Prime Vaccination with Chitosan-Coated Phipps BCG and Boosting with CFP-PLGA against Tuberculosis in a Goat Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
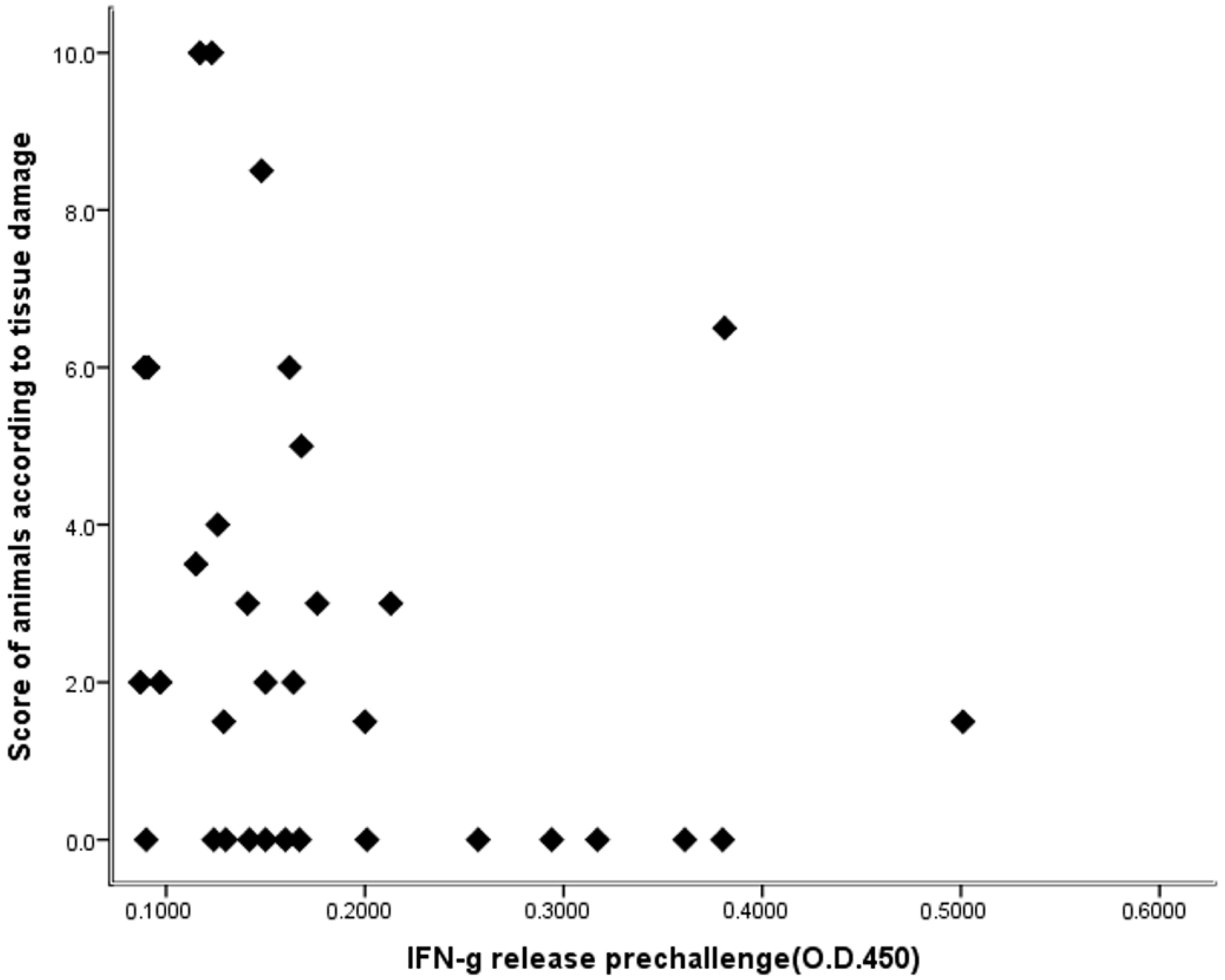
3.1. IFN-γ
3.2. Lesions in the Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Buddle, B.M. Vaccination of cattle against Mycobacterium bovis. Tuberculosis 2001, 81, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Palmer, M.V.; Buddle, B.M.; Vordermeier, H.M. Bovine tuberculosis vaccine research: Historical perspectives and recent advances. Vaccine 2012, 30, 2611–2622. [Google Scholar] [CrossRef]
- OIE. 2018. Available online: http://www.oie.int/en/animal-health-in-the-world/animal-diseases/bovine-tuberculosis/ (accessed on 8 February 2021).
