The Efficacy of a Long-Acting Injectable Selenium Preparation Administered to Pregnant Ewes and Lambs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials & Methods
3. Results
4. Discussion
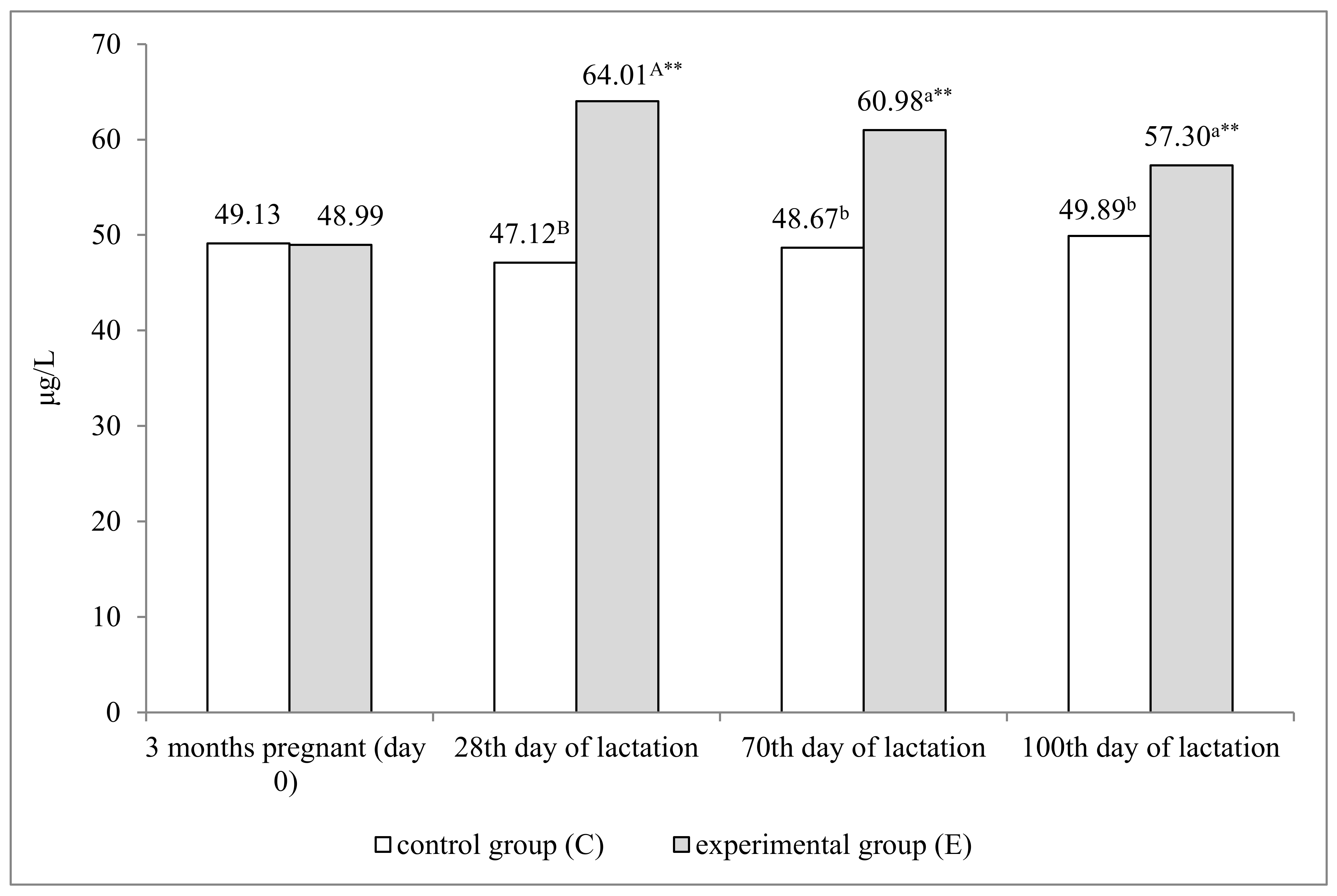
4.1. Selenium Concentration in the Serum and Milk
4.2. Milk Yield and Composition
4.3. Blood Parameters
4.4. Lamb’s Growth Rate and Indicators of Musculature and Fatness
5. Conclusions
- Barium selenate significantly improved the Se status of lambs, regardless of whether it was administered to pregnant ewes (E group) or directly to lambs in the first week of their life (EI group).
