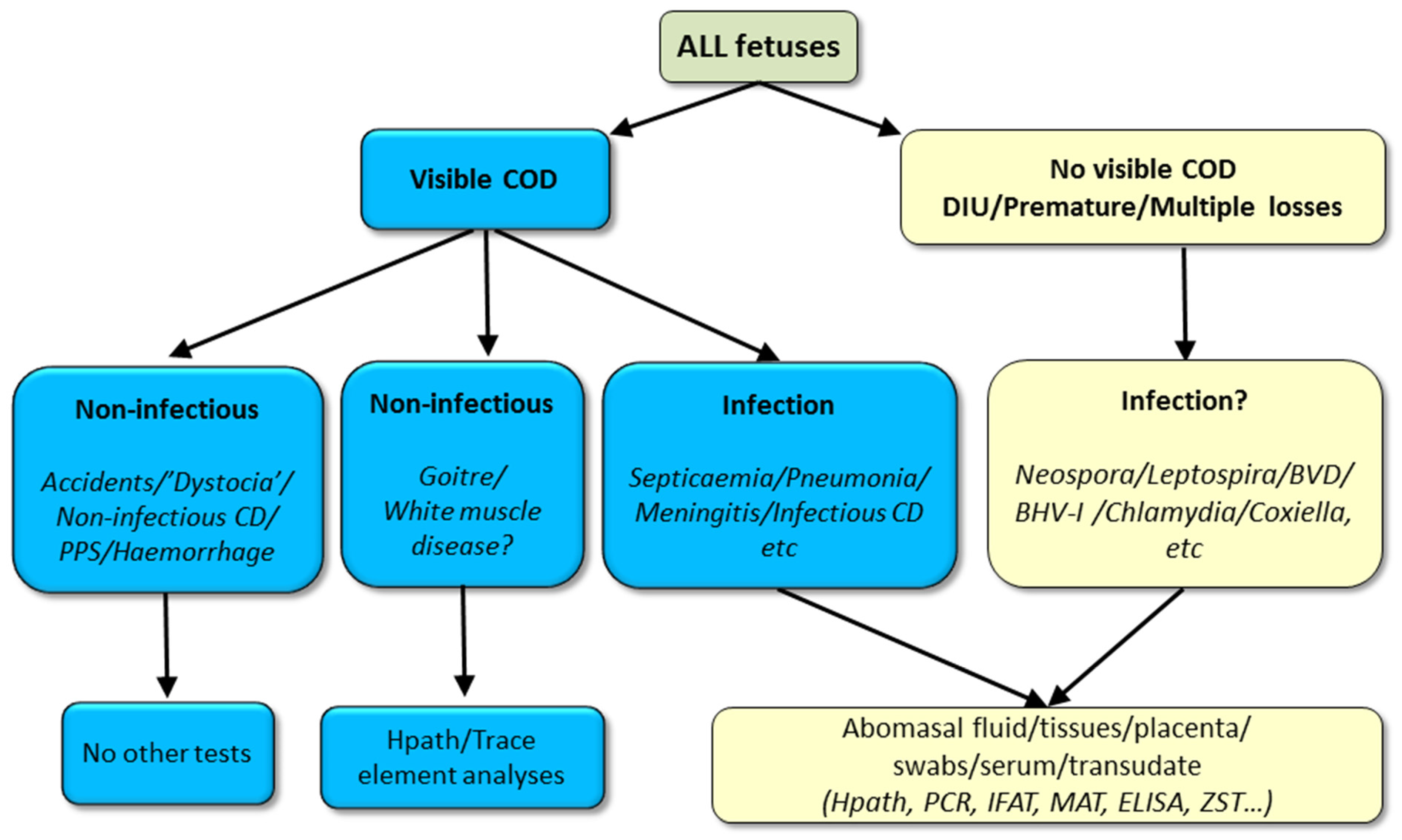
3. Diagnosis of Infectious Causes of Perinatal Mortality
3.1. Herd Health History
3.2. Cohort Sampling
3.3. Examination of the Dam
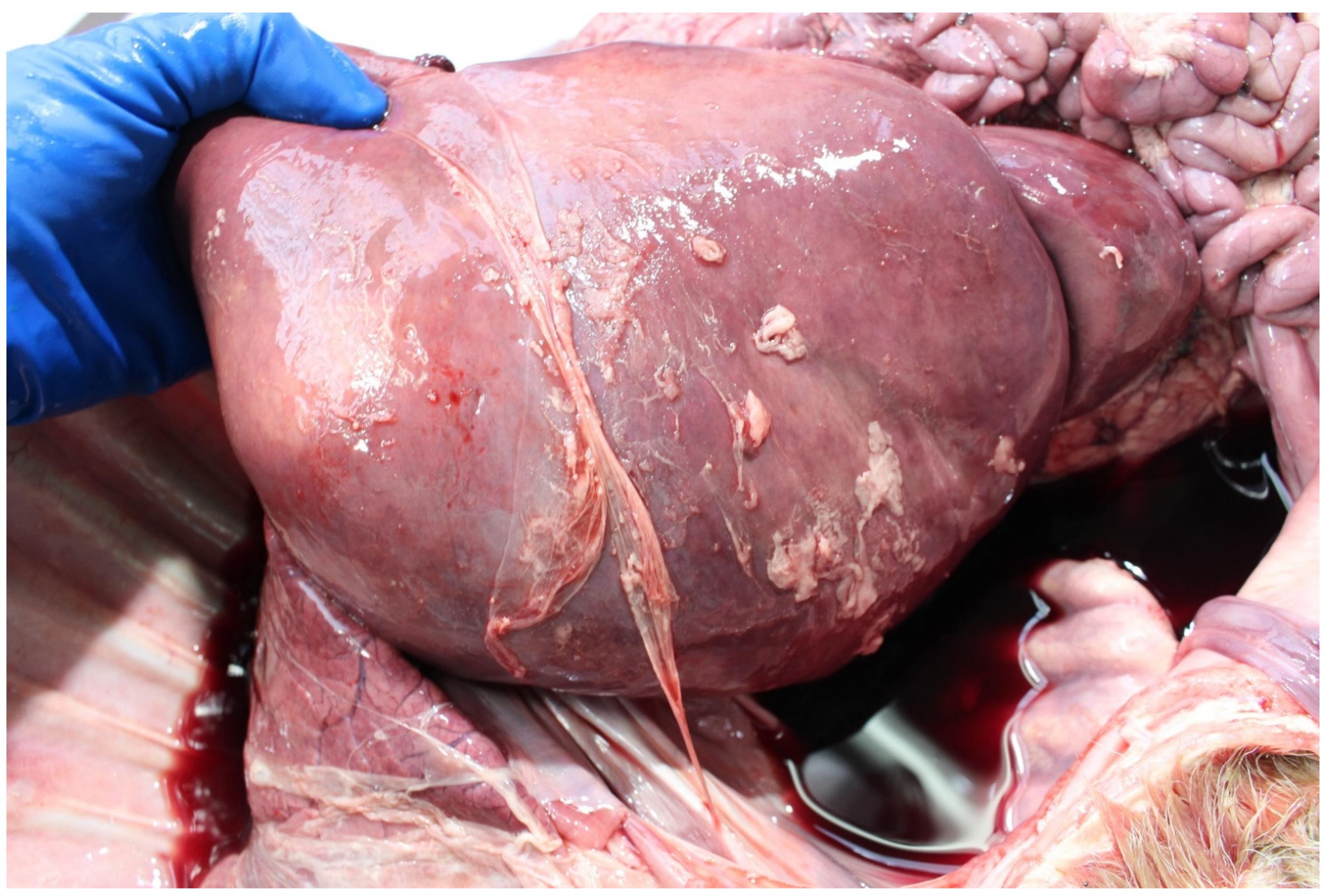
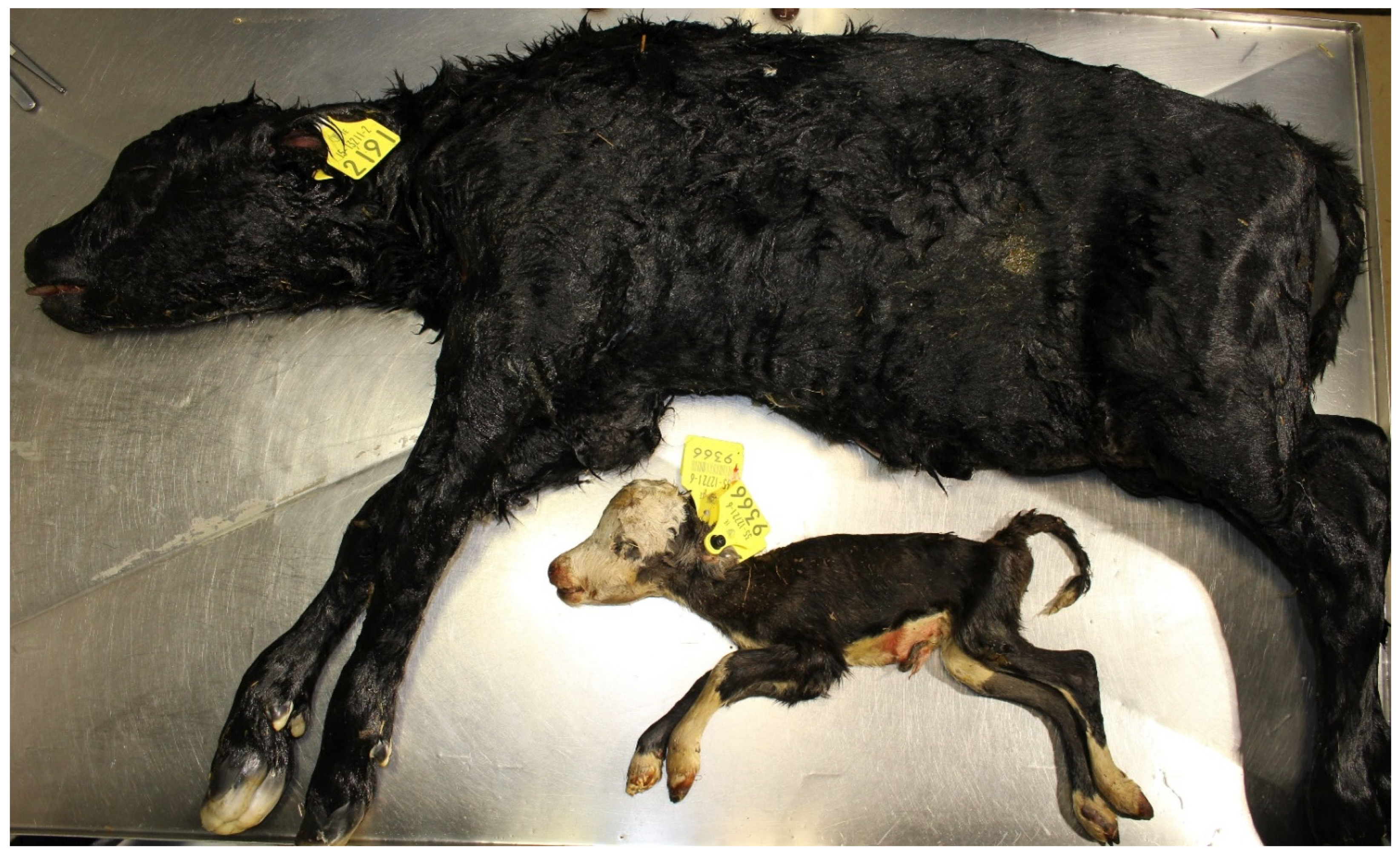
3.4. Examination of the Foetus
3.4.1. Sampling the Carcass
Microbiology Samples
3.4.2. Serology Samples
3.4.3. Histopathology Samples
3.4.4. Standard Microbiology Package
3.4.5. Sample Storage
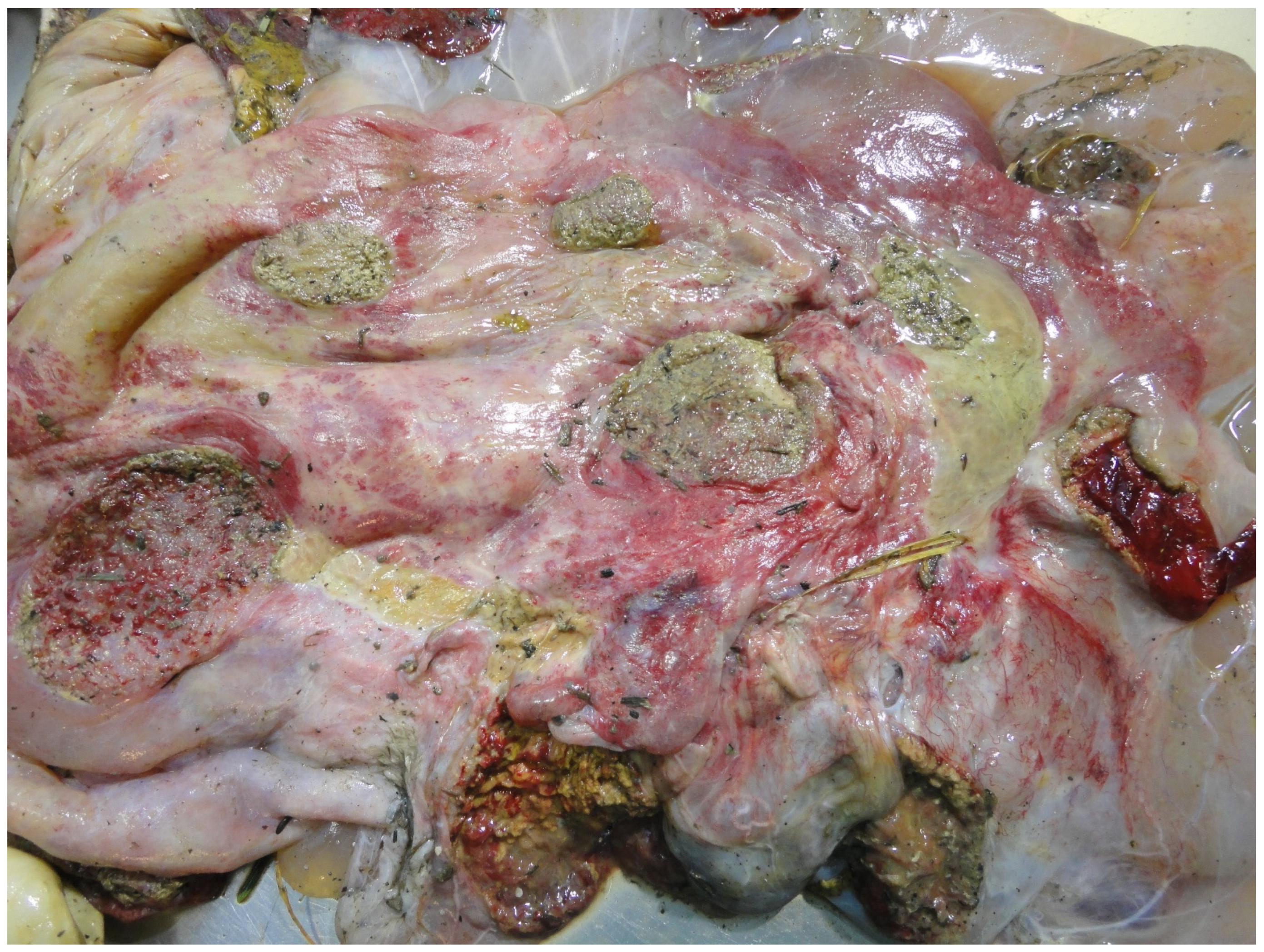
3.5. Examination of the Placenta
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mee, J.F. Why Do So Many Calves Die on Modern Dairy Farms and What Can We Do about Calf Welfare in the Future? Animals 2013, 3, 1036–1057. [Google Scholar] [CrossRef] [PubMed]
- Jawor, P.; Mee, J.F.; Stefaniak, T. Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2. Foetomaternal response to infection and novel diagnostic perspectives. unpublished, manuscript in preparation.