- Chandran, A.; Williams, K.; Mendum, T.; Stewart, G.; Clark, S.; Zadi, S.; Lanni, F.; McLeod, N.; Williams, A.; Villarreal-Ramos, B.; et al. Development of a diagnostic compatible BCG vaccine against Bovine tuberculosis. Sci. Rep. 2019, 9, 17791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.F. Veterinary tuberculosis vaccine development. Clin. Infect. Dis. 2000, 3, S223–S228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddle, B.M.; Pollock, J.M.; Skinner, M.A.; Wedlock, D.N. Development of vaccines to control bovine tuberculosis in cattle and relationship to vaccine development for other intracellular pathogens. Int. J. Parasitol. 2003, 33, 555–566. [Google Scholar] [CrossRef]
- Vordermeier, H.M.; Jones, G.J.; Buddle, B.M.; Hewinson, R.G.; Villarreal-Ramos, B. Bovine Tuberculosis in Cattle: Vaccines, DIVA Tests, and Host Biomarker Discovery. Annu. Rev. Anim. Biosci. 2016, 4, 87–109. [Google Scholar] [CrossRef]
- Alarcon, G.J.C.; Venegas, Y.R.; Narvaez, L.B.; Martínez, O.E.P.; Casanova, L.G.; Gallegos, S.S.; Vargas, A.N.; Ramírez, A.M.O.; Suazo, F.M. Efficacy of a vaccine formula against tuberculosis in cattle. PLoS ONE 2013, 8, e76418. [Google Scholar]
- Buddle, B.M. Tuberculosis vaccines for cattle: The way forward. Expert Rev. Vaccines 2010, 9, 1121–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddle, B. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 1995, 13, 1123–1130. [Google Scholar] [CrossRef]
- Buddle, B.M.; Parlane, N.A.; Keen, D.L.; Aldwell, F.E.; Pollock, J.M.; Lightbody, K.; Andersen, P. Differentiation between Mycobacterium bovis BCG-Vaccinated and M. bovis-Infected Cattle by Using Recombinant Mycobacterial Antigens. Clin. Diagn. Lab. Immunol. 1999, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ameni, G.; Tibbo, M. Kinetics of Interferon-γ (IFN-γ) Release in the Peripheral Blood of Calves Vaccinated with BCG. J. Immunoass. Immunochem. 2002, 23, 245–253. [Google Scholar] [CrossRef]
- Hope, J.C.; Thom, M.L.; Villarreal-Ramos, B.; Vordermeier, H.M.; Hewinson, R.G.; Howard, C.J. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 2005, 139, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Yu, D.H.; Hu, X.D.; Li, S.X.; Zhu, Y.X. A Combined DNA Vaccine-Prime, BCG-Boost Strategy Results in Better Protection Against Mycobacterium bovis Challenge. DNA Cell Biol. 2006, 25, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Wedlock, D.N.; Denis, M.; Vordermeier, H.M.; Hewinson, R.G.; Buddle, B.M. Vaccination of cattle with Danish and Pasteur strains of Mycobacterium bovis BCG induce different levels of IFNγ post-vaccination, but induce similar levels of protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 2007, 118, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Milián-Suazo, F.; Gutiérrez-Pabello, J.A.; Bojorquez-Narváez, L.; Anaya-Escalera, A.M.; Cantó-Alarcón, G.J.; González-Enríquez, J.L.; Campos-Guillén, J. IFN-g response to vaccination against tuberculosis in dairy heifers under commercial settings. Res. Vet. Sci. 2011, 90, 419–424. [Google Scholar] [CrossRef]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R. Vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis bacillus Calmette Guerín. Vaccine 2007, 25, 6589–6597. [Google Scholar] [CrossRef] [Green Version]