- The milk of ewes receiving the Se supplement was characterized by significantly higher fat content and, consequently, higher dry matter concentration.
- The analyzed Se preparation induced significant changes in immunological parameters, thus enhancing defense mechanisms in lambs.
- The preparation in the form of barium selenate (VI) administered to lambs from the EI group had a more stimulating effect on their humoral and cellular immune response than in the lambs from the E group, where the preparation was administered to their mothers.
Author Contributions
Funding
Institutional Review Board Statement
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stec, A.; Mochol, J.; Kurek, Ł.; Wałkuska, G.; Chałabis-Mazurek, A. The influence of different factors on selenium levels in dairy cow herds in the central-eastern region of Poland. Pol. J. Vet. Sci. 2005, 8, 225–229. [Google Scholar] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in cattle: A review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čobanová-Boldižárová, K.; Grešáková, L.; Faix, Š.; Petrovič, V.; Leng, L. Selenium in Sheep Nutrition. In Current Advances in Selenium Research and Applications; Surai, P., Taylor-Pickard, J.A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2008; pp. 209–220. [Google Scholar]
- Koenig, K.M.; Rode, L.M.; Cohen, R.D.; Buckley, W.T. Effects of diet and chemical form of selenium on selenium metabolism in sheep. J. Anim. Sci. 1997, 75, 817–827. [Google Scholar] [CrossRef]
- Lenartova, V.; Holovska, K.; Javorsky, P. The influence on the antioxidant enzyme activity of rumen bacteria Streptococcus bovis and Selenomonas ruminantium. FEMS Microbiol. Ecol. 1998, 27, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Deore, M.; Dumka, V.; Sharma, S.; Srivastava, A. Selenium toxicokinetics after oral and intravenous administration in buffalo calves. Environ. Toxicol. Pharmacol. 2007, 24, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Mynhardt, H.; Van Ryssen, J.B.J.; Coertze, R.J. The effect of heat processing of soybean seed on the metabolism of its selenium in lambs. Animal Feed Sci. Technol. 2006, 128, 122–134. [Google Scholar] [CrossRef]
- Annett, R.W.; Carson, A.F.; Fearon, A.M.; Kilpatrick, D.J. Effects of supplementation with fish oil and barium selenate on performance, carcass characteristics and muscle fatty acid composition of late season lamb finished on grass-based or concentrate-based diets. Animals 2011, 5, 1923–1937. [Google Scholar] [CrossRef]
- Ceballos, A.; Sánchez, J.; Stryhn, H.; Montgomery, J.B.; Barkema, H.W.; Wichtel, J.J. Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J. Dairy Sci. 2009, 92, 324–342. [Google Scholar] [CrossRef] [Green Version]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1995; p. 518. [Google Scholar]
- Lie, R.F.; Schurtz, J.M.; Kenneth, J.P.; Gochonan, N. Cholesterol oxidase-based determination by continuous-flow analysis of total and free cholesterol in serum. Clin. Chem. 1976, 22, 1627–1630. [Google Scholar] [CrossRef]
- McGowan, M.W.; Artiss, J.D.; Stranbergh, D.R.; Zak, B. A peroxidase-coupled method for colorimetric determination of serum triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [CrossRef]
- Huang, X.; Chai, J.; Im, H.; Yarimaga, O.; Yoon, E.; Kim, H. Aspartate aminotransferase (AST, GOT) and alanine aminotransferase (ALT, GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef] [Green Version]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 1994; pp. 816–818. [Google Scholar]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1995; p. 286. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; WB Saunders: St. Louis, MO, USA, 2006; p. 2290. [Google Scholar]
- Siwicki, A.K.; Anderson, D.P. Immunostimulation in Fish: Measuring the Effects of Stimulants by Serological and Immunological Methods; U.S. Fish Wild, IFI: Olsztyn, Poland, 1993; pp. 1–17.
