The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows
Abstract
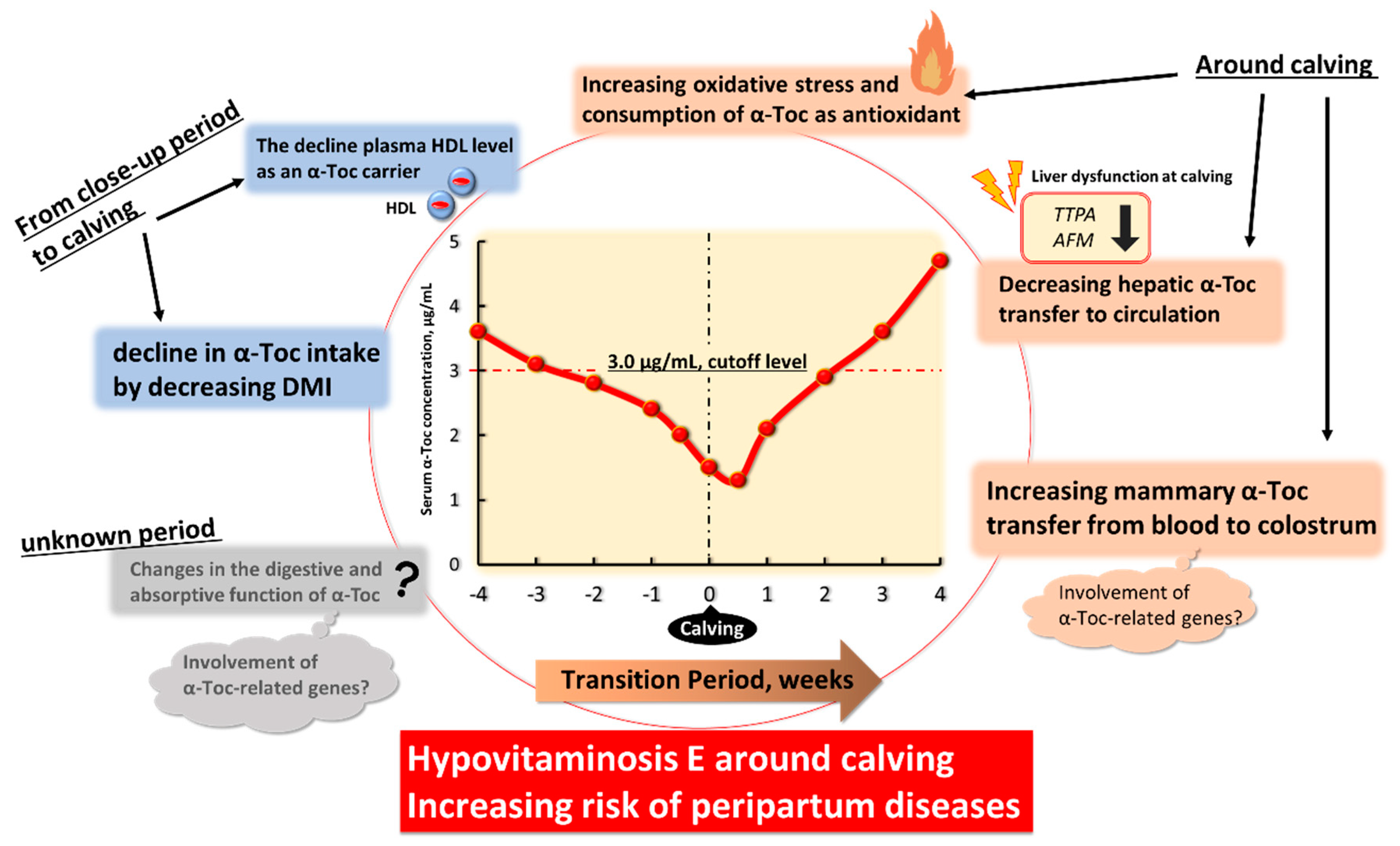
:Simple Summary
Abstract
1. Introduction
2. Vitamin E and VE-Related Molecules
2.1. Vitamin E (α-Tocopherol)
2.2. Alpha-Tocopherol Transfer Protein and Other α-Toc-Related Molecules
2.2.1. Alpha-Tocopherol Transfer Protein
2.2.2. Afamin
2.2.3. Tocopherol-Associated Protein
2.2.4. Scavenger Receptor Class B, Type I
2.2.5. ATP-Binding Cassette Transporter A1
2.2.6. Cytochrome P450 Family 4, Subfamily F, Polypeptide 2
3. Hypovitaminosis E in Transition High-Yield Dairy Cows
3.1. Changes in α-Tocopherol Status in Transition Dairy Cows
3.2. Disease Risk in Hypovitaminosis E and the Effects of α-Toc Supplemantetion in Transition Dairy Cows
3.2.1. Left Displaced Abomasum
3.2.2. Retained Foetal Membranes, Stillbirth, and Reproductive Performance
3.2.3. Udder Health (Mastitis and SCC Values) and Milk Yield
4. Physiological Factors Underlying Decreased Blood α-Toc Level and Hypovitaminosis E in Transition Period
4.1. The Decline in α-Toc Intake by Decreasing DMI from Close-Up Period to Calving
4.2. Changes in the Digestive and Absorptive Functions of α-Toc with Change in the Expression of α-Toc-Related Genes
4.3. The Decline of Plasma HDL Level as an α-Toc Carrier from Close-Up Period to Calving
4.4. Increasing Systemic Oxidative Stress and Consumption of α-Toc as Antioxidant around Calving
4.5. Decreasing Hepatic α-Toc Transfer to Circulation with Change in the Expression of α-Toc-Related Genes
4.6. Increasing Mammary α-Toc Transfer from Blood to Colostrum with Change in the Expression of α-Toc-Related Genes at Calving
5. Foresight
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Miyaji, M.; Nakano, M.; Ishizaki, H.; Matsuyama, H.; Katoh, K.; Roh, S. Changes in the expression of α-tocopherol–related genes in liver and mammary gland biopsy specimens of peripartum dairy cows. J. Dairy Sci. 2018, 101, 5277–5293. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, Y.; Takahashi, T.; Sato, K.; Ardiyanti, A.; Song, S.H.; Sato, R.; Onda, K.; Wada, Y.; Obara, Y.; Suzuki, K.; et al. Changes in circulating adiponectin and metabolic hormone concentrations during periparturient and lactation periods in Holstein dairy cows. Anim. Sci. J. 2012, 83, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Suzuki, Y.; Haga, S.; Yamauchi, E.; Kim, D.; Nishihara, K.; Nakajima, K.; Gotoh, T.; Park, S.; Baik, M.; et al. Downregulated angiopoietin-like protein 8 production at calving related to changes in lipid metabolism in dairy cows. J. Anim. Sci. 2018, 96, 2646–2658. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox Biology in Transition Periods of Dairy Cattle: Role in the Health of Periparturient and Neonatal Animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Dewhurst, R.J.; Friggens, N.C. On the relationship between lactational performance and health: Is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livest. Prod. Sci. 2003, 83, 277–308. [Google Scholar] [CrossRef]
- Mulligan, F.J.; Doherty, M.L. Production diseases of the transition cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef]
- Goff, J.P.; Stabel, J.R. Decreased plasma retinol, α-tocopherol, and zinc concentration during the periparturient period: Effect of milk fever. J. Dairy Sci. 1990, 73, 3195–3199. [Google Scholar] [CrossRef]
- Weiss, W.P.; Todhunter, D.A.; Hogan, J.S.; Smith, K.L. Effect of duration of supplementation of selenium and vitamin E on periparturient dairy cows. J. Dairy Sci. 1990, 73, 3187–3194. [Google Scholar] [CrossRef]
- Weiss, W.P.; Hogan, J.S.; Smith, K.L.; Hoble, K.H. Relationships among selenium, vitamin E, and mammary gland health in commercial dairy herds. J. Dairy Sci. 1990, 73, 381–390. [Google Scholar] [CrossRef]
- Kumagai, H.; Chaipan, Y. Changes of vitamin E status of periparturient dairy cows and newborn calves. Anim. Sci. J. 2004, 75, 541–547. [Google Scholar] [CrossRef]
- Politis, I.; Theodorou, G.; Lampidonis, A.D.; Kominakis, A.; Baldi, A. Short communication: Oxidative status and incidence of mastitis relative to blood α-tocopherol concentrations in the postpartum period in dairy cows. J. Dairy Sci. 2012, 95, 7331–7335. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Fadden, A.N.; Traber, M.G.; Bobe, G. Potential risk indicators of retained placenta and other diseases in multiparous cows. J. Dairy Sci. 2014, 97, 4151–4165. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lytle, K.; Traber, M.G.; Bobe, G. Depleted serum vitamin E concentrations precede left displaced abomasum in early-lactation dairy cows. J. Dairy Sci. 2013, 96, 3012–3022. [Google Scholar] [CrossRef]
- Hogan, J.S.; Weiss, W.P.; Smith, K.L. Role of vitamin E and selenium in host defense against mastitis. J. Dairy Sci. 1993, 76, 2795–2803. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Smith, K.L.; Hogan, J.S.; Weiss, W.P. Dietary vitamin E and selenium affect mastitis and milk quality. J. Anim. Sci. 1997, 75, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Shafer-Weaver, K.; DeRosa, D. Immunobiology of the mammary gland. J. Dairy Sci. 1997, 80, 1851–1865. [Google Scholar] [CrossRef]