- Chambers, M.A.; Carter, S.P.; Wilson, G.J.; Jones, G.; Brown, E.; Hewinson, R.G.; Vordermeier, M. Vaccination against tuberculosis in badgers and cattle: An overview of the challenges, developments and current research priorities in Great Britain. Vet. Rec. 2014, 175, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corner, L.A.L.; Buddle, B.M.; Pfeiffer, D.U.; Morris, R.S. Aerosol vaccination of the brushtail possum (Trichosurus vulpecula) with bacille Calmette-GueÂrin: The duration of protection. Vet. Microbiol. 2001, 81, 181–191. [Google Scholar] [CrossRef]
- Aldwell, F.E.; Tucker, I.G.; de Lisle, G.W.; Buddle, B.M. Oral Delivery of Mycobacterium bovis BCG in a Lipid Formulation Induces Resistance to Pulmonary Tuberculosis in Mice. Infect. Immun. 2003, 71, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Bezos, J.; de Juan, L.; Romero, B.; Álvarez, J.; Mazzucchelli, F.; Mateos, A.; Domínguez, L.; Aranaz, A. Experimental infection with Mycobacterium caprae in goats and evaluation of immunological status in tuberculosis and paratuberculosis co-infected animals. Vet. Immunol. Immunopathol. 2010, 133, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Val, B.; Villarreal-Ramos, B.; Nofrarías, M.; López-Soria, S.; Romera, N.; Singh, M.; Abad, F.X.; Xing, Z.; Vordermeier, H.M.; Domingo, M. Goats Primed with Mycobacterium bovis BCG and Boosted with a Recombinant Adenovirus Expressing Ag85A Show Enhanced Protection against Tuberculosis. Clin. Vaccine Immunol. 2012, 19, 1339–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Val, B.P.; Vidal, E.; Nofrarías, M.; López-Soria, S.; Cardona, P.-J.; Domingo, M. Assessment of Goat Tuberculosis Model for Use in Vaccine Trials. Procedia Vaccinol. 2014, 8, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Pérez de Val, B.; Vidal, E.; López-Soria, S.; Marco, A.; Cervera, Z.; Martín, M.; Mercader, I.; Singh, M.; Raeber, A.; Domingo, M. Assessment of safety and interferon gamma responses of Mycobacterium bovis BCG vaccine in goat kids and milking goats. Vaccine 2016, 34, 881–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, E.; Arrieta-Villegas, C.; Grasa, M.; Mercader, I.; Domingo, M.; Pérez de Val, B. Field evaluation of the efficacy of Mycobacterium bovis BCG vaccine against tuberculosis in goats. BMC Vet. Res. 2017, 13, 252. [Google Scholar] [CrossRef] [Green Version]
- Arrieta-Villegas, C.; Perálvarez, T.; Vidal, E.; Puighibet, Z.; Moll, X.; Canturri, A.; Sevilla, I.A.; Espada, Y.; Juste, R.A.; Domingo, M.; et al. Efficacy of parenteral vaccination against tuberculosis with heat-inactivated Mycobacterium bovis in experimentally challenged goats. PLoS ONE 2018, 13, e0196948. [Google Scholar] [CrossRef]
- Gonzalez-Juarrero, M.; Bosco-Lauth, A.; Podell, B.; Soffler, C.; Brooks, E.; Izzo, A.; Sanchez-Campillo, J.; Bowen, R. Experimental aerosol Mycobacterium bovis model of infection in goats. Tuberculosis 2013, 93, 558–564. [Google Scholar] [CrossRef]
- Gong, W.; Liang, Y.; Wu, X. Animal Models of Tuberculosis Vaccine Research: An Important Component in the Fight against Tuberculosis. BioMed Res. Int. 2020, 4263079. [Google Scholar] [CrossRef]
- Sanchez, J.; Tomás, L.; Ortega, N.; Buendía, A.J.; del Rio, L.; Salinas, J.; Bezos, J.; Caro, M.R.; Navarro, J.A. Microscopical and Immunological Features of Tuberculoid Granulomata and Cavitary Pulmonary Tuberculosis in Naturally Infected Goats. J. Comp. Pathol. 2011, 145, 107–117. [Google Scholar] [CrossRef]