- Siwicki, A.K.; Studnicka, M. Ceruloplasmin activity in carp (Cyprinus carpio). Bamidgeh 1986, 38, 126–129. [Google Scholar]
- Ząbek, K.; Milewski, S.; Wójcik, R.; Siwicki, A.K. The effects of supplementing diets fed to pregnant and lactating ewes with saccharomyces cerevisiae dried yeast. Turk. J. Vet Anim. Sci. 2014, 38, 200–206. [Google Scholar] [CrossRef]
- Junkuszew, A.; Ringdorfer, F. Computer tomography and ultrasound measurement as methods for the prediction of the body composition of lambs. Small Rumin. Res. 2005, 56, 121–125. [Google Scholar] [CrossRef]
- Muñoz, C.; Carson, A.F.; McCoy, M.A.; Dawson, L.E.R.; Irwin, D.; Gordon, A.W.; Kilpatrick, D.J. Effect of supplementation with barium selenate on the fertility, prolificacy and lambing performance of hill sheep. Vet. Rec. 2009, 164, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Čobanová, K.; Faix, Š.; Plachá, I.; Mihaliková, K.; Váradyová, Z.; Kišidayová, S.; Grešáková, Ľ. Effects of different dietary selenium sources on antioxidant status and blood phagocytic activity in sheep. Biol. Trace. Elem. Res. 2017, 175, 339–346. [Google Scholar] [CrossRef]
- Novoselec, J.; Šperanda, M.; Klir, Ž.; Mioč, B.; Steiner, Z.; Antunović, Z. Blood biochemical indicators and concentration of thyroid hormones in heavily pregnant and lactating ewes depending on selenium supplementation. Acta Vet. Brno 2017, 86, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, B.E.; Hernandez, C.E.; Hernandez, L.M.; Tortora-Perez, J.L. Effect of parenteral supplement with sodium selenite on lamb mortality and hematic values of selenium. Agrociencia 2004, 38, 43–51. [Google Scholar]
- Barbé, F.; Chevaux, E.; Castex, M.; Elcoso, G.; Bach, A. Comparison of selenium bioavailability in milk and serum in dairy cows fed different sources of organic selenium. Anim. Prod. Sci. 2020, 60, 269–276. [Google Scholar] [CrossRef]
- Pehrson, B.; Ortman, K.; Madjid, N.; Trafikowska, U. The influence of dietary selenium as selenium yeast or sodium selenite on the concentration of selenium in the milk of suckler cows and on the selenium status of their calves. J. Anim. Sci. 1999, 77, 3371–3376. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, A.; Kruze, J.; Barkema, H.W.; Dohoo, I.R.; Sanchez, J.; Uribe, D.; Wichtel, J.J.; Wittwer, F. Barium selenate supplementation and its effect on intramammary infection in pasture-based dairy cows. J. Dairy Sci. 2010, 93, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Horkỳ, P. Effect of selenium on its content in milk and performance of dairy cows in ecological farming. Potravinarstvo 2015, 9, 324–329. [Google Scholar] [CrossRef]
- Ran, L.; Wu, X.; Shen, X.; Zhang, K.; Ren, F.; Huang, K. Effects of selenium form on blood and milk selenium concentrations, milk component and milk fatty acid composition in dairy cows. J. Sci. Food Agric. 2010, 90, 2214–2219. [Google Scholar] [CrossRef]
- Salman, S.; Dinse, D.; Khol-Parisini, A.; Schafft, H.; Lahrssen-Wiederholt, M.; Schreiner, M.; Scharek-Tedin, L.; Zentek, J. Colostrum and milk selenium, antioxidative capacity and immune status of dairy cows fed sodium selenite or selenium yeast. Arch. Anim. Nutr. 2013, 67, 48–61. [Google Scholar] [CrossRef]
