The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production
Abstract
Simple Summary
Abstract
1. Introduction
2. Scutellaria baicalensis and Its Active Components
3. Biological Functions of S. baicalensis
3.1. Antioxidation
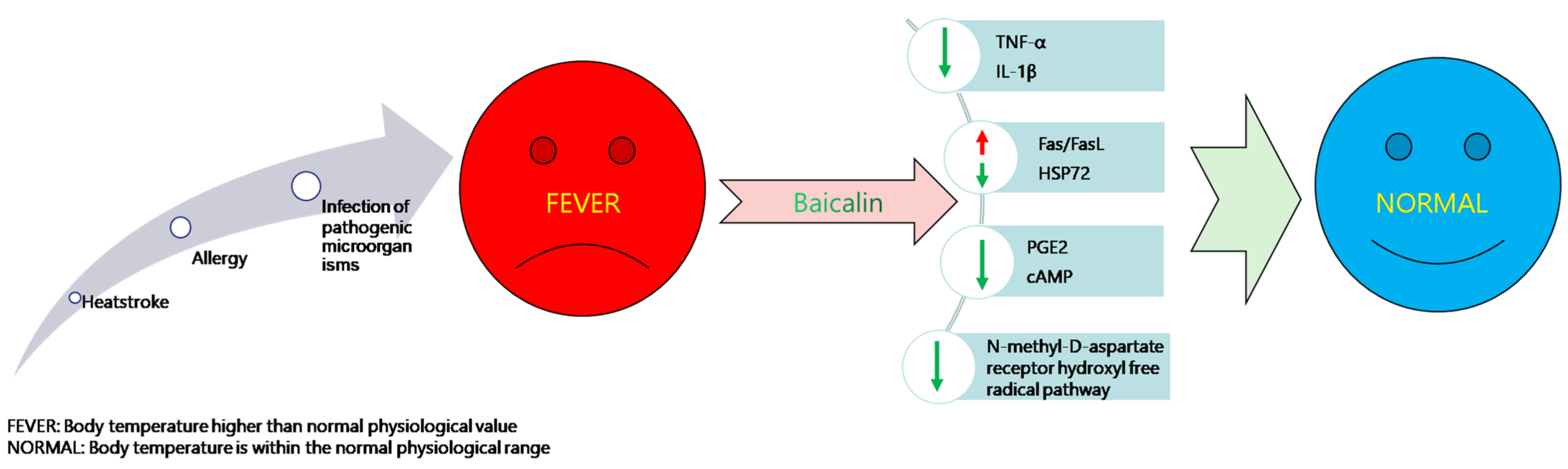
3.2. Antipyretic and Analgesic Effects
3.3. Anti-Inflammatory and Antiallergic Effects
3.4. Antimicrobial Effect
3.5. Antitumor Activity
4. Application of S. baicalensis in Sustainable Animal Production for Better Performance
4.1. Poultry
4.2. Swine
4.3. Ruminants
5. Application of S. baicalensis in Disease Prevention and Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Wallmann, J. Antimicrobial resistance: Challenges ahead. Vet. Rec. 2014, 175, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Antibiotic use in animals. J. Antimicrob. Chemother. 2004, 53, 885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bren, L. Battle of the bugs: Fighting antibiotic resistance. FDA Consum. 2002, 36, 28–34. [Google Scholar] [PubMed]
- Walton, J.R. Antibiotic residues in meat. Br. Vet. J. 1987, 143, 485–486. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Leach, N.; Eckford, S. Waste milk feeding, animal by-products regulations and antibiotic resistance. Vet. Rec. 2013, 172, 166. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- European Food Safety Authority (EFSA). ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals: Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA J. 2017, 15, e04872. [Google Scholar] [CrossRef]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef]
- Liu, S.H.; Chuang, W.C.; Lam, W.; Jiang, Z.; Cheng, Y.C. Safety surveillance of traditional Chinese medicine: Current and future. Drug Saf. 2015, 38, 117–128. [Google Scholar] [CrossRef]
- Storlien, T.M.; Prestløkken, E.; Beauchemin, K.A.; McAllister, T.A.; Iwaasa, A.; Harstad, O.M. Supplementation with crushed rapeseed causes reduction of methane emissions from lactating dairy cows on pasture. Anim. Prod. Sci. 2017, 57, 81–89. [Google Scholar] [CrossRef]
- Shan, C.H.; Guo, J.; Sun, X.; Li, N.; Yang, X.; Gao, Y.; Qiu, D.; Li, X.; Wang, Y.; Feng, M.; et al. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J. Anim. Sci. 2018, 96, 4444–4457. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, S.P.; Luo, D.M.; Zhao, X.L.; Yin, M.J.; Zhou, C.F.; Liu, G.W. Effect of Chinese herbal medicines on rumen fermentation, methanogenesis and microbial flora in vitro. S. Afr. J. Anim. Sci. 2019, 49, 63–70. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, Z.H.; Cao, L.T.; Wang, Y.; Zhou, W.Z.; Zhou, P.; Zuo, F.Y. Effects of traditional Chinese medicine formula on ruminal fermentation, enzyme activities and nutrient digestibility of beef cattle. Anim. Sci. J. 2018, 89, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie Van Leeuwenhoek 2009, 96, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Liu, F.; Wang, Y. Production performance, immunity, and heat stress resistance in Jersey cattle fed a concentrate fermented with probiotics in the presence of a Chinese herbal combination. Anim. Feed Sci. Technol. 2017, 228, 59–65. [Google Scholar] [CrossRef]
- Wang, H.F.; Yang, W.R.; Wang, Y.X.; Yang, Z.B.; Cui, Y.H. The study on the effects of Chinese herbal mixtures on growth, activity of post-ruminal digestive enzymes and serum antioxidant status of beef cattle. Agric. Sci. China 2011, 10, 448–455. [Google Scholar] [CrossRef]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of traditional chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, B.; Bai, C.; Li, G.; Mao, M. Predicting suitable cultivation regions of medicinal plants with Maxent modeling and fuzzy logics: A case study of Scutellaria baicalensis in China. Environ. Earth Sci. 2016, 75, 1–12. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Xing, J.; Chen, X.; Sun, Y.; Luan, Y.; Zhong, D. Interaction of baicalin and baicalein with antibiotics in the gastrointestinal tract. J. Pharm. Pharmacol. 2005, 57, 743–750. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet 2019, 44, 159–168. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, F.; Li, H.D.; Zhao, X.Y. Pharmacokinetic study on baicalin of Qingkailing injection in rats. Zhongguo Zhong Yao Za Zhi 2007, 32, 2534–2538. [Google Scholar]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Z.; Hu, W.; Zhang, W.; Shah, S.W.A.; Ishfaq, M. Baicalin mitigated Mycoplasma gallisepticum-induced structural damage and attenuated oxidative stress and apoptosis in chicken thymus through the Nrf2/HO-1 defence pathway. Vet. Res. 2019, 50, 83. [Google Scholar] [CrossRef]