- Nugent, G.; Yockney, I.J.; Cross, M.L.; Buddle, B.M. Low-dose BCG vaccination protects free-ranging cattle against naturally acquired bovine tuberculosis. Vaccine 2018, 36, 7338–7344. [Google Scholar] [CrossRef]
- Buddle, B.M.; Parlane, N.A.; Wedlock, D.N.; Heiser, A. Overview of Vaccination Trials for Control of Tuberculosis in Cattle, Wildlife and Humans. Transbound. Emerg. Dis. 2013, 60, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddle, B.M. Development of tuberculosis vaccines for cattle. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2010, 2. [Google Scholar] [CrossRef]
- Speth, M.T.; Repnik, U.; Griffiths, G. Layer-by-layer nanocoating of live Bacille-Calmette-Guérin mycobacteria with poly(I:C) and chitosan enhances pro-inflammatory activation and bactericidal capacity in murine macrophages. Biomaterials 2016, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-Based Biocompatible Nanoparticles for Next-Generation Tolerogenic Vaccines against Autoimmune Disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef] [Green Version]
- Bakhru, P.; Sirisaengtaksin, N.; Soudani, E.; Mukherjee, S.; Khan, A.; Jagannath, C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell. Immunol. 2014, 287, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.-A.; Actor, J.K. Lactoferrin modulation of BCG-infected dendritic cell functions. Int. Immunol. 2009, 21, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Costa-Gouveia, J.; Aínsa, J.A.; Brodin, P.; Lucía, A. How can nanoparticles contribute to antituberculosis therapy? Drug Discov. Today 2017, 22, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Dwivedi, P.; Jain, A.; Tyagi, S.; Sahu, T.; Tyagi, R.K. Development of novel carrier(s) mediated tuberculosis vaccine: More than a tour de force. Eur. J. Pharm. Sci. 2014, 62, 227–242. [Google Scholar] [CrossRef]
- Mohan, T.; Verma, P.; Rao, D.N. Novel adjuvants & delivery vehicles for vaccines development: A road ahead. Indian J. Med. Res. 2013, 138, 779–795. [Google Scholar]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Soane, R.J.; Frier, M.; Perkins, A.C.; Jones, N.S.; Davis, S.S.; Illum, L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999, 178, 55–65. [Google Scholar] [CrossRef]
- Zhu, B.D.; Qie, Y.Q.; Wang, J.L.; Zhang, Y.; Wang, Q.Z.; Xu, Y.; Wang, H.H. Chitosan microspheres enhance the immunogenicity of an Ag85B-based fusion protein containing multiple T-cell epitopes of Mycobacterium tuberculosis. Eur. J. Pharm. Biopharm. 2007, 66, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jiang, Q.; Xia, M.; Lu, Y.; Qiu, W.; Zhao, D.; Lu, L.; Peng, G.; Wang, Y. Enhanced Immune Response and Protective Effects of Nano-chitosan-based DNA Vaccine Encoding T Cell Epitopes of Esat-6 and FL against Mycobacterium Tuberculosis Infection. PLoS ONE 2013, 8, e61135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khademi, F.; Taheri, R.-A.; Yousefi Avarvand, A.; Vaez, H.; Momtazi-Borojeni, A.A.; Soleimanpour, S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb. Pathog. 2018, 121, 218–223. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef] [Green Version]