- Grace, N.D. Use of biochemical criteria to diagnose trace element deficiencies in sheep and cattle. In Proceedings of the 9th International Conference on Animal Production, Berlin, Germany, 11–14 September 1997. [Google Scholar]
- Ghany-Hefnawy, A.E.; López-Arellano, A.E.; Revilla-Vázquez, R.; Ramírez-Bribiesca, A.; Tórtora-Pérez, J.L. Interrelationship between fetal and maternal selenium concentrations in small ruminants. Small Rumin. Res. 2007, 73, 174–180. [Google Scholar] [CrossRef]
- Ferreira, G.M.; Petzer, I.M. Injectable organic and inorganic selenium in dairy cows-Effects on milk, blood and somatic cell count levels. Onderstepoort J. Vet. Res. 2019, 86, 1–8. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Jones, A.K.; Bertin, G. Selenium supplementation of lactating dairy cows: Effect on selenium concentration in blood, milk, urine, and feces. J. Dairy Sci. 2006, 89, 3544–3551. [Google Scholar] [CrossRef]
- Heard, J.W.; Stockdale, C.R.; Walker, G.P.; Leddin, C.M.; Dunshea, F.R.; Mclnstosh, G.H.; Shields, P.M.; McKenna, A.; Young, G.P.; Doyle, P.T. Increasing selenium concentration in milk: Effects of amount of selenium from yeast and cereal grain supplements. J. Dairy Sci. 2007, 90, 4117–4127. [Google Scholar] [CrossRef]
- Paschoal, J.J.; Zanetti, M.A.; Del Claro, G.R.; Melo, M.P.D.; Pugine, S.P.; Cunha, J.A. Fatty acid profile and oxidative stability of milk from Holstein cows fed with extruded soybean and organic selenium. Pesqui. Agropecu. Bras. 2007, 42, 1793–1799. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, J.; Montes, P.; Jimenez, A.; Andres, S. Prevention of clinical mastitis with barium selenate in dairy goats from a selenium-deficient area. J. Dairy Sci. 2007, 90, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Saba, F.E.; Saleh, A.A.K.; Al Moafy, A.A. Effect of supplementation with different types of selenium on lactation performance and some blood parameters of Farafra and Saidi ewes and performance of their lambs. Egypt. J. Sheep Goats Sci. 2019, 14, 19–30. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet. World 2018, 11, 562. [Google Scholar] [CrossRef]
- Moosavian, H.R.; Mohri, M.; Seifi, H.A. Effect of parenteral over-supplementation of vitamin A and iron hematology, iron biochemistry, weight gain, and health of neonatal dairy calves. Food Chem. Toxicol. 2010, 48, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, B.J. Effect of selenium supplementation on dairy cattle. J. Anim. Sci. 1992, 70, 3934–3940. [Google Scholar] [CrossRef]
- El-Shahat, K.H.; Abdel Monem, U.M. Effects of dietary supplementation with vitamin E and/or selenium on metabolic and reproductive performance of Egyptian Baladi ewes under subtropical conditions. World App. Sci. J. 2011, 12, 1492–1499. [Google Scholar]
- Abdel-Raheem, S.; Mahmoud, G.; Senosy, W.; El-Sherry, T. Influence of vitamin E and selenium supplementation on the performance, reproductive indices and metabolic status of ossimi ewes. Slov. Vet. Res. 2019, 56 (Suppl. 22), 353–363. [Google Scholar] [CrossRef] [Green Version]
- Sobiech, P. Obraz Hematologiczny, Biochemiczny i Histopatologiczny Pokarmowej Dystrofii Mięśni Koźląt (Hematological, Biochemical and Histopathological Picture of Nutritional Muscular Dystrophy [NMD] in Goat Kids); Monography/dissertation; UWM: Olsztyn, Poland, 2009. [Google Scholar]