- Li, H.; Yang, L. Molecular regulatory mechanism of Nrf2 antioxidant. Chin. J. Bioinform. 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Olagaray, K.E.; Brouk, M.J.; Mamedova, L.K.; Sivinski, S.E.; Liu, H.; Robert, F.; Dupuis, E.; Zachut, M.; Bradford, B.J. Dietary supplementation of Scutellaria baicalensis extract during early lactation decreases milk somatic cells and increases whole lactation milk yield in dairy cattle. PLoS ONE 2019, 14, e0210744. [Google Scholar] [CrossRef]
- Li, Q.; Ge, X. Efffect of baicalin on antipyresis and influence on cytokine. Zhongguo Zhong Yao Za Zhi 2010, 35, 1068–1072. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhang, F.; Fan, S.D. Effects of baicalin on contents of PGE2 and cAMP in hypothalamus of fever rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2002, 18, 139–141. [Google Scholar]
- Guo, X.; Chi, S.; Cong, X.; Li, H.; Jiang, Z.; Cao, R.; Tian, W. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod. Toxicol. 2015, 57, 196–203. [Google Scholar] [CrossRef]
- Chen, D.S.; Cao, J.G.; Zhu, B.; Wang, Z.L.; Wang, T.F.; Tang, J.J. Baicalin attenuates joint pain and muscle dysfunction by inhibiting muscular oxidative stress in an experimental osteoarthritis rat model. Arch. Immunol. Ther. Exp. Warsz 2018, 66, 453–461. [Google Scholar] [CrossRef]
- Lee, J.-K.; Song, Y.-K.; Lim, H.-H. Analgesic and anti-inflammatory effect of Scutellaria baicalensis. Korean J. Orient. Med. 2007, 28, 124–135. [Google Scholar]
- Yimam, M.; Brownell, L.; Hodges, M.; Jia, Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet Suppl. 2012, 9, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Sheng, Y.; Zou, Y.; Bo, L.; Wang, F.; Lou, J.; Fan, X.; Bao, R.; Wu, Y.; et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS ONE 2012, 7, e35523. [Google Scholar] [CrossRef] [PubMed]
- Burnett, B.P.; Jia, Q.; Zhao, Y.; Levy, R.M. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Simujide, A.H.; Aorigele, C.; Chun-Jie, W.; Manda, B. Serotyping of Escherichia coli from healthy cattle and analyses of antimicrobial activities of Chinese herbal drugs on Escherichia coli strains with different serotypes in Hulunbeier, China. Minerva Biotecnol. 2011, 23, 65–70. [Google Scholar]
- Zhao, Q.Y.; Yuan, F.W.; Liang, T.; Liang, X.C.; Luo, Y.R.; Jiang, M.; Qing, S.Z.; Zhang, W.M. Baicalin inhibits Escherichia coli isolates in bovine mastitic milk and reduces antimicrobial resistance. J. Dairy Sci. 2018, 101, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Qian, M.; Tang, S.; Wu, C.; Wang, Y.; He, T.; Chen, T.; Xiao, X. Synergy between baicalein and penicillins against penicillinase-producing Staphylococcus aureus. Int. J. Med. Microbiol. 2015, 305, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, J.; Peng, J.; Zhang, Y.; Wang, L.; Wu, J.; Ye, L.; Fang, C. Effect of baicalin on proliferation and apoptosis in pancreatic cancer cells. Am. J. Transl. Res. 2019, 11, 5645–5654. [Google Scholar]
- Yu, Y.; Pei, M.; Li, L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int. J. Clin. Exp. Med. 2015, 8, 8958–8967. [Google Scholar] [PubMed]