- Gutjahr, A.; Phelip, C.; Coolen, A.-L.; Monge, C.; Boisgard, A.-S.; Paul, S.; Verrier, B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Morton, L.D.; Ulery, B.D. Nanoparticles as synthetic vaccines. Curr. Opin. Biotechnol. 2015, 34, 217–224. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Parumasivam, T.; Chan, J.G.Y.; Lin, L.C.W.; Flórido, M.; West, N.P.; Chan, H.-K.; Britton, W.J. PLGA particulate subunit tuberculosis vaccines promote humoral and Th17 responses but do not enhance control of Mycobacterium tuberculosis infection. PLoS ONE 2018, 13, e0194620. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.J.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Coombes, A.G.A.; Perrie, Y. PLGA microspheres for the delivery of a novel subunit TB vaccine. J. Drug Target. 2008, 16, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Samandi, L.Z.; Wang, Z.; Chen, Z.J.; Gao, J. Synthetic nanovaccines for immunotherapy. J. Control. Release 2017, 263, 200–210. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Z.; Liu, T.; Qian, R.; Wu, T.; Liu, Q.; Shen, A. Polymeric Nanoparticles Engineered as a Vaccine Adjuvant-Delivery System. In Immunization—Vaccine Adjuvant Delivery System and Strategies; Wang, E.N., Wang, T., Eds.; IntechOpen: Hefei, China, 2018. [Google Scholar] [CrossRef] [Green Version]
- Skinner, M.A.; Wedlock, D.N.; Buddle, B.M. Vaccination of animals against Mycobacterium bovis. Rev. Sci. Tech. Off. Int. Epiz. 2001, 20, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Buddle, B.M.; Wards, B.J.; Aldwell, F.E.; Collins, D.M.; de Lisle, G.W. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 2002, 20, 1126–1133. [Google Scholar] [CrossRef]
- Buddle, B.M.; Wedlock, D.N.; Parlane, N.A.; Corner, L.A.L.; de Lisle, G.W.; Skinner, M.A. Revaccination of Neonatal Calves with Mycobacterium bovis BCG Reduces the Level of Protection against Bovine Tuberculosis Induced by a Single Vaccination. Infect. Immun. 2003, 71, 6411–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezos, J.; Casal, C.; Álvarez, J.; Roy, A.; Romero, B.; Rodríguez-Bertos, A.; Bárcena, C.; Díez, A.; Juste, R.; Gortázar, C.; et al. Evaluation of the Mycobacterium tuberculosis SO2 vaccine using a natural tuberculosis infection model in goats. Vet. J. 2017, 223, 60–67. [Google Scholar] [CrossRef]
- Roy, Á.; Risalde, M.A.; Bezos, J.; Casal, C.; Romero, B.; Sevilla, I.; Díez-Guerrier, A.; Rodríguez-Bertos, A.; Domínguez, M.; Garrido, J.; et al. Response of goats to intramuscular vaccination with heat-killed Mycobacterium bovis and natural challenge. Comp. Immunol. Microbiol. Infect. Dis. 2018, 60, 28–34. [Google Scholar] [CrossRef]
- Castillo-Rodal, A.I.; Castañón-Arreola, M.; Hernández-Pando, R.; Calva, J.J.; Sada-Díaz, E.; López-Vidal, Y. Mycobacterium bovis BCG Substrains Confer Different Levels of Protection against Mycobacterium tuberculosis Infection in a BALB/c Model of Progressive Pulmonary Tuberculosis. Infect. Immun. 2006, 74, 1718–1724. [Google Scholar] [CrossRef] [Green Version]
- Khabazzadeh Tehrani, N.; Mahdavi, M.; Maleki, F.; Zarrati, S.; Tabatabaie, F. The role of Montanide ISA 70 as an adjuvant in immune responses against Leishmania major induced by thiol-specific antioxidant-based protein vaccine. J. Parasit. Dis. 2016, 40, 760–767. [Google Scholar] [CrossRef] [Green Version]
- Hope, J.C.; Thom, M.L.; McAulay, M.; Mead, E.; Vordermeier, H.M.; Clifford, D.; Hewinson, R.G.; Villarreal-Ramos, B. Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin. Vaccine Immunol. 2011, 18, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Corner, L.A.L.; Buddle, B.M.; Pfeiffer, D.U.; Morris, R.S. Vaccination of the brushtail possum (Trichosurus vulpecula) against Mycobacterium bovis infection with bacille Calmette-Guérin: The response to multiple doses. Vet. Microbiol. 2002, 84, 327–336. [Google Scholar] [CrossRef]