- Murray, R. Developing an evidence-based diagnostic approach for perinatal mortality in cattle. Hung. Vet. J. 2008, 130, 86–90. [Google Scholar]
- Jawor, P.; Król, D.; Mee, J.F.; Sołtysiak, Z.; Dzimira, S.; Larska, M.; Stefaniak, T. Infection exposure, detection and causes of death in perinatal mortalities in Polish dairy herds. Theriogenology 2017, 103. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S.; Basse, A.; Krogh, H.V.; Christensen, K.; Rønsholt, L. Abortion and calf mortality in Danish cattle herds. Acta Vet. Scand. 1993, 34, 371–377. [Google Scholar] [CrossRef]
- Syrjälä, P.; Anttila, M.; Dillard, K.; Fossi, M.; Collin, K.; Nylund, M.; Autio, T. Causes of bovine abortion, stillbirth and neonatal death in Finland 1999–2006. Acta Vet. Scand. 2007, 49, S3. [Google Scholar] [CrossRef]
- Doherty, M. Serious perinatal mortality in cattle associated with bovine virus diarrhoea-mucosal disease (BVD-MD) virus. lrish Vet. J. 1986, 40, 189–190. [Google Scholar]
- Collins, Á.B.; Doherty, M.L.; Barrett, D.J.; Mee, J.F. Schmallenberg virus: A systematic international literature review (2011-2019) from an Irish perspective. Ir. Vet. J. 2019, 72. [Google Scholar] [CrossRef]
- Fernandez, M.; Campero, C. Pérdidas reproductivas en bovinos causadas por abortos, muertes prematuras, natimortos y neonatos: casuística del período 2006-2007. Rev. Med. 2007, 246–254. [Google Scholar]
- Mee, J.F. (Teagasc, Cork, Ireland). Personal Communication, 2020.
- Kirkbride, C.A. Viral agents and associated lesions detected in a 10-year study of bovine abortions and stillbirths. J. Vet. Diagnostic Investig. 1992, 4, 374–379. [Google Scholar] [CrossRef]
- Khodakaram-Tafti, A.; Ikede, B.O. A retrospective study of sporadic bovine abortions, stillbirths, and neonatal abnormalities in Atlantic Canada, from 1990 to 2001. Can. Vet. J. 2005, 46, 635–637. [Google Scholar]
- Waldner, C.L.; Kennedy, R.I.; Rosengren, L.B.; Pollock, C.M.; Clark, E.G. Gross postmortem and histologic examination findings from abortion losses and calf mortalities in western Canadian beef herds. Can. Vet. J. 2010, 51, 1227–1238. [Google Scholar] [PubMed]
- Norquay, R.; Orr, J.; Norquay, B.; Ellis, K.A.; Mee, J.F.; Reeves, A.; Scholes, S.; Geraghty, T. Perinatal mortality in 23 beef herds in Orkney: Incidence, risk factors and aetiology. Vet. Rec. 2020, 187, 28. [Google Scholar] [CrossRef] [PubMed]
- de Kruif, A.; Benedictus, G. Perinatale sterfte en de geboorte van weinig levenskrachtige kalveren. Tijdschr. Diergeneeskd. 1993, 118, 684–688. [Google Scholar] [PubMed]
- Siguroarson, S.; Vioarsson, H.; Jonsson, M. Orsakir kalfadauoa um buro hja fyrsta kalfs kvigum. Orsakir kalfadauoa hja fyrsta kalfs kvigum. Syyrsla um rannosoknir 2006-2008, Landbunaoarhaaskoll Islands. Rit Lbhl 2008, 19, 34–42. [Google Scholar]
- Ogata, Y.; Nakao, T.; Takahashi, K.; Abe, H.; Misawa, T.; Urushiyama, Y.; Sakai, J. Intrauterine Growth Retardation as a Cause of Perinatal Mortality in Japanese Black Beef Calves. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 1999, 46, 327–334. [Google Scholar] [CrossRef]
- Muskens, J.; Vos, J. Post mortem examinations of aborted and stillborn calves. Reprod. Dom. Anim. 2008, 43 (Suppl. S5), 107. [Google Scholar]
- McCoy, M.; Smyth, J.; Ellis, W.; Kennedy, D. Stillbirth/Perinatal weak calf syndrome. Cattle Pract. 1997, 5, 31–34. [Google Scholar]
- Berglund, B.; Steinbock, L.; Elvander, M. Causes of stillbirth and time of death in Swedish Holstein calves examined post mortem. Acta Vet. Scand. 2003, 44, 111–120. [Google Scholar] [CrossRef]
- Mock, T.; Mee, J.F.; Dettwiler, M.; Rodriguez-Campos, S.; Hüsler, J.; Michel, B.; Häfliger, I.M.; Drögemüller, C.; Bodmer, M.; Hirsbrunner, G. Evaluation of an investigative model in dairy herds with high calf perinatal mortality rates in Switzerland: Perinatal mortality on high risk dairy farms in Switzerland. Theriogenology 2020, 148, 48–59. [Google Scholar] [CrossRef]
- Schefers, J. Why stillborns are still born. Hoard’s Dairym. 2009, 154, 10. [Google Scholar]
- Smyth, J.A.; McNamee, P.T.; Kennedy, D.G.; McCullough, S.J.; Logan, E.F.; Ellis, W.A. Stillbirth/perinatal weak calf syndrome: preliminary pathological, microbiological and biochemical findings. Vet. Rec. 1992, 130, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Zanatto, D.C.S.; Gatto, I.R.H.; Labruna, M.B.; Jusi, M.M.G.; Samara, S.I.; Machado, R.Z.; André, M.R. Coxiella burnetii associated with BVDV (Bovine Viral Diarrhea Virus), BoHV (bovine herpesvirus), Leptospira spp., Neospora caninum, Toxoplasma gondii and trypanosoma vivax in reproductive disorders in cattle. Rev. Bras. Parasitol. Vet. 2019, 28, 245–257. [Google Scholar] [CrossRef]
- Vidal, S.; Kegler, K.; Greub, G.; Aeby, S.; Borel, N.; Dagleish, M.P.; Posthaus, H.; Perreten, V.; Rodriguez-Campos, S. Neglected zoonotic agents in cattle abortion: Tackling the difficult to grow bacteria. BMC Vet. Res. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S.; Krogh, H.V.; Jensen, H.E. A Retrospective Study of Bovine Abortions Associated with Bacillus licheniformis. J. Vet. Med. Ser. B 1995, 42, 225–234. [Google Scholar] [CrossRef]
- Jawor, P.; Stefaniak, T.; Sołtysiak, Z.; Dzimira, S.; Bednarski, M. Salmonella enterica serovar Stanley intrauterine infection in a stillborn calf - case report. Acta Vet. Brno 2013, 82, 363–367. [Google Scholar] [CrossRef]
- Serrano-Pérez, B.; Almería, S.; Mur, R.; Alabart, J.L.; López-Helguera, I.; Garcia-Ispierto, I.; López-Gatius, F. Caracterización de las citoquinas involucradas en la luteolisis del cuerpo luteo de vacas abortadas por la infección experimental con Neospora caninum. In Proceedings of the XVII Jornadas Sobre Producción Animal, Zaragoza, Spain, 30–31 May 2017; pp. 752–754. [Google Scholar]
- Di Blasio, A.; Traversa, A.; Giacometti, F.; Chiesa, F.; Piva, S.; Decastelli, L.; Dondo, A.; Gallina, S.; Zoppi, S. Isolation of Arcobacter species and other neglected opportunistic agents from aborted bovine and caprine fetuses. BMC Vet. Res. 2019, 15, 257. [Google Scholar] [CrossRef]
- Hassig, M. Fetal antibodies against the placenta in miscarriages in cattle. Reprod. Domest. Anim. 1990, 25, 139–140. [Google Scholar]
- Anderson, M.L. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology 2007, 68, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Miguel, C. Bovine abortion, All-Island Animal Disease Surveillance Report 2019; Department of Agriculture, Food and the Marine: Dublin, Ireland, 2020. [Google Scholar]
- Clothier, K.; Anderson, M. Evaluation of bovine abortion cases and tissue suitability for identification of infectious agents in California diagnostic laboratory cases from 2007 to 2012. Theriogenology 2016, 85, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F. Bovine abortion. In Bovine Prenatal, Perinatal and Neonatal Medicine; Szenci, O., Mee, J.F., Bleul, U., Taverne, M.A.M., Eds.; Hungarian Association for Buiatrics: Budapest, Hungary, 2021. [Google Scholar]
- Wheelhouse, N. (Liverpool University, Liverpool, UK). Diplome in Bovine Reproduction. Personal Communication, 2018. [Google Scholar]
- Wheelhouse, N. (Liverpool University, Liverpool, UK). Diplome in Bovine Reproduction. Personal Communication, 2020. [Google Scholar]
- Murray, R.D.; Williams, A.J.; Sheldon, I.M. Field investigation of perinatal mortality in Friesian cattle associated with myocardial degeneration and necrosis. Reprod. Domest. Anim. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Otter, A. Cattle abortions update. Vet. Rec. 2020, 186, 597–598. [Google Scholar] [CrossRef]
- Scottish Agriculture Colleges (SAC) Surveillance report Reproductive tract conditions. Vet. Rec. 2017, 181, 31.