- Jia, Y.; Chen, L.; Guo, S.; Li, Y. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol. Biol. Rep. 2019, 46, 1693–1700. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y.; Li, C.; Wang, H.; Jiang, Z.; Zhang, Z.; Guo, Q.; Song, G.; Bi, K.; Jiang, G. Enhancement of baicalin by hexamethylene bisacetamide on the induction of apoptosis contributes to simultaneous activation of the intrinsic and extrinsic apoptotic pathways in human leukemia cells. Oncol. Rep. 2013, 30, 2071–2080. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Xu, H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Tu, I.H.; Yen, H.T.D.; Cheng, H.W.; Chiu, J.H. Baicalein protects chicken embryonic cardiomyocyte against hypoxia-reoxygenation injury via μ- and δ- but not κ-opioid receptor signaling. Eur. J. Pharmacol. 2008, 588, 251–258. [Google Scholar] [CrossRef]
- Dai, C.S.; Tang, S.S.; Zhang, Q.J.; Li, D.W.; Xiao, X.L. Biological functions of baicalin and baicalein and their application in animal production. China Feed 2015, 18, 11–14. [Google Scholar] [CrossRef]
- Yun, M.Y.; Yang, J.H.; Kim, D.K.; Cheong, K.J.; Song, H.H.; Kim, D.H.; Cheong, K.J.; Kim, Y.I.; Shin, S.C. Therapeutic effects of baicalein on atopic dermatitis-like skin lesions of NC/Nga mice induced by dermatophagoides pteronyssinus. Int. Immunopharmacol. 2010, 10, 1142–1148. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the Quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef]
- Serpa, R.; França, E.J.G.; Furlaneto-Maia, L.; Andrade, C.; Diniz, A.; Furlaneto, M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. J. Med. Microbiol. 2012, 61, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Wang, H.; Shi, K.; Li, J.M.; Zong, Y.; Du, R. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules 2018, 24, 131. [Google Scholar] [CrossRef]
- Smith, J.F.; Starr, E.G.; Goodman, M.A.; Hanson, R.B.; Palmer, T.A.; Woolstenhulme, J.B.; Weyand, J.A.; Marchant, A.D.; Bueckers, S.L.; Nelson, T.K.; et al. Topical application of Wogonin provides a novel treatment of knee osteoarthritis. Front. Physiol. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Seong, R.K.; Kim, J.A.; Shin, O.S. Wogonin, a flavonoid isolated from Scutellaria baicalensis, has anti-viral activities against influenza infection via modulation of AMPK pathways. Acta Virol. 2018, 62, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, D.; Wang, K.X. Study of Scutellaria baicalensis and baicalin against antimicrobial susceptibility of Helicobacter pylori strains in vitro. Zhong Yao Cai 2008, 31, 707–710. [Google Scholar]
- Qiu, F.; Meng, L.; Chen, J.; Jin, H.; Jiang, L. In vitro activity of five flavones from Scutellaria baicalensisin combination with Cefazolin against methicillin resistant Staphylococcus aureus (MRSA). Med. Chem. Res. 2016, 25, 2214–2219. [Google Scholar] [CrossRef]
- Leonova, G.N.; Shutikova, A.L.; Lubova, V.A.; Maistrovskaya, O.S. Inhibitory activity of Scutellaria baicalensis flavonoids against tick-borne encephalitis virus. Bull. Exp. Biol. Med. 2020, 168, 665–668. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, J.; Wang, C.; Shi, W.; Li, D. The therapeutic effect of Yinhuangerchen mixture on avian infectious laryngotracheitis. Poult. Sci. 2018, 97, 2690–2697. [Google Scholar] [CrossRef]
- Shin, H.S.; Bae, M.J.; Choi, D.W.; Shon, D.H. Skullcap (Scutellaria baicalensis) extract and its active compound, wogonin, inhibit ovalbumin-induced Th2-mediated response. Molecules 2014, 19, 2536–2545. [Google Scholar] [CrossRef]
- Bae, M.J.; Shin, H.S.; See, H.J.; Jung, S.Y.; Kwon, D.A.; Shon, D.H. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci. Rep. 2016, 6, 32225. [Google Scholar] [CrossRef]
- Kim, T.W.; Song, I.B.; Lee, H.K.; Kim, M.S.; Ham, S.H.; Cho, J.H.; Lim, J.H.; Yun, H.I. Assessment of dermal safety of Scutellaria baicalensis aqueous extract topical application on skin hypersensitivity. Planta Med. 2013, 79, 959–962. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Son, E.J.; Kim, M.; Heo, Y.M.; Nam, J.B.; Ro, J.Y.; Woo, S.S. Antiallergic herbal composition from Scutellaria baicalensis and Phyllostachys edulis. Planta Med. 2010, 76, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Kim, H.-W.; Kim, S.G.; Jung, S.-H.; Whang, W.-K. Antioxidant and antiallergic activity of compounds from the aerial parts of Scutellaria baicalensis Georgi. Yakhak Hoeji 2006, 50, 136–143. [Google Scholar]