- Weiss, W.P. Requirements of fat-soluble vitamins for dairy cows: A review. J. Dairy Sci. 1998, 81, 2493–2501. [Google Scholar] [CrossRef]
- Hemingway, R.G. The influences of dietary selenium and vitamin E intakes on milk somatic cell counts and mastitis in cows. Vet. Res. Commun. 1999, 23, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.D.; Laven, R.A. Effect of vitamin E supplementation on the health and fertility of dairy cows: A review. Vet. Rec. 2000, 147, 703–708. [Google Scholar] [PubMed]
- Spears, J.W. Micronutrients and immune function in cattle. Proc. Nutr. Soc. 2000, 59, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemingway, R.G. The influences of dietary intakes and supplementation with selenium and vitamin E on reproduction diseases and reproductive efficiency in cattle and sheep. Vet. Res. Commun. 2003, 27, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A. Vitamin E in dairy cows. Livest. Prod. Sci. 2005, 98, 117–122. [Google Scholar] [CrossRef]
- Wilde, D. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 2006, 96, 240–249. [Google Scholar] [CrossRef]
- Vagni, S.; Saccone, F.; Pinotti, L.; Baldi, A. Vitamin E Bioavailability: Past and Present Insights. Food Sci. Nutr. 2011, 2, 1088–1096. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.L.; Li, X.S.; He, B.X. Effects of vitamins and trace-elements supplementation on milk production in dairy cows: A review. Afr. J. Biotechnol. 2011, 10, 2574–2578. [Google Scholar]
- Politis, I. Reevaluation of vitamin E supplementation of dairy cows: Bioavailability, animal health and milk quality. Animals 2012, 6, 1427–1434. [Google Scholar] [CrossRef] [Green Version]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. (Berl.) 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Manoni, M.; Fumagalli, F.; Rovere, N.; Tretola, M.; Baldi, A. The role of micronutrients in high-yielding dairy ruminants: Choline and vitamin E. Ankara Üniv. Vet. Fak. Derg. 2020, 67, 209–214. [Google Scholar] [CrossRef]
- Liu, K.; Luo, H.L.; Yue, D.B.; Ge, S.Y.; Yuan, F.; Yan, L.Y.; Jia, H.N. Molecular cloning and characterization of the sheep α-TTP gene and its expression in response to different vitamin E status. Gene 2012, 494, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, H.L.; Zuo, Z.Y.; Jia, H.N.; Zhang, Y.W.; Chang, Y.F.; Jiao, L.J. Regulation of sheep α-TTP by dietary vitamin E and preparation of monoclonal antibody for sheep α-TTP. Gene 2014, 540, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Nakano, M.; Ishizaki, H.; Roh, S.; Katoh, K. Expression of α-tocopherol-associated genes and α-tocopherol accumulation in Japanese Black (Wagyu) calves with and without α-tocopherol supplementation. J. Anim. Sci. 2015, 93, 4048–4057. [Google Scholar] [CrossRef] [Green Version]
- Mani, O.; Sorensen, M.T.; Sejrsen, K.; Bruckmaier, R.M.; Albrecht, C. Differential expression and localization of lipid transporters in the bovine mammary gland during the pregnancy-lactation cycle. J. Dairy Sci. 2009, 92, 3744–3756. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, H.; Ito, E.; Iwano, H.; Oikawa, S.; Nagahata, H. Effects of vitamin E supplementation on cellular α-tocopherol concentrations of neutrophils in Holstein calves. Can. J. Vet. Res. 2013, 77, 120–125. [Google Scholar]
- Kuhn, M.J.; Putman, A.K.; Sordillo, L.M. Widespread basal cytochrome P450 expression in extrahepatic bovine tissues and isolated cells. J. Dairy Sci. 2020, 103, 625–637. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef]
- Tharwat, M.; Takamizawa, A.; Hosaka, Y.Z.; Endoh, D.; Oikawa, S. Hepatocyte apoptosis in dairy cattle during the transition period. Can. J. Vet. Res. 2012, 76, 241–247. [Google Scholar]
- Finucane, K.A.; McFadden, T.B.; Bond, J.P.; Kennelly, J.J.; Zhao, F.Q. Onset of lactation in the bovine mammary gland: Gene expression profiling indicates a strong inhibition of gene expression in cell proliferation. Funct. Integr. Genomics 2008, 8, 251–264. [Google Scholar] [CrossRef]
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Barella, L.; Muller, P.Y.; Schlachter, M.; Hunziker, W.; Stöcklin, E.; Spitzer, V.; Meier, N.; de Pascual-Teresa, S.; Minihane, A.M.; Rimbach, G. Identification of hepatic molecular mechanisms of action of alpha-tocopherol using global gene expression profile analysis in rats. Biochim. Biophys. Acta 2004, 1689, 66–74. [Google Scholar] [CrossRef]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-Regulatory Activity of α-Tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio, J.M.; Bélanger-Quintana, A.; Suárez, L.; Mayo, D.; Benítez, J.; Díaz, M.; Escobar, H. Ataxia with isolated vitamin E deficiency: Case report and review of the literature. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 206–210. [Google Scholar] [CrossRef]