- Johnston, W.; MacLachlan, G.; Hopkins, G. An outbreak of sudden death and respiratory disease in weaned calves due to Bacillus Licheniformis infection and subsequent abortions and stillbirths. In Proceedings of the 3rd International Symposium World Association Veterinary Laboratory Diagnosticians, Ames, IA, USA, 13–15 June 1983; pp. 107–110. [Google Scholar]
- Benedictus, G. Perinatale kalversterfte met symptomen van het “weak calf syndrome”. Tijdschr. Diergeneeskd. 1989, 114, 1046–1048. [Google Scholar]
- Graham, D.A.; Beggs, N.; Mawhinney, K.; Calvert, V.; Cunningham, B.; Rowan-Layberry, L.; McLaren, I. Comparative evaluation of diagnostic techniques for bovine viral diarrhoea virus in aborted and stillborn fetuses. Vet. Rec. 2009, 164, 56–58. [Google Scholar] [CrossRef]
- Schefers, J.; Munoz-Zanzi, C.; Collins, J.E.; Goyal, S.M.; Ames, T.R. Serological evaluation of precolostral serum samples to detect Bovine viral diarrhea virus infections in large commercial dairy herds. J. Vet. Diagnostic Investig. 2008, 20, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Grooms, D.L. Reproductive consequences of infection with bovine viral diarrhea virus. Vet. Clin. North Am. Food Anim. Pract. 2004, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.A.; Fitzpatrick, D.A.; Ellis, W.A. Stillbirth/perinatal weak calf syndrome: a study of calves infected with Leptospira. Vet. Rec. 1999, 145, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A.; O’Brien, J.J.; Neill, S.D.; Bryson, D.G. Bovine leptospirosis: Experimental serovar hardjo infection. Vet. Microbiol. 1986, 11, 293–299. [Google Scholar] [CrossRef]
- Delooz, L.; Czaplicki, G.; Gregoire, F.; Dal Pozzo, F.; Pez, F.; Kodjo, A.; Saegerman, C. Serogroups and genotypes of Leptospira spp. strains from bovine aborted foetuses. Transbound. Emerg. Dis. 2018, 65, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bartley, P.M.; Guido, S.; Mason, C.; Stevenson, H.; Chianini, F.; Carty, H.; Innes, E.A.; Katzer, F. Detection of Neospora caninum DNA in cases of bovine and ovine abortion in the South-West of Scotland. Parasitology 2019, 146, 979–982. [Google Scholar] [CrossRef]
- Brickell, J.S.; McGowan, M.M.; Wathes, D.C. Association between Neospora caninum seropositivity and perinatal mortality in dairy heifers at first calving. Vet. Rec. 2010, 167, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, M.C.; Hietala, S.K.; Claudia Muiioz-Zanzi, C. Stillbirths Associated with Neospora caninum and BVDV type II in Dairy Heifers. In Proceedings of the American Association of Bovine Practitioners Proceedings of the Annual Conference, Vancouver, BC, Canada, 13–15 September 2001; p. 154. [Google Scholar]
- Graham, D.A.; Smyth, J.A.; McLaren, I.E.; Ellis, W.A. Stillbirth/perinatal weak calf syndrome: serological examination for evidence of Neospora caninum infection. Vet. Rec. 1996, 139, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Innes, E.A.; Andrianarivo, A.G.; Björkman, C.; Williams, D.J.L.; Conrad, P.A. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 2002, 18, 497–504. [Google Scholar] [CrossRef]
- Buxton, D.; McAllister, M.M.; Dubey, J.P. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002, 18, 546–552. [Google Scholar] [CrossRef]
- Pospíšil, Z.; Krejčí, J.; Machatková, M.; Zendulková, D.; Lány, P.; Číhal, P. The efficacy of an inactivated IBR vaccine in the prevention of intra-uterine infection and its use in a disease-control programme. J. Vet. Med. Ser. B 1996, 43, 15–21. [Google Scholar] [CrossRef]
- Egyed, L.; Sassi, G.; Tibold, J.; Mádl, I.; Szenci, O. Symptomless intrauterine transmission of bovine herpesvirus 4 to bovine fetuses. Microb. Pathog. 2011, 50, 322–325. [Google Scholar] [CrossRef]