- Zhou, Y.J.; Wang, H.; Sui, H.H.; Li, L.; Zhou, C.L.; Huang, J.J. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic Rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm. Res. 2016, 65, 603–612. [Google Scholar] [CrossRef]
- Liu, J.J.; Huang, T.S.; Cheng, W.F.; Lu, F.J. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int. J. Cancer 2003, 106, 559–565. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure-antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Tong, R.; Mehendale, S.R.; Wang, C.Z.; Shao, Z.; Yuan, C.S. Comparison of antioxidant effects of various Scutellaria baicalensis fractions and the potential role of catalase upregulation. Am. J. Chin. Med. 2009, 37, 621–623. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Cong, X.; Wang, X.; Jiang, Z.; Zhang, Q.; Qi, X.; Gao, S.; Cao, R.; Tian, W. Baicalin attenuates lipopolysaccharide induced inflammation and apoptosis of cow mammary epithelial cells by regulating NF-κB and HSP72. Int. Immunopharmacol. 2016, 40, 139–145. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, N.; Li, D.; Liang, D.; Liu, Z.; Li, F.; Fu, Y.; Cao, Y.; Deng, X.; Yang, Z. Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int. Immunopharmacol. 2013, 16, 125–130. [Google Scholar] [CrossRef]
- Tian, H.; Wang, X.P.; Zhang, F.L. Protective effects of baicalein on the gastric mucosa of rats with chronic atrophic gastritis. Tradit. Chin. Med. J. 2015, 14, 62–64. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.S. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Li, S.; Ou, Y.W.; Ou, Q. Effects of Scutellaria baicalensis on activity and biofilm formation of Klebsiella pneumoniae. Chin. Med. Sci. J. 2016, 31, 180–184. [Google Scholar] [CrossRef]
- Sohail, A.; Bhat, W.F.; Bhat, S.A.; Furkan, M.; Shah, A.; Bano, B. Investigating the preventive effects of baicalin and gallocatechin against glyoxal-induced cystatin aggregation. J. Biomol. Struct. Dyn. 2018, 36, 3791–3802. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, Y.; Zhang, W.; Guo, Q. Baicalein suppresses the proliferation and invasiveness of colorectal cancer cells by inhibiting Snail-induced epithelial-mesenchymal transition. Mol. Med. Rep. 2020, 21, 2544–2552. [Google Scholar] [CrossRef]
- Song, J.; Zhou, Y.Z.; Pang, Y.Y.; Gao, L.; Du, G.H.; Qin, X.M. The anti-aging effect of Scutellaria baicalensis Georgi flowers extract by regulating the glutamine-glutamate metabolic pathway in D-galactose induced aging rats. Exp. Gerontol. 2020, 134, 110843. [Google Scholar] [CrossRef]
- Ren, L.; Liu, W.; Wang, C.; Yang, Y.; Huang, X.; Wang, C.; Li, Y. The ancient Chinese formula Longdan Xiegan Tang improves antipsychotic-induced hyperprolactinemia by repairing the hypothalamic and pituitary TGF-β1 signaling in rats. J. Ethnopharmacol. 2020, 254, 112572. [Google Scholar] [CrossRef]
- Nam, J.E.; Jo, S.Y.; Ahn, C.W.; Kim, Y.S. Baicalin attenuates fibrogenic process in human renal proximal tubular cells (HK-2) exposed to diabetic milieu. Life Sci. 2020, 254, 117742. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Zhao, X. Baicalin inhibits human cervical cancer cells by suppressing protein kinase C/signal transducer and activator of transcription (PKC/STAT3) signaling pathway. Med. Sci. Monit. 2018, 24, 1955–1961. [Google Scholar] [CrossRef]
- Han, Y.H.; Kee, J.Y.; Kim, D.S.; Mun, J.G.; Park, S.H.; Kim, Y.J.; Um, J.Y.; Hong, S.H. Arctii Fructus inhibits colorectal cancer cell proliferation and MMPs mediated invasion via AMPK. Am. J. Chin. Med. 2017, 45, 1309–1325. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Cheng, J.; Yu, B.; Duan, C.; Peng, J. Baicalin, a Chinese herbal medicine, inhibits the proliferation and migration of human non-small cell lung carcinoma (NSCLC) cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Med. Sci. Monit. 2018, 24, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Yang, D.; Chen, Y.; Liu, W. Baicalin suppresses lung cancer growth by targeting PDZ-binding kinase/T-LAK cell-originated protein kinase. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, Z.; Chen, P.; Wang, J.; Zhou, Y.; Huang, J. Baicalin inhibits growth and induces apoptosis of human osteosarcoma cells by suppressing the AKT pathway. Oncol. Lett. 2019, 18, 3188–3194. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Luo, X.; Zhang, Q.; Song, L. Baicalin, a potent inhibitor of NF-κB signaling pathway, enhances chemosensitivity of breast cancer cells to docetaxel and inhibits tumor growth and metastasis both in vitro and in vivo. Front. Pharmacol. 2020, 11, 879. [Google Scholar] [CrossRef]