- Sobiech, P.; Kuleta, Z. Usefulness of some biochemical indicators in detection of early stages of nutritional muscular dystrophy in lambs. Small Rumin. Res. 2002, 45, 209–215. [Google Scholar] [CrossRef]
- Sousa, C.P.; De Azevedo, J.T.; Silva, A.M.; Viegas, C.A.; Rei, R.L.; Gomes, M.E.; Dias, I.R. Serum total and bone alkaline phosphatase levels and their correlation with serum minerals over the lifespan of sheep. Acta Vet. Hung. 2014, 62, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rock, M.J.; Kincaid, R.I.; Carstens, G.E. Effects of prenatal source and level of dietary selenium on passive immunity and thermometabolism of newborn lambs. Small Rumin. Res. 2000, 40, 129–138. [Google Scholar] [CrossRef]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Horning, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nut. 2007, 98, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.R. Mechanisms by which selenium influences immune responses. Arch. Immunol. Ther. Exp. 2007, 55, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Ponnampalam, E.N.; Celi, P.; Hopkins, D.L.; Leury, B.J.; Dunshea, F.R. High dietary vitamin E and selenium improves feed intake and weight gain of finisher lambs and maintains redox homeostasis under hot conditions. Small Rumin. Res. 2016, 137, 17–23. [Google Scholar] [CrossRef]
- Soliman, E.B. Changes in productive performance, hemato-biochemical indices, immune and antioxidant status of growing Ossimi lambs subjected to vitamins A and E administration. Egypt. J. Sheep Goats Sci. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Huang, Y.B.; Sun, Y.W.; Zhou, J.Q.; GUO, L. Effects of organic selenium sources on lamb’s growth performance and its antioxidative activities. Anim. Husb. Feed Sci. 2009, 9, 13. [Google Scholar]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Mohri, M.; Ehsani, A.; Norouzian, M.A.; Bami, M.H.; Seifi, H.A. Parenteral selenium and vitamin e supplementation to lambs: Hematology, serum biochemistry, performance, and relationship with other trace elements. Biol. Trace Elem. Res. 2011, 139, 308–316. [Google Scholar] [CrossRef]
- Alimohamady, R.; Aliarabi, H.; Bahari, A.; Dezfoulian, A.H. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol. Trace Elem. Res. 2013, 154, 45–54. [Google Scholar] [CrossRef]
- Sushma, K.; Reddy, Y.R.; Kumari, N.N.; Reddy, P.B.; Raghunandan, T.; Sridhar, K. Effect of selenium supplementation on performance, cost economics, and biochemical profile of Nellore ram lambs. Vet. World 2015, 8, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Vignola, G.; Lambertini, L.; Mazzone, G.; Giammarco, M.; Tassinari, M.; Martelli, G.; Bertin, G. Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci. 2009, 81, 678–685. [Google Scholar] [CrossRef]
- Mudgal, V.; Garg, A.K.; Dass, R.S. Effect of dietary selenium and copper supplementation on growth and nutrient utilization in buffalo (Bubalus bubalis) calves. Anim. Nutr. Feed Technol. 2007, 7, 79–88. [Google Scholar]