- Yokota, T.; Igarashi, K.; Uchihara, T.; Jishage, K.; Tomita, H.; Inaba, A.; Li, Y.; Arita, M.; Suzuki, H.; Mizusawa, H.; et al. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. USA 2001, 98, 15185–15190. [Google Scholar] [CrossRef] [Green Version]
- Azzi, A.; Ricciarelli, R.; Zingg, J.M. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.K.; Nørgaard, J.V.; Lauridsen, C. Bioavailability of alpha-tocopherol stereoisomers in rats depends on dietary doses of all-rac- or RRR-alpha-tocopheryl acetate. Br. J. Nutr. 2006, 95, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.L.; Ludwig, M.I. Relative vitamin E potency of natural and of synthetic alpha-tocopherol. J. Biol. Chem. 1949, 179, 1111–1115. [Google Scholar] [CrossRef]
- National Academy Press. Nutrient Requirements of Dairy Cattle; National Academy Press: Washington, WA, USA, 2001. [Google Scholar]
- Meglia, G.E.; Jensen, S.K.; Lauridsen, C.; Waller, K.P. Alpha-tocopherol concentration and stereoisomer composition in plasma and milk from dairy cows fed natural or synthetic vitamin E around calving. J. Dairy Res. 2006, 73, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Lashkari, S.; Kristensen, N.B. Pharmacokinetics of α-tocopherol stereoisomers in plasma and milk of cows following a single dose injection of all-rac-α-tocopheryl acetate. Food Chem. 2020, 310, 125931. [Google Scholar] [CrossRef]
- Sadri, H.; Dänicke, S.; Meyer, U.; Rehage, J.; Frank, J.; Sauerwein, H. Tocopherols and tocotrienols in serum and liver of dairy cows receiving conjugated linoleic acids or a control fat supplement during early lactation. J. Dairy Sci. 2015, 98, 7034–7043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidiroglou, N.; Laflamme, L.F.; McDowell, L.R. Blood plasma and tissue concentrations of vitamin E in beef cattle as influenced by supplementation of various tocopherol compounds. J. Anim. Sci. 1988, 66, 3227–3234. [Google Scholar] [CrossRef]
- Sato, Y.; Hagiwara, K.; Arai, H.; Inoue, K. Purification and characterization of the α-tocopherol transfer protein from rat liver. FEBS Lett. 1991, 288, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Arai, H.; Miyata, A.; Tokita, S.; Yamamoto, K.; Tanabe, T.; Inoue, K. Primary structure of alpha-tocopherol transfer protein from rat liver. Homology with cellular retinaldehyde-binding protein. J. Biol. Chem. 1993, 268, 17705–17710. [Google Scholar] [CrossRef]
- Arita, M.; Sato, Y.; Miyata, A.; Tanabe, T.; Takahashi, E.; Kayden, H.J.; Arai, H.; Inoue, K. Human α-tocopherol transfer protein: cDNA cloning, expression and chromosomal localization. Biochem. J. 1995, 306, 437–443. [Google Scholar] [CrossRef]
- Ouahchi, K.; Arita, M.; Kayden, H.; Hentati, F.; Hamida, M.B.; Sokol, R.; Arai, H.; Inoue, K.; Mandel, J.L.; Koenig, M. Ataxia with isolated vitamin E deficiency is caused by mutations in the α-tocopherol transfer protein. Nat. Genet. 1995, 9, 141–145. [Google Scholar] [CrossRef]
- Hentati, A.; Deng, H.X.; Hung, W.Y.; Nayer, M.; Ahmed, M.S.; He, X.; Tim, R.; Stumpf, D.A.; Siddique, T. Human α-tocopherol transfer protein: Gene structure and mutations in familial vitamin E deficiency. Ann. Neurol. 1996, 39, 295–300. [Google Scholar] [CrossRef]
- Arita, M.; Nomura, K.; Arai, H.; Inoue, K. α-Tocopherol transfer protein stimulates the secretion of α-tocopherol from a cultured liver cell line through a brefeldin A-insensitive pathway. Proc. Natl. Acad. Sci. USA 1997, 94, 12437–12441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, N.; Ohto, U.; Hiramatsu, T.; Urabe, M.; Uchida, Y.; Satow, Y.; Arai, H. Impaired α-TTP-PIPs interaction underlies familial vitamin E deficiency. Science 2013, 340, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Morley, S.; Wilson, K.; Nava, P.; Atkinson, J.; Manor, D. Intracellular trafficking of vitamin E in hepatocytes: The role of tocopherol transfer protein. J. Lipid Res. 2005, 46, 2072–2082. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Atkinson, J.; Manor, D. Biochemical consequences of heritable mutations in the alpha-tocopherol transfer protein. Biochemistry 2006, 45, 8236–8242. [Google Scholar] [CrossRef] [PubMed]
- Kono, N.; Arai, H. Intracellular Transport of Fat-Soluble Vitamins A and E. Traffic 2015, 16, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Ghelfi, M.; Atkinson, J.; Parker, R.; Qian, J.; Carlin, C.; Manor, D. Vitamin E and phosphoinositides regulate the intracellular localization of the hepatic α-tocopherol transfer protein. J. Biol. Chem. 2016, 291, 17028–17039. [Google Scholar] [CrossRef] [Green Version]
- Irías-Mata, A.; Sus, N.; Flory, S.; Stock, D.; Woerner, D.; Podszun, M.; Frank, J. α-Tocopherol transfer protein does not regulate the cellular uptake and intracellular distribution of α- and γ-tocopherols and -tocotrienols in cultured liver cells. Redox Biol. 2018, 19, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fechner, H.; Schlame, M.; Guthmann, F.; Stevens, P.A.; Rustow, B. α- and δ-tocopherol induce expression of hepatic α-tocopherol-transfer-protein mRNA. Biochem. J. 1998, 331, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Arai, H.; Arita, M.; Sato, Y.; Ogihara, T.; Inoue, K.; Mino, M.; Tamai, H. Effect of α-tocopherol status on α-tocopherol transfer protein expression and its messenger RNA level in rat liver. Free Radic. Res. 1998, 28, 87–92. [Google Scholar] [CrossRef]