- Zeng, L.; Dong, J.; Yu, W.; Huang, J.; Liu, B.; Feng, X. Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm. Pharmacol. Ther. 2010, 23, 411–419. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.; Weng, Y.; Yu, Y.; Zhang, D.; Fan, W.; Dai, R.; Hu, Z. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 2004, 74, 2467–2478. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, N.; Ling, Y.; Gao, Y.; Wang, L.; Sun, Y.; Qi, Q.; Feng, F.; Liu, W.; Liu, W.; et al. Wogonoside inhibits lipopolysaccharide-induced angiogenesis in vitro and in vivo via toll-like receptor 4 signal transduction. Toxicology 2009, 259, 10–17. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, Y.; Lin, B.; Qin, Y.; Hui, H.; Li, Z.; You, Q.; Lu, N.; Guo, Q. Oroxylin A, a classical natural product, shows a novel inhibitory effect on angiogenesis induced by lipopolysaccharide. Pharmacol. Rep. 2012, 64, 1189–1199. [Google Scholar] [CrossRef]
- Zhou, M.; Song, X.; Huang, Y.; Wei, L.; Li, Z.; You, Q.; Guo, Q.; Lu, N. Wogonin inhibits H2O2-induced angiogenesis via suppressing PI3K/Akt/NF-κB signaling pathway. Vasc. Pharmacol. 2014, 60, 110–119. [Google Scholar] [CrossRef]
- Fan, J.H.; Yang, Y.L.; Gu, H.B.; Jiang, Y.X.; Li, S.M.; Dou, X.H. Study on the germicidal efficacy and safety evaluation of a Chinese medicine semen disinfectant. Chin. J. Vet. Drug 2020, 54, 41–48. [Google Scholar]
- Zhou, Y.; Mao, S.; Zhou, M. Effect of the flavonoid baicalein as a feed additive on the growth performance, immunity, and antioxidant capacity of broiler chickens. Poult. Sci. 2019, 98, 2790–2799. [Google Scholar] [CrossRef]
- Króliczewska, B.; Zawadzki, W.; Skiba, T.; Kopec, W.; Kroliczewski, J. The influence of baical skullcap root (Scutellaria baicalensis radix) in the diet of broiler chickens on the chemical composition of the muscles, selected performance traits of the animals and the sensory characteristics of the meat. Vet. Med. 2008, 53, 373–380. [Google Scholar] [CrossRef]
- Rusinek-Prystupa, E.; Lechowski, J.; Żukiewicz-Sobczak, W.; Sobczak, P.; Zawiślak, K. Influence of citrosept addition to drinking water and Scutellaria baicalensis root extract on the content of selected mineral elements in blood plasma of Turkey hens. Ann. Agric. Environ. Med. 2014, 21, 595–600. [Google Scholar] [CrossRef][Green Version]
- An, B.K.; Kwon, H.S.; Lee, B.K.; Kim, J.Y.; You, S.J.; Kim, J.M.; Kang, C.W. Effects of dietary skullcap (Scutellaria baicalensis) extract on laying performance and lipid oxidation of chicken eggs. Asian-Australas. J. Anim. Sci. 2010, 23, 772–776. [Google Scholar] [CrossRef]
- Varmuzova, K.; Matulova, M.E.; Gerzova, L.; Cejkova, D.; Gardan-Salmon, D.; Panhéleux, M.; Robert, F.; Sisak, F.; Havlickova, H.; Rychlik, I. Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella enteritidis infection. Poult. Sci. 2015, 94, 2049–2058. [Google Scholar] [CrossRef]
- Lv, H.Y.; Li, M.; Wang, Z.M.; Liang, W.; Nie, W.; Guo, Y.M. Effects of Lonicera japonica and Scutellaria baicalensis extracts on growth performance, immune organ development and antioxidant function of Broilers. Chin. J. Anim. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Liang, Y.; Ren, C.C.; Jing, D.; Teng, Z.C.; Bi, H.M. Effects of flavonoids from Scutellaria baicalensis Georgi on growth performance and intestinal microflora of broilers. J. Tradit. Chin. Vet. Med. 2012, 31, 39–42. [Google Scholar] [CrossRef]
- Li, G.Z.; Diao, X.P. Effect of adding fermented Scutellaria in feed on production performance of weaned piglets. China Feed 2012, 34, 27–28. [Google Scholar] [CrossRef]