- Francis, J. Control of infection with the bovine tubercle bacillus. Lancet 1950, 1, 34–39. [Google Scholar] [CrossRef]
- Vordermeier, H.M.; Chambers, M.A.; Buddle, B.M.; Pollock, J.M.; Hewinson, R.G. Progress in the development of vaccines and diagnostic reagents to control tuberculosis in cattle. Vet. J. 2006, 171, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.A.; Buddle, B.M.; Wedlock, D.N.; Keen, D.; de Lisle, G.W.; Tascon, R.E.; Candido Ferraz, J.; Lowrie, D.B.; Cockle, P.J.; Vordermeier, H.M.; et al. A DNA Prime-Mycobacterium bovis BCG Boost Vaccination Strategy for Cattle Induces Protection against Bovine Tuberculosis. Infect. Immun. 2003, 71, 4901–4907. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, J.C.; Stavropoulos, E.; Yang, M.; Coade, S.; Espitia, C.; Lowrie, D.B.; Colston, M.J.; Tascon, R.E. A Heterologous DNA Priming-Mycobacterium bovis BCG Boosting Immunization Strategy Using Mycobacterial Hsp70, Hsp65, and Apa Antigens Improves Protection against Tuberculosis in Mice. Infect. Immun. 2004, 72, 6945–6950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McShane, H.; Hill, A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005, 7, 962–967. [Google Scholar] [CrossRef] [PubMed]
- McShane, H.; Pathan, A.A.; Sander, C.R.; Goonetilleke, N.P.; Fletcher, H.A.; Hill, A.V.S. Boosting BCG with MVA85A: The first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis 2005, 85, 47–52. [Google Scholar] [CrossRef]
- Vordermeier, H.M.; Rhodes, S.G.; Dean, G.; Goonetilleke, N.; Huygen, K.; Hill, A.V.S.; Hewinson, R.G.; Gilbert, S.C. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 2004, 112, 461–470. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Denis, M.; Skinner, M.A.; Koach, J.; de Lisle, G.W.; Vordermeier, H.M.; Hewinson, R.G.; van Drunen Littel-van den Hurk, S.; Babiuk, L.A.; Hecker, R.; et al. Vaccination of Cattle with a CpG Oligodeoxynucleotide-Formulated Mycobacterial Protein Vaccine and Mycobacterium bovis BCG Induces Levels of Protection against Bovine Tuberculosis Superior to Those Induced by Vaccination with BCG Alone. Infect. Immun. 2005, 73, 3540–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddle, B.M.; Aldwell, F.E.; Skinner, M.A.; Lisle GW de Denis, M.; Vordermeier, H.M.; Hewinson, R.G.; Wedlock, D.N. Effect of oral vaccination of cattle with lipid-formulated BCG on immune responses and protection against bovine tuberculosis. Vaccine 2005, 23, 3581–3589. [Google Scholar] [CrossRef]
- Logan, K.E.; Chambers, M.A.; Hewinson, R.G.; Hogarth, P.J. Frequency of IFN-γ producing cells correlates with adjuvant enhancement of bacille Calmette-Guèrin induced protection against Mycobacterium bovis. Vaccine 2005, 23, 5526–5532. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Skinner, M.A.; Parlane, N.A.; Vordermeier, H.M.; Hewinson, R.G.; de Lisle, G.W.; Buddle, B.M. Vaccination with DNA vaccines encoding MPB70 or MPB83 or a MPB70 DNA prime-protein boost does not protect cattle against bovine tuberculosis. Tuberculosis 2003, 83, 339–349. [Google Scholar] [CrossRef]