- Shaw, H.M.; Huang, C.J. Liver α-tocopherol transfer protein and its mRNA are differentially altered by dietary vitamin E deficiency and protein insufficiency in rats. J. Nutr. 1998, 128, 2348–2354. [Google Scholar] [CrossRef] [Green Version]
- Rengaraj, D.; Truong, A.D.; Hong, Y.; Pitargue, F.M.; Kim, J.H.; Hong, Y.H.; Han, J.Y.; Kil, D.Y. Identification and expression analysis of alpha tocopherol transfer protein in chickens fed diets containing different concentrations of alpha-tocopherol. Res. Vet. Sci. 2019, 123, 99–110. [Google Scholar] [CrossRef]
- Kaempf-Rotzoll, D.E.; Igarashi, K.; Aoki, J.; Jishage, K.; Suzuki, H.; Tamai, H.; Linderkamp, O.; Arai, H. α-tocopherol transfer protein is specifically localized at the implantation site of pregnant mouse uterus. Biol. Reprod. 2002, 67, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misaki, K.; Takitani, K.; Ogihara, T.; Inoue, A.; Kawakami, C.; Kuno, T.; Kawamura, N.; Miyake, M.; Nakagawa, T.; Tamai, H. Alpha-tocopherol content and α-tocopherol transfer protein expression in leukocytes of children with acute leukemia. Free Radic. Res. 2003, 37, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kaempf-Rotzoll, D.E.; Horiguchi, M.; Hashiguchi, K.; Aoki, J.; Tamai, H.; Linderkamp, O.; Arai, H. Human placental trophoblast cells express α-tocopherol transfer protein. Placenta 2003, 24, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Vasu, V.T.; Yokohama, W.; Corbacho, A.M.; Phung, A.; Lim, Y.; Aung, H.H.; Cross, C.E.; Davis, P.A. Lung vitamin E transport processes are affected by both age and environmental oxidants in mice. Toxicol. Appl. Pharmacol. 2007, 222, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, Y.; Komatsu-Tanaka, M.; Hirose, N.; Kaji, Y.; Saeki, K. Molecular Cloning and Expression Analysis of Bovine Alpha-tocopherol Transfer Protein (α-TTP). Annu. Res. Rev. 2017, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Zuo, Z.Y.; Luo, H.L.; Liu, K.; Jia, H.N.; Zhang, Y.W.; Jiao, L.J.; Chang, Y.F. Dietary vitamin E affects α-TTP mRNA levels in different tissues of the Tan sheep. Gene 2014, 541, 1–7. [Google Scholar] [CrossRef]
- Haga, S.; Ishizaki, H.; Suzuki, T. Effects of orally administered Rhizopus oryzae aqueous extracts in the close-up dry period on postpartum serum liver injury markers and hepatic gene expression levels in dairy cows. J. Farm Anim. Infect. Dis. 2020, 9, 69–81. (In Japanese) [Google Scholar]
- Voegele, A.F.; Jerković, L.; Wellenzohn, B.; Eller, P.; Kronenberg, F.; Liedl, K.R.; Dieplinger, H. Characterization of the vitamin E-binding properties of human plasma afamin. Biochemistry 2002, 41, 14532–14538. [Google Scholar] [CrossRef]
- Jerkovic, L.; Voegele, A.F.; Chwatal, S.; Kronenberg, F.; Radcliffe, C.M.; Wormald, M.R.; Lobentanz, E.M.; Ezeh, B.; Eller, P.; Dejori, N.; et al. Afamin is a novel human vitamin E-binding glycoprotein characterization and in vitro expression. J. Proteome Res. 2005, 4, 889–899. [Google Scholar] [CrossRef]
- Lichenstein, H.S.; Lyons, D.E.; Wurfel, M.M.; Johnson, D.A.; McGinley, M.D.; Leidli, J.C.; Trollinger, D.B.; Mayer, J.P.; Wright, S.D.; Zukowski, M.M. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J. Biol. Chem. 1994, 269, 18149–18154. [Google Scholar] [CrossRef]
- Hubalek, M.; Buchner, H.; Mörtl, M.G.; Schlembach, D.; Huppertz, B.; Firulovic, B.; Köhler, W.; Hafner, E.; Dieplinger, B.; Wildt, L.; et al. The vitamin E-binding protein afamin increases in maternal serum during pregnancy. Clin. Chim. Acta 2014, 434, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Seeber, B.; Morandell, E.; Lunger, F.; Wildt, L.; Dieplinger, H. Afamin serum concentrations are associated with insulin resistance and metabolic syndrome in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2014, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Varga, V.E.; Lőrincz, H.; Szentpéteri, A.; Juhász, L.; Seres, I.; Paragh, G., Jr.; Balla, J.; Paragh, G.; Harangi, M. Changes in serum afamin and vitamin E levels after selective LDL apheresis. J. Clin. Apher. 2018, 33, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Erol, S.A.; Tanacan, A.; Anuk, A.T.; Tokalioglu, E.O.; Biriken, D.; Keskin, H.L.; Moraloglu, O.T.; Yazihan, N.; Sahin, D. Evaluation of maternal serum afamin and vitamin E levels in pregnant women with COVID-19 and its association with composite adverse perinatal outcomes. J. Med. Virol. 2021, 93, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, I.; Bernhart, E.; Wintersperger, A.; Hammer, A.; Waltl, S.; Malle, E.; Sperk, G.; Wietzorrek, G.; Dieplinger, H.; Sattler, W. Afamin is synthesized by cerebrovascular endothelial cells and mediates α-tocopherol transport across an in vitro model of the blood–brain barrier. J. Neurochem. 