- Smith, M.W.; Miller, R.B.; Svoboda, I.; Lawson, K.F. Efficacy of an intranasal infectious bovine rhinotracheitis vaccine for the prevention of abortion in cattle. Can. Vet. J. 1978, 19, 63–71. [Google Scholar]
- Wilson, T.E. Observations and comments on two outbreaks of abortion associated with IBR virus infection. Can. Vet. J. La Rev. Vet. Can. 1974, 15, 227–229. [Google Scholar]
- Flammini, C.; Allegri, G. Osservazioni su un focolaio di aborto da virus IBR nella bovina. Nuova Vet. 1972, 48, 224. [Google Scholar]
- Miller, R.B.; Smith, M.W.; Lawson, K.F. Some lesions observed in calves born to cows exposed to the virus of infectious bovine rhinotracheitis in the last trimester of gestation. Can. J. Comp. Med. Rev. Can. Médecine Comparée 1978, 42, 438–445. [Google Scholar]
- Wheelhouse, N.; Mearns, R.; Willoughby, K.; Wright, E.; Turnbull, D.; Longbottom, D. Evidence of members of the Chlamydiales in bovine abortions in England and Wales. Vet. Rec. 2015, 176, 465. [Google Scholar] [CrossRef] [PubMed]
- Wheelhouse, N.; Flockhart, A.; Aitchison, K.; Livingstone, M.; Finlayson, J.; Flachon, V.; Sellal, E.; Dagleish, M.P.; Longbottom, D. Experimental challenge of pregnant cattle with the putative abortifacient Waddlia chondrophila. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Borel, N.; Ruhl, S.; Casson, N.; Kaiser, C.; Pospischil, A.; Greub, G. Parachlamydia spp. and related Chlamydia-like organisms and bovine abortion. Emerg. Infect. Dis. 2007, 13, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M.; Gharbi, Y.; Hassena, A.B.; Slima, A.B.; Mallek, Z.; Gautier, M.; Greub, G.; Gdoura, R.; Fendri, I. Survey of infectious etiologies of bovine abortion during mid- to late gestation in dairy herds. PLoS ONE 2014, 9, e91549. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Chlamydophila abortus. Vet. Irel. J. 2013, 3, 262. [Google Scholar]
- Nielsen, K.T.; Nielsen, S.S.; Agger, J.F.; Christoffersen, A.B.; Agerholm, J.S. Association between antibodies to Coxiella burnetii in bulk tank milk and perinatal mortality of Danish dairy calves. Acta Vet. Scand. 2011, 53, 1–8. [Google Scholar] [CrossRef]
- Seo, M.G.; Ouh, I.O.; Kwak, D. Herd prevalence and genotypes of Coxiella burnetii in dairy cattle bulk tank milk in Gyeongsang provinces of South Korea. Trop. Anim. Health Prod. 2018, 50, 1399–1404. [Google Scholar] [CrossRef]
- Ryan, E.D.; Kirby, M.; Collins, D.M.; Sayers, R.; Mee, J.F.; Clegg, T. Prevalence of Coxiella burnetii (Q fever) antibodies in bovine serum and bulk-milk samples. Epidemiol. Infect. 2011, 139, 1413–1417. [Google Scholar] [CrossRef][Green Version]
- Freick, M.; Konrath, A.; Enbergs, H.; Walraph, J.; Weber, J.; Eulenberger, K. Detection of Coxiella burnetii DNA and anti-Coxiella burnetii IgG antibodies in precolostral blood samples of stillborn calves in an endemically infected Holstein dairy herd. Folia Microbiol. 2018, 63, 253–260. [Google Scholar] [CrossRef]
- Fecteau, G.; Smith, B.P.; George, L.W. Septicemia and Meningitis in the Newborn Calf. Vet. Clin. North Am. Food Anim. Pract. 2009, 25, 195–208. [Google Scholar] [CrossRef]
- Knudtson, W.U.; Kirkbride, C.A. Fungi associated with bovine abortion in the northern plains states (USA). J. Vet. Diagnostic Investig. 1992, 4, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Whitman, K.J.; Bono, J.L.; Clawson, M.L.; Loy, J.D.; Bosilevac, J.M.; Arthur, T.M.; Ondrak, J.D. Genomic-based identification of environmental and clinical Listeria monocytogenes strains associated with an abortion outbreak in beef heifers. BMC Vet. Res. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Szacawa, E.; Jawor, P.; Dudek, K.; Bednarek, D.; Stefaniak, T. Screening for Mollicutes microorganisms in perinatal calf mortality cases in Polish dairy herds. Pol. J. Vet. Sci. 2018, 21, 441–444. [Google Scholar] [CrossRef]