- Aghwan, Z.A.; Sazili, A.Q.; Kadhim, K.K.; Alimon, A.R.; Goh, Y.M.; Adeyemi, K.D. Effects of dietary supplementation of selenium and iodine on growth performance, carcass characteristics and histology of thyroid gland in goats. Anim. Sci. J. 2016, 87, 690–696. [Google Scholar] [CrossRef]
- Kumar, N.; Garg, A.K.; Dass, R.S.; Chaturvedi, V.K.; Mudgal, V.; Varshney, V.P. Selenium supplementation influences growth performance, antioxidant status and immune response in lambs. Anim. Feed Sci. Technol. 2009, 153, 77–87. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Mohamed, M.Y. Effect of Different Dietary Selenium Sources Supplementation on Nutrient Digestibility, Productive Performance and Some Serum Biochemical Indices in Sheep. Egypt. J. Nutr. Feeds 2018, 21, 53–64. [Google Scholar] [CrossRef]
- Kumar, N.; Garg, A.K.; Mudgal, V.; Dass, R.S.; Chaturvedi, V.K.; Varshney, V.P. Effect of different levels of selenium supplementation on growth rate, nutrient utilization, blood metabolic profile, and immune response in lambs. Biol. Trace Elem. Res. 2008, 126, 44–56. [Google Scholar] [CrossRef]
- Mojapelo, M.M.; Lehloenya, K.C. Effect of selenium supplementation on attainment of puberty in Saanen male goat kids. Theriogenology 2019, 138, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gabryszuk, M.; Klewiec, J. Effect of injecting 2- and 3-year-old ewes with selenium and selenium-vitamin E on reproduction and rearing of lambs. Small Rumin. Res. 2002, 43, 127–132. [Google Scholar] [CrossRef]
- Zarbalizadeh-Saed, A.; Seifdavati, J.; Abdi-Benemar, H.; Salem, A.Z.; Barbabosa-Pliego, A.; Camacho-Diaz, L.M.; Fadayifar, A.; Seyed-Sharifi, R. Effect of Slow-Release Pellets of Selenium and Iodine on Performance and Some Blood Metabolites of Pregnant Moghani Ewes and Their Lambs. Biol. Trace Elem. Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Soliman, E.B.; AKI, A.E.M.; Kassab, A.Y. Combined effect of vitamin E and selenium on some productive and physiological characteristics of ewes and their lambs during suckling period. Egypt. J. Sheep Goat Sci. 2012, 7, 31–42. [Google Scholar] [CrossRef]
- Pisek, L.; Travnicek, J.; Salat, J.; Kroupova, V.; Soch, M. Changes in white blood cells in sheep blood during selenium supplementation. Vet. Med. 2008, 53, 255–259. [Google Scholar] [CrossRef] [Green Version]

| Specification | CJ® Concentrate | Cereal Straw | Meadow Hay | Dried Beet Pulp |
|---|---|---|---|---|
| Chemical Composition | ||||
| Dry matter (%) | 88.99 | 91.01 | 84.98 | 93.11 |
| Crude ash (%) | 5.59 | 7.01 | 9.79 | 4.81 |
| Crude protein (%) | 19.32 | 4.22 | 7.25 | 5.05 |
| Crude fat (%) | 3.42 | 1.54 | 0.82 | 0.82 |
| Crude fiber (%) | 6.92 | 43.2 | 28.62 | 14.03 |
| Selenium (mg/kg) | 0.15 | 0.025 | 0.023 | 0.047 |
| Gross energy MJ kg−1 | 16.14 | 16.89 | 16.22 | 15.88 |
| Parameter | Lactation Day 28 | Lactation Day 70 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (n = 10) | E (n = 10) | EI (n = 10) | C (n = 10) | E (n = 10) | EI (n = 10) | Group | Date | Group × Date | ||
| Milk yield (mL/d) | 1528.4 | 1567.8 | 1537.3 | 1056.2 | 1083.6 | 1047.7 | 32.97 | 0.053 | 0.095 | 0.748 |
| Dry matter (%) | 3.82 | 16.36 | 15.66 | 15.82 b | 16.72 a | 16.12 | 1.096 | 0.021 | 0.110 | 0.994 |
| Fat (%) | 5.05 | 5.67 | 5.27 | 5.29 b | 6.02 a | 5.64 | 0.620 | 0.056 | 0.151 | 0.969 |