2009, 108, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Herdt, T.H.; Smith, J.C. Blood-lipid and lactation-stage factors affecting serum vitamin E concentrations and vitamin E cholesterol ratios in dairy cattle. J. Vet. Diagn. Investig. 1996, 2, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, A.; Zimmer, S.; Spycher, S.E.; Azzi, A. Identification of a novel cytosolic tocopherol-binding protein: Structure, specificity, and tissue distribution. IUBMB Life 1999, 48, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Stocker, A.; Sarbolouki, M.N.; Spycher, S.E.; Sassoon, J.; Azzi, A. A novel human tocopherol-associated protein: Cloning, in vitro expression, and characterization. J. Biol. Chem. 2000, 275, 25672–25680. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, J.; Iwamoto, T.; Kida, S.; Masushige, S.; Yamada, K.; Esashi, T. Tocopherol-associated protein is a ligand-dependent transcriptional activator. Biochem. Biophys. Res. Commun. 2001, 285, 295–299. [Google Scholar] [CrossRef]
- Porter, T.D. Supernatant protein factor and tocopherol-associated protein: An unexpected link between cholesterol synthesis and vitamin E (review). J. Nutr. Biochem. 2003, 14, 3–6. [Google Scholar] [CrossRef]
- Ikeda, T.; Murakami, M.; Funaba, M. Expression of tocopherol-associated protein in mast cells. Clin. Diagn. Lab. Immunol. 2004, 11, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ring, B.Z.; Seitz, R.S.; Ross, D.T.; Woolf, K.; Beck, R.A.; Hicks, D.G.; Yeh, S. Expression of a-Tocopherol-Associated protein (TAP) is associated with clinical outcome in breast cancer patients. BMC Clin. Pathol. 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardones, P.; Strobel, P.; Miranda, S.; Leighton, F.; Quiñones, V.; Amigo, L.; Rozowski, J.; Krieger, M.; Rigotti, A. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J. Nutr. 2002, 132, 443–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem. 2006, 281, 4739–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Ulatowski, L.M. Vitamin E: Mechanism of transport and regulation in the CNS. IUBMB Life 2019, 71, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Argov, N.; Moallem, U.; Sklan, D. Lipid transport in the developing bovine follicle: Messenger RNA expression increases for selective uptake receptors and decreases for endocytosis receptors. Biol. Reprod. 2004, 71, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Argov, N.; Moallem, U.; Sklan, D. Summer heat stress alters the mRNA expression of selective-uptake and endocytotic receptors in bovine ovarian cells. Theriogenology 2005, 64, 1475–1489. [Google Scholar] [CrossRef]
- Rajapaksha, W.R.; McBride, M.; Robertson, L.; O’Shaughnessy, P.J. Sequence of the bovine HDL-receptor (SR-BI) cDNA and changes in receptor mRNA expression during granulosa cell luteinization in vivo and in vitro. Mol. Cell. Endocrinol. 1997, 134, 59–67. [Google Scholar] [CrossRef]
- Knight, B.L. ATP-binding cassette transporter A1: Regulation of cholesterol efflux. Biochem. Soc. Trans. 2004, 32, 124–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 2005, 1735, 1–19. [Google Scholar] [CrossRef]
- Liu, M.; Chung, S.; Shelness, G.S.; Parks, J.S. Hepatic ABCA1 and VLDL triglyceride production. Biochim. Biophys. Acta 2012, 1821, 770–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shichiri, M.; Takanezawa, Y.; Rotzoll, D.E.; Yoshida, Y.; Kokubu, T.; Ueda, K.; Tamai, H.; Arai, H. ATP-binding cassette transporter A1 is involved in hepatic alpha-tocopherol secretion. J. Nutr. Biochem. 2010, 21, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kielar, D.; Dietmaier, W.; Langmann, T.; Aslanidis, C.; Probst, M.; Naruszewicz, M.; Schmitz, G. Rapid quantification of human ABCA1 mRNA in various cell types and tissues by real-time reverse transcription-PCR. Clin. Chem. 2001, 47, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Langmann, T.; Mauerer, R.; Zahn, A.; Moehle, C.; Probst, M.; Stremmel, W.; Schmitz, G. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin. Chem. 2003, 49, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Vaughan, A.M.; Stocker, R. ATP-binding cassette transporter A1 mediates cellular secretion of α-tocopherol. J. Biol. Chem. 2001, 276, 39898–39902. [Google Scholar] [CrossRef] [Green Version]
- Farke, C.; Viturro, E.; Meyer, H.H.D.; Albrecht, C. Identification of the bovine cholesterol efflux regulatory protein ABCA1 and its expression in various tissues. J. Anim. Sci. 2006, 84, 2887–2894. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, C.; Wei, Z.; Wang, Y.; Zhang, X.; Fu, Y. Activation of liver X receptors inhibit LPS-induced inflammatory response in primary bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2018, 197, 87–92. [Google Scholar] [CrossRef]
- Schlegel, G.; Ringseis, R.; Keller, J.; Schwarz, F.J.; Eder, K. Changes in the expression of hepatic genes involved in cholesterol homeostasis in dairy cows in the transition period and at different stages of lactation. J. Dairy Sci. 2012, 95, 3826–3836. [Google Scholar] [CrossRef] [Green Version]