- Trichard, C.J.; Jacobsz, E.P. Mycoplasmas recovered from bovine genitalia, aborted foetuses and placentas in the Republic of South Africa. Onderstepoort J. Vet. Res. 1985, 52, 105–110. [Google Scholar] [PubMed]
- Ball, H.J.; Neill, S.D.; Ellis, W.A.; O’Brien, J.J.; Ferguson, H.W. The isolation of mycoplasma from bovine foetuses and their dams. Br. Vet. J. 1978, 134, 584–589. [Google Scholar] [CrossRef]
- Langford, E.V. Mycoplasma recovered from bovine male and female genitalia and aborted foeti. Proc. Am. Assoc. Vet. Lab. Diagn. 1976, 19, 221–232. [Google Scholar]
- Bocklisch, H.; Pfützner, H.; Martin, J.; Templin, G.; Kreusel, S. Mycoplasma-bovis-Aborte bei Kühen nach experimenteller Infektion. Arch. Exp. Veterinarmed. 1986, 40, 48–55. [Google Scholar]
- Stalheim, O.H.; Proctor, S.J. Experimentally induced bovine abortion with Mycoplasma agalactiae subsp bovis. Am. J. Vet. Res. 1976, 37, 879–883. [Google Scholar]
- Sánchez-Miguel, C.; Crilly, J.; Grant, J.; Mee, J.F. Sensitivity, specificity and predictive probability values of serum agglutination test titres for the diagnosis of Salmonella Dublin culture-positive bovine abortion and stillbirth. Transbound. Emerg. Dis. 2017. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Nielsen, L.R.; Toft, N.; Houe, H. Association between bulk-tank milk Salmonella antibody level and high calf mortality in Danish dairy herds. J. Dairy Sci. 2010, 93, 304–310. [Google Scholar] [CrossRef]
- Wernike, K.; Holsteg, M.; Schirrmeier, H.; Hoffmann, B.; Beer, M. Natural infection of pregnant cows with Schmallenberg virus—A follow-up study. PLoS ONE 2014, 9, e98223. [Google Scholar] [CrossRef]
- Semambo, D.K.; Ayliffe, T.R.; Boyd, J.S.; Taylor, D.J. Early abortion in cattle induced by experimental intrauterine infection with pure cultures of Actinomyces pyogenes. Vet. Rec. 1991, 129, 12–16. [Google Scholar] [CrossRef]
- Wolf-Jäckel, G.A.; Hansen, M.S.; Larsen, G.; Holm, E.; Agerholm, J.S.; Jensen, T.K. Diagnostic studies of abortion in Danish cattle 2015-2017. Acta Vet. Scand. 2020, 62. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F. Economic and welfare consequence of perinatal mortality in dairy calves. In Bovine Prenatal, Perinatal and Neonatal Medicine; Szenci, O., Mee, J.F., Bleul, U., Taverne, M.A.M., Eds.; Hungarian Association for Buiatrics: Budapest, Hungary, 2021; in press. [Google Scholar]
- Fávero, J.F.; de Araújo, H.L.; Lilenbaum, W.; Machado, G.; Tonin, A.A.; Baldissera, M.D.; Stefani, L.M.; Da Silva, A.S. Bovine leptospirosis: Prevalence, associated risk factors for infection and their cause-effect relation. Microb. Pathog. 2017, 107, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Norquay, R. Perinatal Losses in Beef Herds in Orkney: Assessing Incidence and Associated Pathology from General Practice. MVM Thesis, University of Glasgow, Glasgow, UK, 2017. [Google Scholar]
- Hogan, I.; Crowe, B.; Doherty, M.; Fagan, J.; Kennedy, E.; Conneely, M.; Lorenz, I. Optimisation of the zinc sulphate turbidity test for the determination of immune status. Vet. Rec. 2016, 178, 169. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F. Denormalizing poor dairy youngstock management: dealing with “farm-blindness”. J. Anim. Sci. 2020, 98, S140–S149. [Google Scholar] [CrossRef] [PubMed]
- Karstrup, C.C.; Klitgaard, K.; Jensen, T.K.; Agerholm, J.S.; Pedersen, H.G. Presence of bacteria in the endometrium and placentomes of pregnant cows. Theriogenology 2017, 99, 41–47. [Google Scholar] [CrossRef]
- Muskens, J.; Wouda, W.; von Bannisseht-Wijsmuller, T.; van Maanen, C. Prevalence of Coxiella burnetii infections in aborted fetuses and stillborn calves. Vet. Rec. 2012, 170, 260. [Google Scholar] [CrossRef]
- Mee, J.F.; Sanchez-Miguel, C.; Nosov, M.; Gilmore, J. Are we misdiagnosing many Neospora and Leptospira-positive bovine aborted and stillborn fetuses using fetal serology? (Abstract). Reprod. Domest. Anim. 2016, 51, 116. [Google Scholar]