- Zhao, P.; Li, H.; Lei, Y.; Li, T.; Kim, S.; Kim, I. Effect of fermented medicinal plants on growth performance, nutrient digestibility, fecal noxious gas emissions, and diarrhea score in weanling pigs. J. Sci. Food Agric. 2016, 96, 1269–1274. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, Y.S.; Chiou, M.T.; Su, C.H.; Chen, D.S.; Tsai, C.E.; Yu, B.; Hsu, Y.M. Application of Scutellariae radix, Gardeniae fructus, and probiotics to prevent Salmonella enterica serovar choleraesuis infection in swine. Evid.-Based Complementary Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; He, X.; Jiao, N.; Zhang, X.; Qiu, K.; Piao, X.; Yin, J. The involvement of NF-κB/P38 pathways in Scutellaria baicalensis extracts attenuating of Escherichia coli K88-induced acute intestinal injury in weaned piglets. Br. J. Nutr. 2019, 122, 152–161. [Google Scholar] [CrossRef]
- Fu, S.; Zhuang, F.; Guo, L.; Qiu, Y.; Xiong, J.; Ye, C.; Liu, Y.; Wu, Z.; Hou, Y.; Hu, C.A.A. Effect of baicalin-aluminum complexes on fecal microbiome in piglets. Int. J. Mol. Sci. 2019, 20, 2390. [Google Scholar] [CrossRef]
- Liao, Y.J.; Cui, Y.M.; Hu, X.R.; Zheng, Y.W.; Lu, H.M.; Huang, D.; Deng, Y.H.; Deng, X.M. Treatment test of skullcap injection on the artificial infection of swine edema disease. Chin. J. Vet. Sci. 2016, 36. [Google Scholar] [CrossRef]
- Lv, J.F.; Wen, A.Y.; Ning, K.J.; Li, L.; Jiang, J.P.; Lu, Z.X.; Liu, C.; Ying, R.H.; Feng, B.M. Effects and mechanism of baicalin for treating diarrheic piglets. Chin. J. Vet. Sci. 2016, 36, 1401–1405. [Google Scholar] [CrossRef]
- Gheisar, M.M.; Cheong, J.Y.; Zhao, P.; Kim, I.H. Evaluating the influence of dietary phytogenic blends on gestating and lactating sows and suckling piglets. Anim. Prod. Sci. 2018, 50, 2071–2075. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, scutellaria baicalensis) as alternative performance enhancers in broilers. J. Poult. Sci. 2015, 52, 119–126. [Google Scholar] [CrossRef]
- Liu, W.C.; Kim, I.H. Influence of extract mixture from Scutellaria baicalensis and Lonicera japonica on egg production, nutrient digestibility, blood profiles and egg quality in laying hens reared in hot humid season. Anim. Nutr. Feed Technol. 2017, 17, 137–146. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhang, W.; Hu, W.; Waqas Ali Shah, S.; Liu, Y.; Wang, J.; Wu, Z.; Ahmad, I.; Li, J. Antagonistic effects of baicalin on Mycoplasma gallisepticum-induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infect. Drug Resist. 2019, 12, 3075–3089. [Google Scholar] [CrossRef]
- Króliczewska, B.; Graczyk, S.; Króliczewski, J.; Pliszczak-Król, A.; Miśta, D.; Zawadzki, W. Investigation of the immune effects of Scutellaria baicalensis on blood leukocytes and selected organs of the chicken’s lymphatic system. J. Anim. Sci. Biotechnol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Pi, S.H.; Kim, I.H. Growth performance, blood profile, nutrient digestibility and meat quality of broilers fed on diets supplemented with Scutellaria baicalensis extract. Eur. Poult. Sci. 2016, 80, 1–10. [Google Scholar] [CrossRef]
- Liao, X.D.; Wen, Q.; Zhang, L.Y.; Lu, L.; Zhang, L.Y.; Luo, X.G. Effect of dietary supplementation with flavonoid from Scutellaria baicalensis Georgi on growth performance, meat quality and antioxidative ability of broilers. J. Integr. Agric. 2018, 17, 1165–1170. [Google Scholar] [CrossRef]
- Wang, W.W.; Jia, H.J.; Zhang, H.J.; Wang, J.; Lv, H.Y.; Wu, S.G.; Qi, G.H. Supplemental plant extracts from Flos lonicerae in combination with Baikal skullcap Attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019, 10, 1681. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of probiotic bacteria-fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, Scutellaria baicalensis) as performance enhancers in growing pigs. Anim. Sci. J. 2015, 86, 603–609. [Google Scholar] [CrossRef]
- Liu, W.C.; Pi, S.H.; Kim, I.H. Effects of Scutellaria baicalensis and Lonicera japonica extract mixture supplementation on growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs. Ital. J. Anim. Sci. 2016, 15, 446–452. [Google Scholar] [CrossRef]