- Kessler, E.C.; Gross, J.J.; Bruckmaier, R.M.; Albrecht, C. Cholesterol metabolism, transport, and hepatic regulation in dairy cows during transition and early lactation. J. Dairy Sci. 2014, 97, 5481–5490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farke, C.; Meyer, H.H.; Bruckmaier, R.M.; Albrecht, C. Differential expression of ABC transporters and their regulatory genes during lactation and dry period in bovine mammary tissue. J. Dairy Res. 2008, 75, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, O.; Körner, M.; Sorensen, M.T.; Sejrsen, K.; Wotzkow, C.; Ontsouka, C.E.; Friis, R.R.; Bruckmaier, R.M.; Albrecht, C. Expression, localization, and functional model of cholesterol transporters in lactating and nonlactating mammary tissues of murine, bovine, and human origin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 642–654. [Google Scholar] [CrossRef] [Green Version]
- Mani, O.; Körner, M.; Ontsouka, C.E.; Sorensen, M.T.; Sejrsen, K.; Bruckmaier, R.M.; Albrecht, C. Identification of ABCA1 and ABCG1 in milk fat globules and mammary cells—implications for milk cholesterol secretion. J. Dairy Sci. 2011, 94, 1265–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chu, S.; Wang, X.; Fan, Y.; Zhan, T.; Arbab, A.A.I.; Li, M.; Zhang, H.; Mao, Y.; Loor, J.J.; et al. MicroRNA-106b Regulates Milk Fat Metabolism via ATP Binding Cassette Subfamily A Member 1 (ABCA1) in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 3981–3990. [Google Scholar] [CrossRef]
- Kikuta, Y.; Kusunose, E.; Kondo, T.; Yamamoto, S.; Kinoshita, H.; Kusunose, M. Cloning and expression of a novel form of leukotriene B4 omega-hydroxylase from human liver. FEBS Lett. 1994, 348, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Kikuta, Y.; Miyauchi, Y.; Kusunose, E.; Kusunose, M. Expression and molecular cloning of human liver leukotriene B4 omega-hydroxylase (CYP4F2) gene. DNA Cell Biol. 1999, 18, 723–730. [Google Scholar] [CrossRef]
- Kikuta, Y.; Kusunose, E.; Kusunose, M. Characterization of human liver leukotriene B(4) omega-hydroxylase P450 (CYP4F2). J. Biochem. 2000, 127, 1047–1052. [Google Scholar] [CrossRef]
- Sontag, T.J.; Parker, R.S. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002, 277, 25290–25296. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.; Leist, M.; Petrzika, M.; Gassmann, B.; Brigelius-Flohé, R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 1995, 62, 1527S–1534S. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R. Vitamin E and drug metabolism. Biochem. Biophys. Res. Commun. 2003, 305, 737–740. [Google Scholar] [CrossRef]
- Abe, C.; Ikeda, S.; Yamashita, K. Dietary sesame seeds elevate alpha-tocopherol concentration in rat brain. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Takitani, K.; Inoue, K.; Koh, M.; Miyazaki, H.; Kishi, K.; Inoue, A.; Tamai, H. Alpha-Tocopherol status and altered expression of alpha-tocopherol-related proteins in streptozotocin-induced type 1 diabetes in rat models. J. Nutr. Sci. Vitaminol. (Tokyo) 2014, 60, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.H.; Savas, U.; Griffin, K.J.; Johnson, E.F. Regulation of human cytochrome P450 4F2 expression by sterol regulatory element-binding protein and lovastatin. J. Biol. Chem. 2007, 282, 5225–5236. [Google Scholar] [CrossRef] [Green Version]
- Bartolini, D.; Torquato, P.; Barola, C.; Russo, A.; Rychlicki, C.; Giusepponi, D.; Bellezza, G.; Sidoni, A.; Galarini, R.; Svegliati-Baroni, G.; et al. Nonalcoholic fatty liver disease impairs the cytochrome P-450-dependent metabolism of alpha-tocopherol (vitamin E). J. Nutr. Biochem. 2017, 47, 120–131. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Mavangira, V.; Sordillo, L.M. Invited review: Cytochrome P450 enzyme involvement in health and inflammatory-based diseases of dairy cattle. J. Dairy Sci. 2021, 104, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007, 27, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; Takada, T.; Yamanashi, Y.; Suzuki, H. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol. Pharmacol. 2008, 74, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Vitamin E intestinal absorption: Regulation of membrane transport across the enterocyte. IUBMB Life 2019, 71, 416–423. [Google Scholar] [CrossRef]
- Weiss, W.P.; Hogan, J.S.; Smith, K.L.; Todhunter, D.A.; Williams, S.N. Effect of supplementing periparturient cows with vitamin E on distribution of alpha-tocopherol in blood. J. Dairy Sci. 1992, 75, 3479–3485. [Google Scholar] [CrossRef]
- Weiss, W.P.; Hogan, J.S.; Smith, K.L.; Williams, S.N. Effect of dietary fat and vitamin E on alpha-tocopherol and beta-carotene in blood of peripartum cows. J. Dairy Sci. 1994, 77, 1422–1429. [Google Scholar] [CrossRef]