- Elias, D.; Akuffo, H.; Britton, S. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, A.; Irwin, S.; Izzo, A.A.; Basaraba, R.J.; Orme, I.M. Memory T Lymphocytes Generated by Mycobacterium bovis BCG Vaccination Reside within a CD4 CD44lo CD62 Ligandhi Population. Infect. Immun. 2005, 73, 7759–7764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langermans, J.A.M.; Andersen, P.; van Soolingen, D.; Vervenne, R.A.W.; Frost, P.A.; van der Laan, T.; van Pinxteren, L.A.H.; van den Hombergh, J.; Kroon, S.; Peekel, I.; et al. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: Implications for primate models in tuberculosis vaccine research. Proc. Natl. Acad. Sci. USA 2001, 98, 11497–11502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
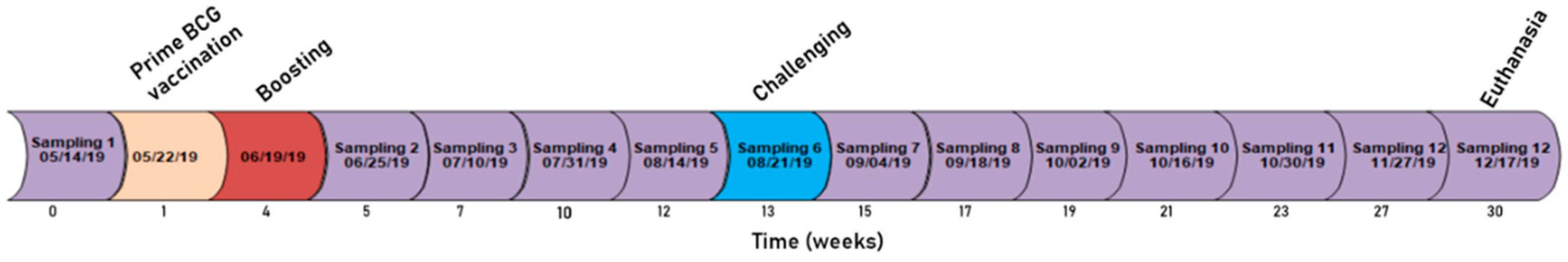


| Group Number | Priming Formulation | Boosting Formulation | Boosting Adjuvant |
|---|---|---|---|
| 1 | None | None | None |
| 2 | BCG | None | None |
| 3 | BCG | CFP | MontanideTM |
| 4 | Chitosan-coated BCG | CFP + Chitosan + PLGA | MontanideTM |
| 5 | Chitosan- coated BCG | CFP + Chitosan | MontanideTM |
| Score | Score Definition |
|---|---|
| 0 | No visible lesions |
| 1–1.9 | Few lesions (≤20) in lung or lymph nodes |
| 2–2.9 | Few lesions (≤20) in lung and lymph nodes |
| 3–3.9 | Between 21 and 50 lesions in lung or lymph nodes |
| 4–4.9 | Between 21 and 50 lesions in lung and lymph nodes |
| 5–5.9 | Multiple lesions (between 51 and 100) localized in lung or lymph nodes |
| 6–6.9 | Multiple lesions (between 51 and 100) localized in lung and lymph nodes |
| 7–7.9 | Multiple lesions (≥101) disseminated in lung or lymph nodes |
| ≥8 | Multiple lesions (≥101) disseminated in lung and lymph nodes. |
| Sampling Week | Experimental Group | Mean * IFN-γ Release (OD 450 nm) | Standard Deviation | 95% CI | p-Value |
|---|---|---|---|---|---|
| 1 | 1 | 0.1866 a | 0.2419 | −0.0672; 0.4405 | 0.713 |
| Prime BCG vaccination | 2 | 0.0914 a | 0.0164 | 0.0762; 0.1066 | |
| 3 | 0.0970 a | 0.0366 | 0.0361; 0.1308 | ||
| 4 | 0.1661 a | 0.2277 | −0.0440; 0.3767 | ||
| 5 | 0.1101 a | 0.0751 | 0.0313; 0.1890 | ||
| 3 | 1 | 0.01453 a | 0.0350 | 0.1085; 0.1821 | 0.25 |
| Groups 3–5 boosting | 2 | 0.2711 a | 0.0350 | −0.0340; 0.5763 | |
| 3 | 0.3282 a | 0.1801 | 0.1616; 0.4949 | ||
| 4 | 0.2578 a | 0.1251 | 0.1421; 0.3736 | ||
| 5 | 0.1245 a | 0.0331 | 0.0897; 0.1592 | ||
| 6 | 1 | 0.1235 a | 0.0367 | 0.0849; 0.1620 | 0.357 |
| All groups challenging | 2 | 0.1377 a | 0.0772 | 0.0662; 0.2091 | |
| 3 | 0.2185 a | 0.1406 | 0.0884; 0.3486 | ||
| 4 | 0.2197 a | 0.2122 | 0.0233; 0.460 | ||
| 5 | 0.1158 a | 0.0275 | 0.0869; 0.1447 | ||
| 8 | 1 | 0.2021b | 0.0180 | 0.1831; 0.2211 | 0.0001 |
| 2 | 0.0947 a | 0.0106 | 0.0849; 0.1044 | ||
| 3 | 0.1114 a | 0.0352 | 0.0788; 0.1440 | ||
| 4 | 0.0925 a | 0.0366 | 0.0586; 0.1264 | ||
| 5 | 0.1495 a,b | 0.0663 | 0.0798; 0.2191 | ||
| 11 | 1 | 0.1755 a | 0.0436 | 0.1296; 0.2213 | 0.05 |
| 2 | 0.3252 a,b | 0.0759 | 0.2550; 0.3954 | ||
| 3 | 0.5140 b | 0.1366 | 0.3877; 0.6402 | ||
| 4 | 0.3640 a,b | 0.2260 | 0.1750; 0.5529 | ||
| 5 | 0.4252 a,b | 0.3734 | −0.0385; 0.8889 | ||
| 13 | 1 | 0.0988 a | 0.0204 | 0.0773; 0.1203 | 0.049 |
| 2 | 0.2364 a | 0.1450 | 0.1022; 0.3705 | ||
| 3 | 0.2405 a | 0.0820 | 0.1647; 0.3164 | ||
| 4 | 0.1848 a | 0.1010 | 0.0914; 0.2783 | ||
| 5 | 0.1475 a | 0.0401 | 0.1053; 0.1896 | ||
| 16 | 1 | 0.2556 a | 0.1852 | 0.0612; 0.4501 | 0.002 |
| 2 | 0.4712 a,b | 0.2387 | 0.2504; 0.6921 | ||
| 3 | 0.3667 a,b | 0.2463 | 0.1389; 0.5945 | ||
| 4 | 0.7475 b | 0.4321 | 0.3478; 1.1472 | ||
| 5 | 0.1013 a | 0.04951 | 0.0493; 0.1532 | ||
| 18 | 1 | 0.0933 a | 0.0205 | 0.0717; 0.1149 | 0.002 |