- Guo, Q.; Xuan, M.F.; Luo, Z.B.; Wang, J.X.; Han, S.Z.; Ri, M.H.; Choe, Y.G.; Hwang, K.M.; Yin, X.J.; Kang, J.D. Baicalin improves the in vitro developmental capacity of pig embryos by inhibiting apoptosis, regulating mitochondrial activity and activating sonic hedgehog signaling. Mol. Hum. Reprod. 2019, 25, 538–549. [Google Scholar] [CrossRef]
- Yausheva, E.V.; Duskaev, G.K.; Levakhin, G.I.; Nurzhanov, B.S.; Yuldashbaev, Y.A.; Rysaev, A.F.; Rakhmatullin, S.G.; Inchagova, K.S. Evaluation of the effects of plant extracts on cattle rumen microbiome. IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012165. [Google Scholar] [CrossRef]
- Gao, X.; Guo, M.; Li, Q.; Peng, L.; Liu, H.; Zhang, L.; Bai, X.; Wang, Y.; Li, J.; Cai, C. Plasma metabolomic profiling to reveal antipyretic mechanism of Shuang-huang-lian injection on yeast-induced pyrexia rats. PLoS ONE 2014, 9, e100017. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhang, W.; Liu, Y.; Wang, J.; Wu, Z.; Shah, S.W.; Li, R.; Miao, Y.; Chen, C.; Li, J. Baicalin attenuated Mycoplasma gallisepticum-induced immune impairment in chicken bursa of Fabricius through modulation of autophagy and inhibited inflammation and apoptosis. J. Sci. Food Agric. 2021, 101, 880–890. [Google Scholar] [CrossRef]
- Wu, Z.; Fan, Q.; Miao, Y.; Tian, E.; Ishfaq, M.; Li, J. Baicalin inhibits inflammation caused by coinfection of Mycoplasma gallisepticum and Escherichia coli involving IL-17 signaling pathway. Poult. Sci. 2020, 99, 5472–5480. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Chen, L.; Lian, J.; Xie, Z.; Ye, Y.; Lv, H.; Li, H.; et al. Effect of baicalin on bacterial secondary infection and inflammation caused by H9N2 AIV infection in chickens. Biomed. Res. Int. 2020, 2020, 2524314. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Xu, J.; Ye, C.; Fu, S.; Hu, C.A.; Qiu, Y.; Liu, Y. Protective effects of baicalin on peritoneal tight junctions in piglets challenged with Glaesserella parasuis. Molecules 2021, 26, 1268. [Google Scholar] [CrossRef]
- Jia, F.; Ma, W.; Zhang, X.; Wang, D.; Zhou, X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 2731–2742. [Google Scholar] [CrossRef]
- Perruchot, M.H.; Gondret, F.; Robert, F.; Dupuis, E.; Quesnel, H.; Dessauge, F. Effect of the flavonoid baicalin on the proliferative capacity of bovine mammary cells and their ability to regulate oxidative stress. PeerJ 2019, 7, e6565. [Google Scholar] [CrossRef]
- Zheng, C.; Pei, T.; Huang, C.; Chen, X.; Bai, Y.; Xue, J.; Wu, Z.; Mu, J.; Li, Y.; Wang, Y. A novel systems pharmacology platform to dissect action mechanisms of traditional Chinese medicines for bovine viral diarrhea disease. Eur. J. Pharm. Sci. 2016, 94, 33–45. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhao, Y.L.; Qin, J.H. Research on the efficacy of Chinese herbal compound for bovine viral diarrhea. Prog. Vet. Med. 2010, 31, 117–119. [Google Scholar] [CrossRef]
- Yang, F.; Yue, H.; Xu, S.J.; Luo, T.T.; Hou, W. Estimation of the antivirus effects of Chinese herbal compound in vitro. J. Southwest Univ. Natl. Nat. Sci. Ed. 2009, 35, 529–531. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, R.; Hu, Y.; Zhu, T.; Ma, T.; Wu, H.; Hu, L. Anti-NDV activity of baicalin from a traditional Chinese medicine in vitro. J. Vet. Med Sci. 2016, 78, 819–824. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Shi, J.; Zeng, L.; Wang, D.; Wu, Y.; Hu, Y.; Liu, J. Assessment of the effect of baicalin on duck virus hepatitis. Curr. Mol. Med. 2019, 19, 376–386. [Google Scholar] [CrossRef]


| Type of Product | Active Components | Biological Functions | Application | Target | References |
|---|---|---|---|---|---|
| Active compound | Baicalin | Antioxidation | Thymus protection | Chicken | [23,24,25] |
| Antipyretic effect | Reducing mastitis incidence | Dairy cow, rat | [26,27,28,29] | ||
| Analgesic effect | - | Rat | [30,31,32] | ||
| Anti-inflammation | Pyemiapyaemia Cardiovascular disease 1 | Human, mouse | [33] [34] | ||