- Weiss, W.P.; Hogan, J.S.; Todhunter, D.A.; Smith, K.L. Effect of vitamin E supplementation in diets with a low concentration of selenium on mammary gland health of dairy cows. J. Dairy Sci. 1997, 80, 1728–1737. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Batra, T.R.; Zhao, X. Bioavailability of vitamin E compounds and the effect of supplementation on release of superoxide and hydrogen peroxide by bovine neutrophils. J. Dairy Sci. 1997, 80, 187–193. [Google Scholar] [CrossRef]
- Chawla, R.; Kaur, H. Plasma antioxidant vitamin status of periparturient cows supplemented with α-tocopherol and β-carotene. Anim. Feed Sci. Technol. 2004, 114, 279–285. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Herdt, T.H.; Seymour, W.M.; Duffield, T.F.; Leslie, K.E. Peripartum serum vitamin E, retinol, and beta-carotene in dairy cattle and their associations with disease. J. Dairy Sci. 2004, 87, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Calderon, F.; Chauveau-Duriot, B.; Martin, B.; Graulet, B.; Doreau, M.; Nozière, P. Variations in carotenoids, vitamins A and E, and color in cow’s plasma and milk during late pregnancy and the first three months of lactation. J. Dairy Sci. 2007, 90, 2335–2346. [Google Scholar] [CrossRef]
- Strickland, J.M.; Wisnieski, L.; Herdt, T.H.; Sordillo, L.M. Serum retinol, beta-carotene, and alpha-tocopherol as biomarkers for disease risk and milk production in periparturient dairy cows. J. Dairy Sci. 2021, 104, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Ashutosh; Chandra, G.; Singh, A.K. Heat shock protein 70, oxidative stress, and antioxidant status in periparturient crossbred cows supplemented with alpha-tocopherol acetate. Trop. Anim. Health Prod. 2013, 45, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, R.J.; Goselink, R.M.; Dobbelaar, P.; Nielen, M.; Newbold, J.R.; van Werven, T. The relationship between oxidative damage and vitamin E concentration in blood, milk, and liver tissue from vitamin E supplemented and nonsupplemented periparturient heifers. J. Dairy Sci. 2008, 91, 977–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, N.; Wathes, D.C.; Lawrence, K.E.; McGowan, M.; Laven, R.A. The effect of parenteral supplementation of vitamin E with selenium on the health and productivity of dairy cattle in the UK. Vet. J. 2008, 177, 381–387. [Google Scholar] [CrossRef]
- Weiss, W.P.; Hogan, J.S.; Wyatt, D.J. Relative bioavailability of all-rac and RRR vitamin E based on neutrophil function and total alpha-tocopherol and isomer concentrations in periparturient dairy cows and their calves. J. Dairy Sci. 2009, 92, 720–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politis, I.; Hidiroglou, M.; Batra, T.R.; Gilmore, J.A.; Gorewit, R.C.; Scherf, H. Effects of vitamin E on immune function of dairy cows. Am. J. Vet. Res. 1995, 56, 179–184. [Google Scholar] [PubMed]
- Politis, I.; Hidiroglou, N.; White, J.H.; Gilmore, J.A.; Williams, S.N.; Scherf, H.; Frigg, M. Effects of vitamin E on mammary and blood leukocyte function, with emphasis on chemotaxis, in periparturient dairy cows. Am. J. Vet. Res. 1996, 57, 468–471. [Google Scholar]
- Politis, I.; Hidiroglou, N.; Cheli, F.; Baldi, A. Effects of vitamin E on urokinase-plasminogen activator receptor expression by bovine neutrophils. Am. J. Vet. Res. 2001, 62, 1934–1938. [Google Scholar] [CrossRef]
- Politis, I.; Bizelis, I.; Tsiaras, A.; Baldi, A. Effect of vitamin E supplementation on neutrophil function, milk composition and plasmin activity in dairy cows in a commercial herd. J. Dairy Res. 2004, 71, 273–278. [Google Scholar] [CrossRef]
- McNaughton, A.P.; Murray, R.D. Structure and function of the bovine fetomaternal unit in relation to the causes of retained fetal membranes. Vet. Rec. 2009, 165, 615–622. [Google Scholar] [CrossRef]
- Bourne, N.; Laven, R.; Wathes, D.C.; Martinez, T.; McGowan, M. A meta-analysis of the effects of Vitamin E supplementation on the incidence of retained foetal membranes in dairy cows. Theriogenology 2007, 67, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, A.; Saranjam, N.; Amuoghli Tabrizi, B. Antioxidant Concentration Status in the Serum of Cows with Left Displacement Abomasom. Glob. Vet. 2011, 7, 478–481. [Google Scholar]
- Pontes, G.C.; Monteiro, P.L., Jr.; Prata, A.B.; Guardieiro, M.M.; Pinto, D.A.; Fernandes, G.O.; Wiltbank, M.C.; Santos, J.E.; Sartori, R. Effect of injectable vitamin E on incidence of retained fetal membranes and reproductive performance of dairy cows. J. Dairy Sci. 2015, 98, 2437–2449. [Google Scholar] [CrossRef]
- Moghimi-Kandelousi, M.; Alamouti, A.A.; Imani, M.; Zebeli, Q. A meta-analysis and meta-regression of the effects of vitamin E supplementation on serum enrichment, udder health, milk yield, and reproductive performance of transition cows. J. Dairy Sci. 2020, 103, 6157–6166. [Google Scholar] [CrossRef]
- Goff, J.P.; Kimura, K.; Horst, R.L. Effect of mastectomy on milk fever, energy, and vitamins A, E, and beta-carotene status at parturition. J. Dairy Sci. 2002, 85, 1427–1436. [Google Scholar] [CrossRef]
- Miyaji, M.; Haga, S.; Matsuyama, H.; Hosoda, K. Effect of feeding brown rice instead of corn on lactation performance and blood metabolites in periparturient dairy cows. Anim. Feed Sci. Technol. 2016, 219, 234–240. [Google Scholar] [CrossRef]