| Protein (%) | 4.72 | 5.12 | 4.80 | 4.95 | 5.28 | 4.91 | 0.174 | 0.075 | 0.321 | 0.750 |
| Lactose (%) | 5.23 | 5.26 | 5.29 | 5.28 | 5.35 | 5.26 | 0.061 | 0.835 | 0.582 | 0.743 |
| SCC (103/mL) | 272.00 | 201.50 | 226.00 | 140.10 | 134.50 | 150.40 | 12.97 | 0.363 | 0.111 | 0.584 |
| Selenium (μg/L) | 0.134 | 0.164 | 0.121 | 0.245 | 0.325 | 0.241 | 0.001 | 0.542 | 0.058 | 0.366 |
| Parameter | 4–7 Days of Age (Day 0) | 28 Day of Age | 70 Day of Age | 100 Day of Age | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | Group | Date | Group × Date | ||
| WBC(109/L) | 8.38 | 8.42 | 8.41 | 8.43 | 8.46 | 8.83 | 8.44 | 8.52 | 9.17 | 8.41 | 8.59 | 9.79 | 4.06 | 0.132 | 0.095 | 0.167 |
| RBC(1012/L) | 10.28 | 10.33 | 10.32 | 10.38 | 10.45 | 10.59 | 10.39 | 10.42 | 10.92 | 10.37 | 10.48 | 11.10 | 0.958 | 0.280 | 0.169 | 0.106 |
| HGB (g/dL) | 11.28 | 11.33 | 11.35 | 11.33 | 11.51 | 11.63 | 11.29 | 11.67 | 12.04 | 11.28 | 11.70 | 12.01 | 0.622 | 0.101 | 0.194 | 0.652 |
| HCT (%) | 34.83 | 34.87 | 34.79 | 35.12 | 35.16 | 35.11 | 35.02 | 35.36 | 35.28 | 35.25 | 35.62 | 35.53 | 4.83 | 0.521 | 0.156 | 0.247 |
| MCV (fl) | 34.97 | 34.88 | 34.92 | 35.83 | 34.50 | 33.84 | 35.92 | 34.41 | 33.58 | 34.91 | 34.02 | 32.36 | 4.98 | 0.332 | 0.920 | 0. 496 |
| MCH (pg) | 10.28 | 10.27 | 10.29 | 10.33 | 10.29 | 10.29 | 10.32 | 10.30 | 10.29 | 10.33 | 10.29 | 10.27 | 0.598 | 0.277 | 0.068 | 0.428 |
| MCHC(g/dL) | 31.98 | 32.51 | 32.84 | 32.51 | 32.60 | 33.06 | 32.94 | 33.21 | 33.18 | 33.12 | 33.44 | 33.51 | 1.64 | 0.077 | 0.376 | 0.204 |
| PLT (109/L) | 653.71 | 633.12 | 663.78 | 663.12 | 648.45 | 689.82 | 689.33 | 673.19 | 708.22 | 673.24 | 685.91 | 699.14 | 24.70 | 0.780 | 0.158 | 0.093 |
| MPV (fl) | 8.27 | 8.46 | 8.12 | 9.29 | 9.45 | 9.02 | 9.34 | 9.51 | 9.38 | 9.02 | 9.41 | 9.44 | 0.797 | 0.135 | 0.112 | 0.289 |
| Parameter | 4–7 Days of Age (Day 0) | 28 Day of Age | 70 Day Of Age | 100 Day of Age | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | Group | Date | Group× Date | ||
| GLU (mmol/L) | 4.75 | 4.80 | 4.79 | 4.76 | 4.82 | 4.83 | 4.72 | 4.81 | 4.84 | 4.74 | 4.83 | 4.89 | 0.170 | 0.090 | 0.900 | 0.119 |
| TP (g/L) | 48.22 | 57.34 | 48.21 | 47.12 B | 60.45 B | 69.87 A** | 48.91 B | 55.33 B | 67.37 A | 48.03 B | 47.19 B | 68.45 A* | 29.6 | 0.002 | 0.002 | 0.694 |
| AST (U/L) | 75.04 | 64.16 | 74.08 | 82.96 | 64.22 | 64.18 | 87.28 | 64.21 | 64.02 | 90.59 | 64.34 | 64.12 | 36.70 | 0.057 | 0.101 | 0.114 |
| ALP (U/L) | 325.64 | 338.92 | 329.55 | 350.87 | 365.39 | 352.64 | 399.25 | 372.44 | 392.39 | 380.72 | 398.58 | 402.56 | 115.86 | 0.657 | 0.218 | 0.157 |
| LDH (U/L) | 1040.00 | 938.54 | 1002.71 | 1187.37 | 962.56 | 972.59 | 1222.45 | 973.92 | 994.13 | 1253.37 | 983.15 | 997.98 | 229.19 | 0.714 | 0.140 | 0.615 |
| GGT (U/L) | 56.82 | 57.34 | 57.92 | 57.88 | 57.45 | 58.02 | 59.32 | 60.02 | 59.89 | 59.98 | 60.12 | 60.08 | 11.88 | 0.716 | 0.178 | 0.229 |
| Chol (mmol/L) | 2.02 | 2.15 | 2.14 | 2.09 | 2.20 | 2.22 | 2.07 | 2.25 | 2.28 | 2.09 | 2.23 | 2.28 | 0.679 | 0.323 | 0.145 | 0.515 |
| TG (mmol/L) | 0.42 | 0.36 | 0.38 | 0.45 | 0.37 | 0.37 | 0.47 | 0.37 | 0.36 | 0.52 a | 0.39 b | 0.38 b | 0.030 | 0.017 | 0.169 | 0.761 |