- Mee, J.F. Investigation of bovine abortion and stillbirth/perinatal mortality—Similar diagnostic challenges, different approaches. Ir. Vet. J. 2020, 73, 1–13. [Google Scholar] [CrossRef]
- Jamaluddin, A.A.; Case, J.T.; Hird, D.W.; Blanchard, P.C.; Peauroi, J.R.; Anderson, M.L. Dairy cattle abortion in California: Evaluation of diagnostic laboratory data. J. Vet. Diagnostic Investig. 1996, 8, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Guido, S.; Katzer, F.; Nanjiani, I.; Milne, E.; Innes, E.A. Serology-Based Diagnostics for the Control of Bovine Neosporosis. Trends Parasitol. 2016, 32, 131–143. [Google Scholar] [CrossRef]
- Mee, J.F. A practitioner’s guide to post-mortem examination of an aborted or stillborn calf. Livestock 2016, 21, 38–43. [Google Scholar] [CrossRef]
- Geraghty, T.; Murphy, A.; Mee, J.F.; Orr, J. How to investigate a stillbirth on farm. In Pract. 2021, in press. [Google Scholar]
- Mee, J.F.; Ley, R. How to set up a postmortem facility in a farm animal practice. UK Vet Livest. 2021, in press. [Google Scholar]
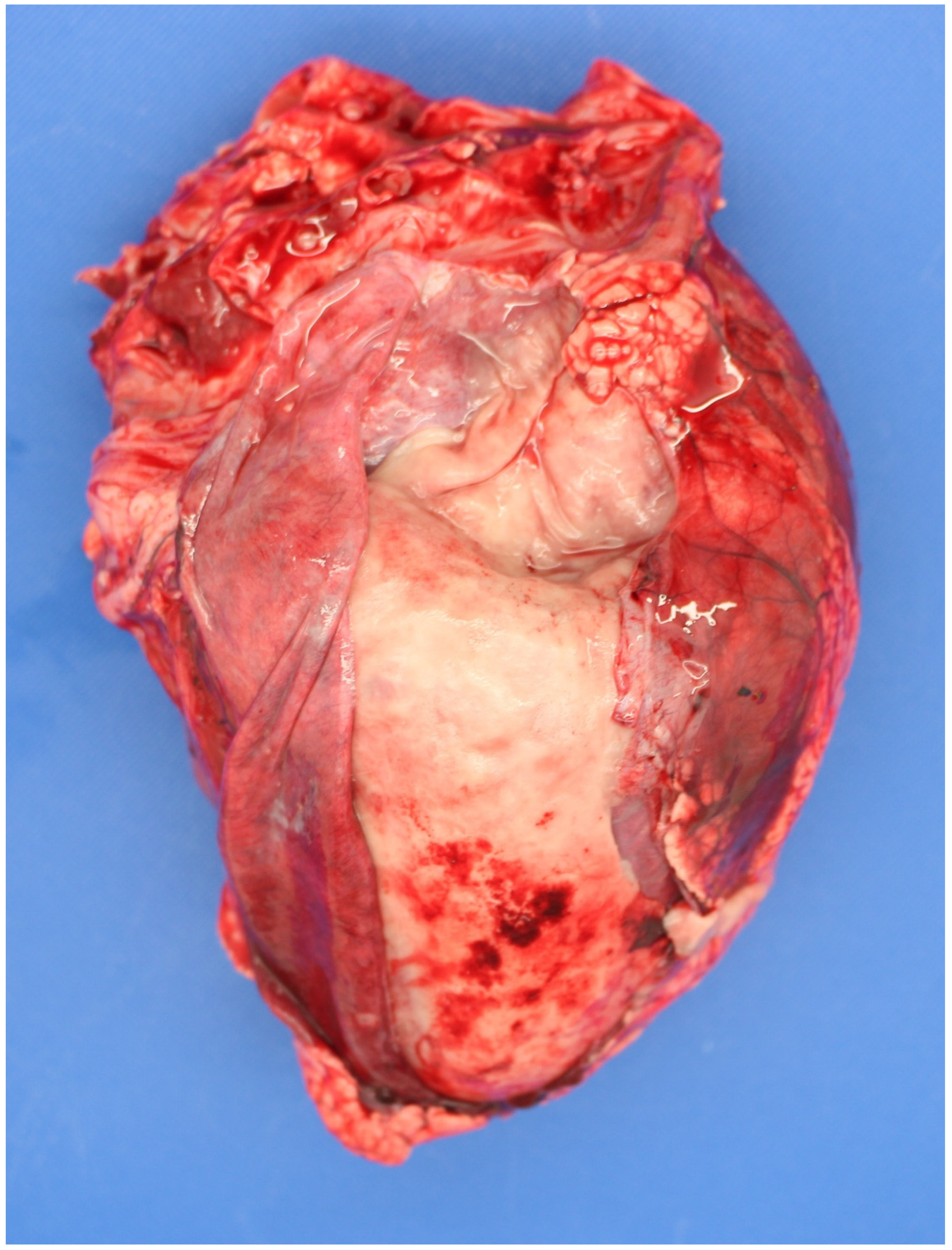
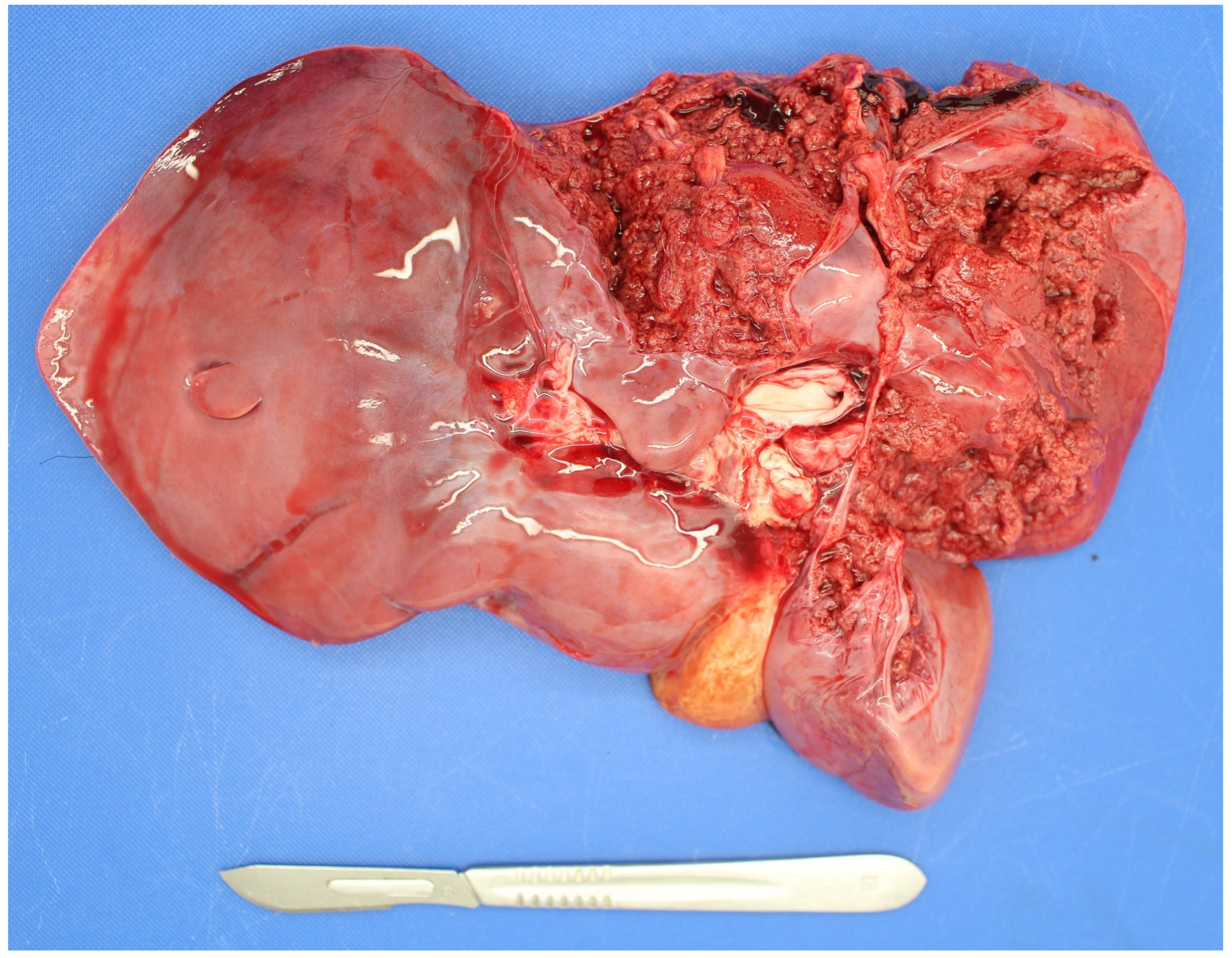
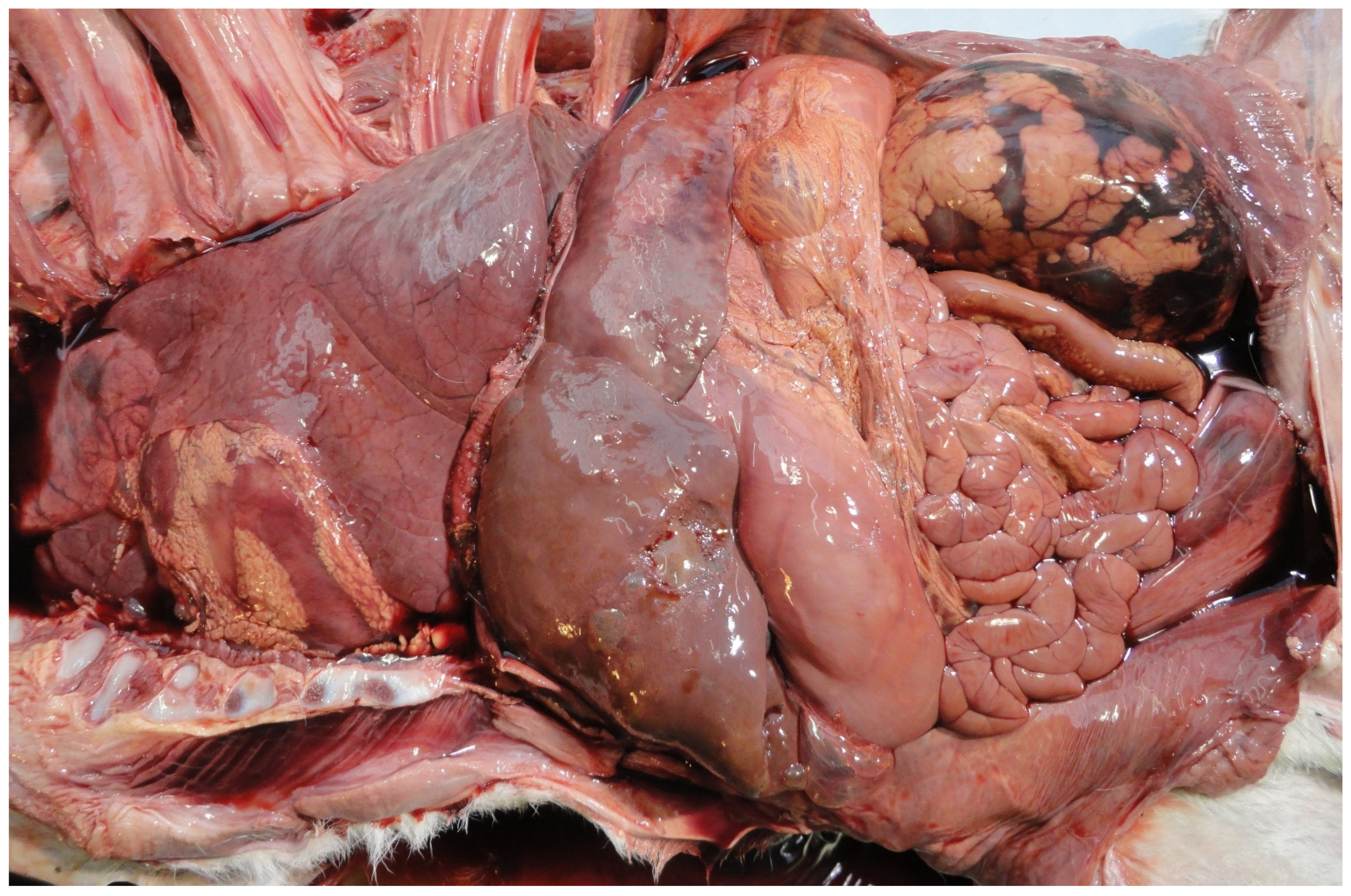

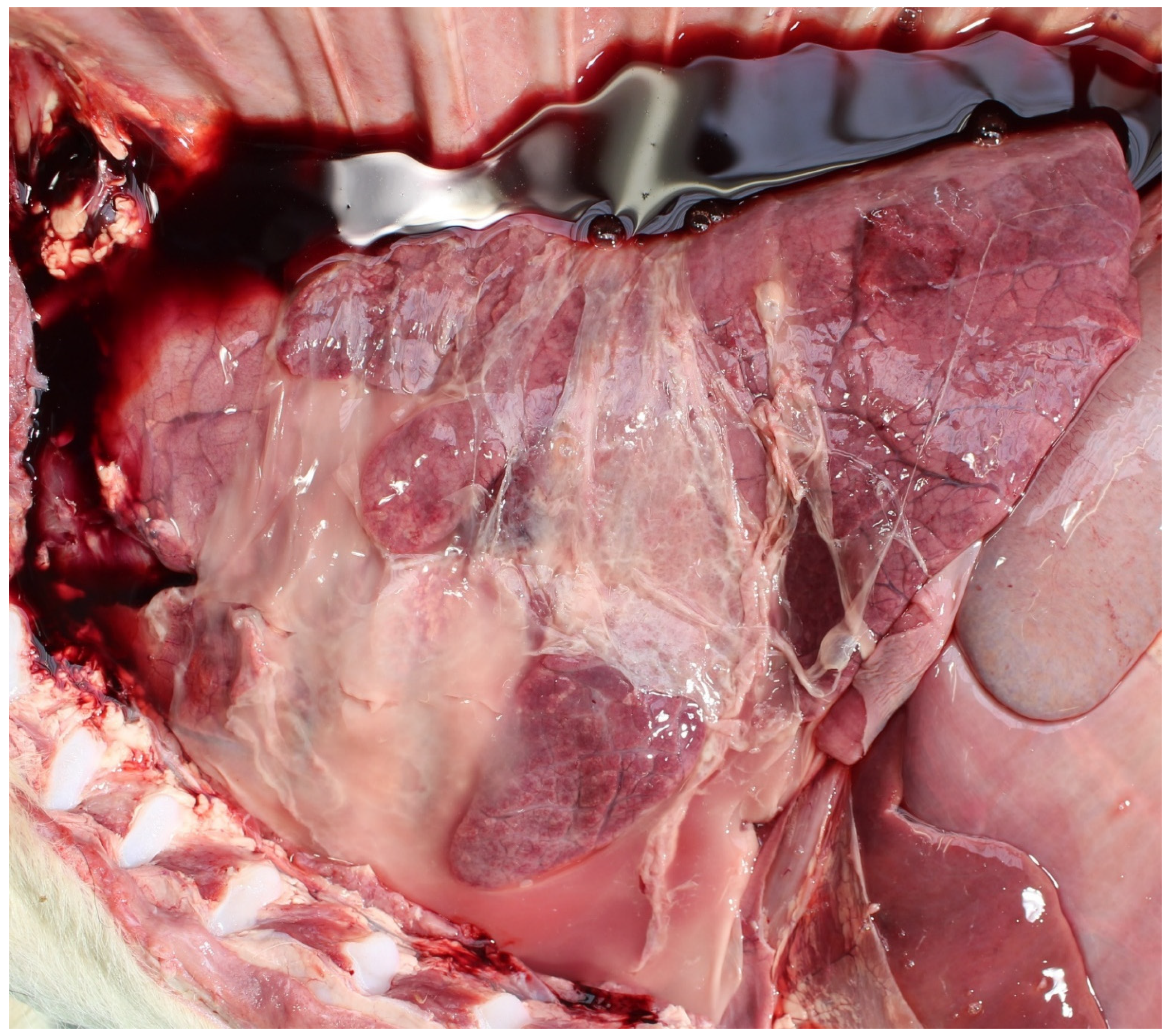
| Country | Perinate Definition | Calves (No.) | Calf Type | Dystocia | Anoxia | Congenital Defects | Infection | Other | Unknown | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Argentina | Gestation >260 d—stillborn | 19 | Dairy | 21.1 | NR 1 | NR | 10.6 | 5.3 | 68.4 | [9] |
| Belgium | Gestation fullterm—died ≤24 h | NR | Dairy & Beef | 7 | 55 | 6 | 18 | 4 | 10 | [15] |
| Canada | Gestation >8 mths—died <1 h | 560 | Beef | 40.2 | NR | 4.3 | 4.3 | 31 | 20.2 | [13] |
| Denmark | Stillbirth | 130 | Dairy & Beef | 9 | 81 | 1.5 | 8 | 0.1 | 0 | [5] |
| Finland | Gestation fullterm—died ≤24 h | 148 | Dairy | 43 | NR 2 | 10 | 14 | 8 | 25 | [6] |
| Iceland | Gestation fullterm—died ≤24 h | 129 | Dairy | 34 | 37 | NR | 12 | 13 | 3.9 | [16] |
| Ireland | Gestation ≥260 d—died ≤48 h | 1345 | Dairy | 33 | 10 | 11 | 9 | 28 | 9 | [10] |
| Japan | Stillbirth | 155 | Beef | 21 | NR | 3.9 | NR | 5.1 | 69.7 | [17] |
| Netherlands | Stillbirth | 193 | Dairy | 4 | 37 | 8.3 | 7.3 | 6 | 42 | [18] |
| Nr. Ireland | Gestation fullterm—died ≤10 min. | 365 | Dairy | 23 | 46 | NR | 31 | NR | NR | [19] |
| Poland | Gestation fullterm—died ≤6 h | 121 | Dairy | NR | NR | NR | 9.9 | NR | NR | [4] |
| Scotland | Gestation fullterm—died ≤48 h | 54 | Beef | 37 2 | NR | 11.1 | 35.2 | 3.7 | 13.0 | [14] |
| Sweden | Gestation fullterm—died ≤24 h | 76 | Dairy | 46.1 | NR | 5.3 | 2.6 | 10.5 | 35.5 | [20] |
| Switzerland 3 | Gestation ≥260 d—died ≤48 h | 47 | Dairy | 4.3 | NR | 0 | 34 | 4.2 | 57.5 | [21] |
| USA | Gestation fullterm—died ≤48 h | 60 | Dairy | 25 | 28.5 | 3.3 | 5 | 6.6 | 31.6 | [22] |
| Primary | Secondary |
|---|---|
| Akabane virus | Absidia spp. |
| Anaplasma phagocytophilium | Arcobacteria spp. |
| Aspergillus spp. | Acinetobacter species |
| Bluetongue virus | Actinomyces spp. |
| Brucella abortus | Aerococcus spp. |
| Bovine adenovirus 5 | Aeromonas hydrophila |
| Bovine herpes viruses 1 and 4 | Bacillus spp. |
| Bovine viral diarrhoea virus | Chlamydia-related organisms (CRO), e.g., Parachlamydia-like organisms (PCO), Waddlia spp. |
| Bacillus licheniformis | Citrobacter youngae |
| Campylobacter foetus spp. | Escherichia spp. |
| Chlamydophila abortus | Fusobacterium spp |
| Clostridium perfringens | Hafnia alvei |
| Coxiella burntiii | Histophilus somni |
| Leptospira spp. | Klebsiella pneumonia |
| Listeria monocytogenes | Listeria spp. |
| Mycoplasma spp. | Mannheimia spp. |
| Neospora caninum | Neisseria, |
| Parainfluenza 3 virus | Nocardia spp. |
| Salmonella spp. | Pantoea agglomerans |
| Schmallenberg virus | Pasteurella spp. |