- Hymøller, L.; Jensen, S.K. Stability in the rumen and effect on plasma status of single oral doses of vitamin D and vitamin E in high-yielding dairy cows. J. Dairy Sci. 2010, 93, 5748–5757. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ozai, R.; Sugino, T.; Kawashima, K.; Kushibiki, S.; Kim, Y.H.; Sato, S. Changes in peripheral blood oxidative stress markers and hepatic gene expression related to oxidative stress in Holstein cows with and without subacute ruminal acidosis during the periparturient period. J. Vet. Med. Sci. 2020, 82, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Alderson, N.E.; Mitchell, G.E., Jr.; Little, C.O.; Warner, R.E.; Tucker, R.E. Preintestinal disappearance of vitamin E in ruminants. J. Nutr. 1971, 101, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Bernabucci, U.; Ronchi, B.; Basiricò, L.; Pirazzi, D.; Rueca, F.; Lacetera, N.; Nardone, A. Abundance of mRNA of apolipoprotein B100, apolipoprotein E, and microsomal triglyceride transfer protein in liver from periparturient dairy cows. J. Dairy Sci. 2004, 87, 2881–2888. [Google Scholar] [CrossRef] [Green Version]
- Pilotto, A.; Savoini, G.; Baldi, A.; Invernizzi, G.; De Vecchi, C.; Theodorou, G.; Koutsouli, P.; Politis, I. Short communication: Associations between blood fatty acids, beta-hydroxybutyrate, and alpha-tocopherol in the periparturient period in dairy cows: An observational study. J. Dairy Sci. 2016, 99, 8121–8126. [Google Scholar] [CrossRef]
- Katoh, N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002, 64, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; O’Boyle, N.; Gandy, J.C.; Corl, C.M.; Hamilton, E. Shifts in thioredoxin reductase activity and oxidant status in mononuclear cells obtained from transition dairy cattle. J. Dairy Sci. 2007, 90, 1186–1192. [Google Scholar] [CrossRef]
- Konvičná, J.; Vargová, M.; Paulíková, I.; Kováč, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animals 2013, 7, 1374–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, S.; Prasdini, W.A.; Djati, M.S.; Prasetyawan, S.; Ciptad, G. Malondialdehyde (MDA) level and protein profile of serum after calving towards the provision of selenium-vitamin E™ on dairy cow frisian holstein (FH). J. Phys. Conf. Ser. 2019, 1146. [Google Scholar] [CrossRef] [Green Version]
- Khatti, A.; Mehrotra, S.; Patel, P.K.; Singh, G.; Maurya, V.P.; Mahla, A.S.; Chaudhari, R.K.; Das, G.K.; Singh, M.; Sarkar, M.; et al. Supplementation of vitamin E, selenium and increased energy allowance mitigates the transition stress and improves postpartum reproductive performance in the crossbred cow. Theriogenology 2017, 104, 142–148. [Google Scholar] [CrossRef]
- Gessner, D.K.; Schlegel, G.; Ringseis, R.; Schwarz, F.J.; Eder, K. Up-regulation of endoplasmic reticulum stress induced genes of the unfolded protein response in the liver of periparturient dairy cows. BMC Vet. Res. 2014, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef] [Green Version]
- Hidiroglou, M. Mammary transfer of vitamin E in dairy cows. J. Dairy Sci. 1989, 72, 1067–1071. [Google Scholar] [CrossRef]
- Linzell, J.L. Mammary blood flow and methods of identifying and measuring precursors of milk. In Lactation: A Comprehensive Treatise; Larson, B.L., Smith, V.R., Eds.; Academic Press: New York, NY, USA, 1974; Volume 1, pp. 143–225. [Google Scholar]
- Delamaire, E.; Guinard-Flament, J. Longer milking intervals alter mammary epithelial permeability and the udder’s ability to extract nutrients. J. Dairy Sci. 2006, 89, 2007–2016. [Google Scholar] [CrossRef]
- Ontsouka, E.C.; Albrecht, C. Cholesterol transport and regulation in the mammary gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, T.A.; Lippolis, J.D. Developmental changes in the milk fat globule membrane proteome during the transition from colostrum to milk. J. Dairy Sci. 2008, 91, 2307–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffari, M.H.; Bernhöft, K.; Etheve, S.; Immig, I.; Hölker, M.; Sauerwein, H.; Schweigert, F.J. Technical note: Rapid field test for the quantification of vitamin E, beta-carotene, and vitamin A in whole blood and plasma of dairy cattle. J. Dairy Sci. 2019, 102, 11744–11750. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haga, S.; Ishizaki, H.; Roh, S. The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows. Animals 2021, 11, 1088. https://doi.org/10.3390/ani11041088
Haga S, Ishizaki H, Roh S. The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows. Animals. 2021; 11(4):1088. https://doi.org/10.3390/ani11041088
Chicago/Turabian StyleHaga, Satoshi, Hiroshi Ishizaki, and Sanggun Roh. 2021. "The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows" Animals 11, no. 4: 1088. https://doi.org/10.3390/ani11041088
APA StyleHaga, S., Ishizaki, H., & Roh, S. (2021). The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows. Animals, 11(4), 1088. https://doi.org/10.3390/ani11041088