| Parameter | 4–7 Days of Age (Day 0) | 28 Day of Age | 70 Day of Age | 100 Day of Age | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | C (n = 12) | E (n = 12) | EI (n = 12) | Group | Time | Group× Time | ||
| Lysozyme (mg/L) | 0.92 | 0.94 | 1.02 | 0.92 b | 0.94 b | 1.05 a | 0.86 B | 0.98 B | 1.12 A** | 0.90 B | 1.02 B* | 1.16 A** | 0.022 | 0.001 | 0.001 | 0.228 |
| Ceruloplasmin (U/L) | 51.55 | 51.89 | 52.01 | 50.30 b | 51.82 b | 53.09 a* | 52.26 B | 52.02 B | 56.81 A** | 51.27 B | 52.45 B | 56.23 A** | 9.00 | 0.004 | 0.002 | 0.890 |
| Gamma globulin (g/L) | 26.62 | 26.23 | 26.15 | 23.80 b | 26.89 b | 32.87 a* | 23.90 B | 26.54 B | 35.06 A** | 23.55 B | 26.38 B | 35.81 A** | 5.33 | 0.004 | 0.001 | 0.082 |
| RBA (OD 620 nm) | 0.52 | 0.53 | 0.63 | 0.49 | 0.53 | 0.59 | 0.48 B | 0.56 B | 0.61 A | 0.51 B | 0.58 B* | 0.63 A | 0.074 | 0.007 | 0.614 | 0.720 |
| PKA (OD 620 nm) | 0.39 | 0.40 | 0.43 | 0.37 b | 0.45 b* | 0.48 a | 0.37 b | 0.44 b | 0.49 a* | 0.35 B | 0.46 B* | 0.52 A** | 0.013 | 0.004 | 0.001 | 0.350 |
| MTT-ConA (RI) | 1.11 | 1.09 | 1.09 | 1.11 b | 1.18 b** | 1.19 a** | 1.13 | 1.17 ** | 1.19 ** | 1.13 b | 1.20 b** | 1.21 a** | 0.205 | 0.034 | 0.002 | 0.839 |
| MTT-LPS (RI) | 1.02 | 1.03 | 1.08 | 0.94 b | 1.10 b | 1.16 a* | 0.90 B* | 1.12 B* | 1.28 A** | 0.98 | 1.08 | 1.15 | 0.055 | 0.012 | 0.049 | 0.080 |
| Parameter | Group | SEM | p-Value | ||
|---|---|---|---|---|---|
| C (n = 12) | E (n = 12) | EI (n = 12) | |||
| Initial body weight (kg)—2 days of age | 5.24 | 5.28 | 5.31 | 0.654 | 0.980 |
| Average body weight (kg)—4–7 days of age | 6.08 | 6.16 | 6.26 | 0.698 | 0.916 |
| Final body weight (kg)—100 days of age | 28.44 b | 29.57 | 31.47 a | 12.09 | 0.011 |
| Average daily gain—ADG (g) | 239.12 b | 250.34 | 269.71 a | 101.10 | 0.073 |
| m.l.d. at 100 days of age: | |||||
| height (cm) | 2.18 B | 2.26 | 2.38 A | 0.048 | 0.007 |
| width (cm) | 5.63 Bb | 6.14 a | 6.31 A | 0.228 | 0.005 |
| cross-sectional area (cm2) | 8.63 Bb | 10.04 a | 10.28 A | 1.68 | 0.009 |
| Fat thickness over the loin eye at 100 days of age (cm) | 0.21 | 0.25 | 0.21 | 0.003 | 0.149 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewski, S.; Sobiech, P.; Błażejak-Grabowska, J.; Wójcik, R.; Żarczyńska, K.; Miciński, J.; Ząbek, K. The Efficacy of a Long-Acting Injectable Selenium Preparation Administered to Pregnant Ewes and Lambs. Animals 2021, 11, 1076. https://doi.org/10.3390/ani11041076
Milewski S, Sobiech P, Błażejak-Grabowska J, Wójcik R, Żarczyńska K, Miciński J, Ząbek K. The Efficacy of a Long-Acting Injectable Selenium Preparation Administered to Pregnant Ewes and Lambs. Animals. 2021; 11(4):1076. https://doi.org/10.3390/ani11041076
Chicago/Turabian StyleMilewski, Stanisław, Przemysław Sobiech, Justyna Błażejak-Grabowska, Roman Wójcik, Katarzyna Żarczyńska, Jan Miciński, and Katarzyna Ząbek. 2021. "The Efficacy of a Long-Acting Injectable Selenium Preparation Administered to Pregnant Ewes and Lambs" Animals 11, no. 4: 1076. https://doi.org/10.3390/ani11041076
APA StyleMilewski, S., Sobiech, P., Błażejak-Grabowska, J., Wójcik, R., Żarczyńska, K., Miciński, J., & Ząbek, K. (2021). The Efficacy of a Long-Acting Injectable Selenium Preparation Administered to Pregnant Ewes and Lambs. Animals, 11(4), 1076. https://doi.org/10.3390/ani11041076