| 2 | 1.1730 b | 0.8178 | 0.4166; 1.9293 | ||
| 3 | 0.6585 a,b | 0.4943 | 0.2014; 1.1157 | ||
| 4 | 0.4650 a,b | 0.3746 | 0.1184; 0.8115 | ||
| 5 | 0.1053 a | 0.0307 | 0.0730; 0.1375 | ||
| 20 | 1 | 0.7015 a,b | 0.2699 | 0.4181; 0.9848 | 0.050 |
| 2 | 1.022 a,b | 0.6780 | 0.3949; 1.6490 | ||
| 3 | 0.7787 a,b | 0.5017 | 0.3146; 1.2427 | ||
| 4 | 1.4491 b,c | 0.8747 | 0.6401; 2.2581 | ||
| 5 | 0.4566 a,b | 0.3581 | 0.0807; 0.8325 | ||
| 22 | 1 | 0.2473 a | 0.1335 | 0.1072; 0.3874 | 0.001 |
| 2 | 0.7285 a,b | 0.5109 | 0.2560; 1.2011 | ||
| 3 | 0.4198 a,b | 0.3974 | 0.0522; 0.7874 | ||
| 4 | 1.2011 b | 0.7193 | 0.5357; 1.8665 | ||
| 5 | 0.1071 a | 0.0343 | 0.0711; 0.1432 | ||
| 24 | 1 | 0.4270 a | 0.2106 | 0.2059; 0.6480 | 0.137 |
| 2 | 0.3648 a | 0.2789 | 0.1068; 0.6228 | ||
| 3 | 0.1421 a | 0.0583 | 0.0881; 0.1960 | ||
| 4 | 0.4712 a | 0.4152 | 0.0872; 0.8553 | ||
| 5 | 0.2450 a | 0.1186 | 0.1204; 0.3695 | ||
| 28 | 1 | 0.3918 a | 0.3512 | 0.0232; 0.7604 | 0.158 |
| 2 | 0.1785 a | 0.0802 | 0.1043; 0.2528 | ||
| 3 | 0.1441 a | 0.0780 | 0.0719; 0.2163 | ||
| 4 | 0.2178 a | 0.1717 | 0.0590; 0.3676 | ||
| 5 | 0.2430 a | 0.0725 | 0.1668; 0.3191 | ||
| 30 | 1 | 0.0688 a | 0.0138 | 0.0542; 0.0833 | 0.180 |
| Euthanasia | 2 | 0.1361 a | 0.0556 | 0.0847; 0.1875 | |
| 3 | 0.1174 a | 0.0529 | 0.0684; 0.1663 | ||
| 4 | 0.1205 a | 0.0498 | 0.0744; 0.1667 | ||
| 5 | 0.0665 a | 0.0079 | 0.0581; 0.0748 |
| Experimental Group | Animals with Lesion */Animals Challenged (%) | Average Lesion Score ** | 95% CI |
|---|---|---|---|
| 1 | 6/6 (100) | 6.33 b ± 3.2 | 2.97; 9.69 |
| 2 | 6/7 (86) | 3.00 ab ± 2.2 | 0.98; 5.01 |
| 3 | 2/7 (28) | 1.07 a ± 2.2 | −1.00; 3.14 |
| 4 | 2/7 (28) | 1.43 a ± 3.2 | −1.51; 4.35 |
| 5 | 4/6 (67) | 1.75 a ± 1.5 | 0.20; 3.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Magallanes, Y.G.; Durán-Aguilar, M.; Sosa-Gallegos, S.L.; Álvarez, Á.H.; Andrade-Santillán, F.A.; Bárcenas-Reyes, I.; González-Ruíz, S.; Rodríguez-Hernández, E.; Cantó-Alarcón, G.J.; Milián-Suazo, F. Prime Vaccination with Chitosan-Coated Phipps BCG and Boosting with CFP-PLGA against Tuberculosis in a Goat Model. Animals 2021, 11, 1046. https://doi.org/10.3390/ani11041046
Contreras-Magallanes YG, Durán-Aguilar M, Sosa-Gallegos SL, Álvarez ÁH, Andrade-Santillán FA, Bárcenas-Reyes I, González-Ruíz S, Rodríguez-Hernández E, Cantó-Alarcón GJ, Milián-Suazo F. Prime Vaccination with Chitosan-Coated Phipps BCG and Boosting with CFP-PLGA against Tuberculosis in a Goat Model. Animals. 2021; 11(4):1046. https://doi.org/10.3390/ani11041046
Chicago/Turabian StyleContreras-Magallanes, Yesenia Guadalupe, Marina Durán-Aguilar, Susana L. Sosa-Gallegos, Ángel H. Álvarez, Fátima A. Andrade-Santillán, Isabel Bárcenas-Reyes, Sara González-Ruíz, Elba Rodríguez-Hernández, Germinal J. Cantó-Alarcón, and Feliciano Milián-Suazo. 2021. "Prime Vaccination with Chitosan-Coated Phipps BCG and Boosting with CFP-PLGA against Tuberculosis in a Goat Model" Animals 11, no. 4: 1046. https://doi.org/10.3390/ani11041046
APA StyleContreras-Magallanes, Y. G., Durán-Aguilar, M., Sosa-Gallegos, S. L., Álvarez, Á. H., Andrade-Santillán, F. A., Bárcenas-Reyes, I., González-Ruíz, S., Rodríguez-Hernández, E., Cantó-Alarcón, G. J., & Milián-Suazo, F. (2021). Prime Vaccination with Chitosan-Coated Phipps BCG and Boosting with CFP-PLGA against Tuberculosis in a Goat Model. Animals, 11(4), 1046. https://doi.org/10.3390/ani11041046