| Antibacterial effect | Escherichia coli | Dairy cow | [35,36,37,38] | ||
| Antitumor activity | Pancreatic cancer 1 Liver cancer 1 Colon cancer 1 Leukemia 1 | Human | [39] [40] [41] [42] | ||
| Baicalein | Antioxidation | Protection against H2O2-induced oxidative injury of neuronal cells | Human | [19] [23,43,44,45] | |
| Heart protection | Chicken | ||||
| Anti-inflammation | Treatment of skin diseases | Mouse | [46] | ||
| Antimicrobial effect | Staphylococcus aureus and E.coli | Animals | [47,48] | ||
| Candida | [49,50] | ||||
| Antitumor | Induction of tumor cell death | Human | [51] | ||
| Wogonin | Cartilage protection | Treatment of arthritis 1 | Mouse | [52] | |
| Anti-inflammation | Treatment of skin diseases | Animals | [53] | ||
| Antivirus | Treatment of avian influenza | Chicken | [54] | ||
| Extract | S. baicalensis extract | Antibacterial effect | Klebsiella pneumoniae and Pseudomonas aeruginosa | - | [55,56] |
| Antivirus | Avian infectious laryngotracheitis, tick borne encephalitis 1 | Animals | [57,58] | ||
| Root | S. baicalensis | Antiallergic effect | Treatment of pruritus | Animals | [59,60,61,62,63,64,65] |
| Inhibition of angiogenesis | Treatment of cancer 1 | Human | [66] |
| Type of Animal | Age of Animals | Source | Dose or Concentration | Main Effects | References |
|---|---|---|---|---|---|
| Broilers | 7–42 d | Baicalein | 100–200 mg/kg diet | Improve growth performance, immunity, and antioxidant activity | [93] |
| Broilers | 1–42 d | S. baicalensis roots | 5, 10, 15 g/kg diet | Increase broiler body weight and feed conversion efficiency | [94] |
| Turkey hens | 42–63 d | S. baicalensis extract | 0.009, 0.018, 0.036 mL/kg BW 1 | Alter the contents of sodium, potassium, calcium, magnesium, copper, zinc, and iron in plasma and affect meat quality | [95] |
| Laying hens | - | S. baicalensis extract | 5 g/kg diet | Increase egg weight, decrease microbial content in the cecum, reduce the amount of propylene glycol in eggs and delay lipid oxidation in eggs | [96] |
| Chicken | 9–24 d | Curcuma longa and S. baicalensis extracts | 2 g/kg diet | Reduce intestinal inflammation and improve production performance | [97] |
| Broilers | 1–42 d | S. baicalensis and Lonicera japonica extracts | 500 mg/kg diet | Improve growth performance, promote the development of immune organs, and improve the antioxidant function | [98] |
| Broilers | 1–49 d | Baicalin | 20 mg/kg diet | Improve growth performance, blood parameters, nutrient digestibility, and meat quality | [99] |
| Weaned piglets | 28–56 d | Fermented Scutellaria | 1.5 mg/kg diet | Enhance appetite, increase average daily intake, reduce feed-to-weight ratio and diarrhea rate, and improve the feed reward | [100] |
| Weaned piglets | - | S. baicalensis extract | - | Improve growth performance and manipulate intestinal microflora | [101,102,103,104] |
| Weaned piglets | 28–35 d | Baicalin | 10 mg/kg BW, 1 time/d, 5 d; intramuscular injection | Prevent swine edema disease | [105] |
| Piglets | 5–25 d | Baicalin | 212.5 mg/time, 2 times/d, 5 d; oral administration | Prevent piglet diarrhea | [106] |
| Pregnant and lactating sows | - | Mixed herbs containing S. baicalensis | - | Decrease weight loss and improve litter performance | [107] |
| Dairy cows | - | S. baicalensis extract | 100 g/kg diet | Reduce incidence of mastitis and improve milk yield | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production. Animals 2021, 11, 1039. https://doi.org/10.3390/ani11041039
Yin B, Li W, Qin H, Yun J, Sun X. The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production. Animals. 2021; 11(4):1039. https://doi.org/10.3390/ani11041039
Chicago/Turabian StyleYin, Baishuang, Wei Li, Hongyu Qin, Jinyan Yun, and Xuezhao Sun. 2021. "The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production" Animals 11, no. 4: 1039. https://doi.org/10.3390/ani11041039
APA StyleYin, B., Li, W., Qin, H., Yun, J., & Sun, X. (2021). The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production. Animals, 11(4), 1039. https://doi.org/10.3390/ani11041039