| Trichomonas foetus foetus | Providencia stuartii |
| Trueperella pyogenes | Serratia spp. |
| Staphylococcus spp. | |
| Streptococcus spp. | |
| Ureaplasma spp. | |
| Yeasts | |
| Yersinia spp. |
| Country | Infected Calves (No.) | Infection(%) | Bac. lich. | Cox. burn. | Fungi | Lepto. spp. | List. spp. | Neo. cani. | Tru. pyog. | Salm. spp. | Viruses | Other inf. | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Argentina | 19 | 10.6 | NR | NR | NR | NR | NR | 5.3 | NR | NR | NR | 5.3 | [9] |
| Belgium | NR | 18 | NR | NR | NR | NR | NR | NR | NR | NR | 12 | 6 | [15] |
| Canada | 560 | 4.3 | NR | NR | NR | NR | 0.4 | 0.2 | NR | NR | 0.2 | 3.5 | [13] |
| Denmark | 130 | 8 | 1 | NR | NR | NR | NR | NR | NR | NR | 7 | NR | [5] |
| Finland | 148 | 14 | 5 | NR | NR | NR | NR | 2 | 0.7 | NR | NR | 6.3 | [6] |
| Iceland | 129 | 12 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 12 | [16] |
| Ireland | 1345 | 9 | 0.80 | NR | NR | 0.6 | 0.2 | 1.5 | 0.9 | 0.2 | 0.8 | 4 | [10] |
| Netherlands | 193 | 7.3 | 1.6 | NR | NR | NR | NR | NR | NR | 1.6 | 4.1 | NR | [18] |
| Nr. Ireland | 365 | 31 | NR | NR | NR | 13.4 | NR | NR | NR | NR | NR | 17.6 | [19] |
| Poland | 121 | 9.9 | NR | NR | NR | NR | NR | 7.4 | NR | NR | NR | 1.7 | [4] |
| Scotland | 54 | 35.2 | 5.5 | NR | 3.7 | 1.9 | NR | NR | NR | NR | NR | 24.1 | [14] |
| Sweden | 76 | 2.6 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 2.6 | [20] |
| Switzerland | 47 | 77 | NR | 31.9 | 8.5 | 6.4 | NR | 2.1 | NR | NR | NR | 28.1 | [21] |
| USA | 60 | 5 | NR | NR | NR | NR | NR | NR | NR | NR | 5 | NR | [22] |
| For Investigation of | Standard Samples | Ancillary Samples | Comments |
|---|---|---|---|
| Failure of passive transfer of immunoglobulins | Perinate blood | NA ** | Only test calves >24 h old, e.g., ZST test |
| Foetopathogenic bacteria and fungi (e.g., Aspergillus spp., B. licheniformis, L. monocytogenes, T. pyogenes, S. Dublin) | Foetal stomach contents (FSC), Placenta (ideally collected from dam to reduce contamination) | Foetal lung, liver, gall bladder, kidney, brain, eyelid. Dam vaginal swab, placentome, blood. | Ancillary samples where FSC/placenta unavailable/contaminated. |
| Neospora caninum | Foetal brain, serum | Foetal heart. Placenta. Dam/cohort bloods | Fresh brain/placenta for PCR, fixed brain or heart/placenta for histopathology if PCR positive |
| Leptospira Hardjo | Foetal kidney, serum | Dam/cohort bloods | Foetal sample dependent upon lab. tests |
| BVDv | Foetal ear, spleen, thymus, serum | Foetal kidney. Dam/cohort bloods | Foetal sample dependent upon lab. Tests |
| BHV-I | Foetal liver, serum. | Foetal kidney. Placenta. Dam/cohort bloods | Foetal PCR/histopathology preferred tests |
| Gross lesions (e.g., foetal pneumonia) | Affected foetal organ | As required | As appropriate (e.g., bacteriology, histopath) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mee, J.F.; Jawor, P.; Stefaniak, T. Role of Infection and Immunity in Bovine Perinatal Mortality: Part 1. Causes and Current Diagnostic Approaches. Animals 2021, 11, 1033. https://doi.org/10.3390/ani11041033
Mee JF, Jawor P, Stefaniak T. Role of Infection and Immunity in Bovine Perinatal Mortality: Part 1. Causes and Current Diagnostic Approaches. Animals. 2021; 11(4):1033. https://doi.org/10.3390/ani11041033
Chicago/Turabian StyleMee, John F., Paulina Jawor, and Tadeusz Stefaniak. 2021. "Role of Infection and Immunity in Bovine Perinatal Mortality: Part 1. Causes and Current Diagnostic Approaches" Animals 11, no. 4: 1033. https://doi.org/10.3390/ani11041033
APA StyleMee, J. F., Jawor, P., & Stefaniak, T. (2021). Role of Infection and Immunity in Bovine Perinatal Mortality: Part 1. Causes and Current Diagnostic Approaches. Animals, 11(4), 1033. https://doi.org/10.3390/ani11041033






