Microvascularization and Expression of Fibroblast Growth Factor and Vascular Endothelial Growth Factor and Their Receptors in the Mare Oviduct
Abstract
:Simple Summary
Abstract
1. Introduction
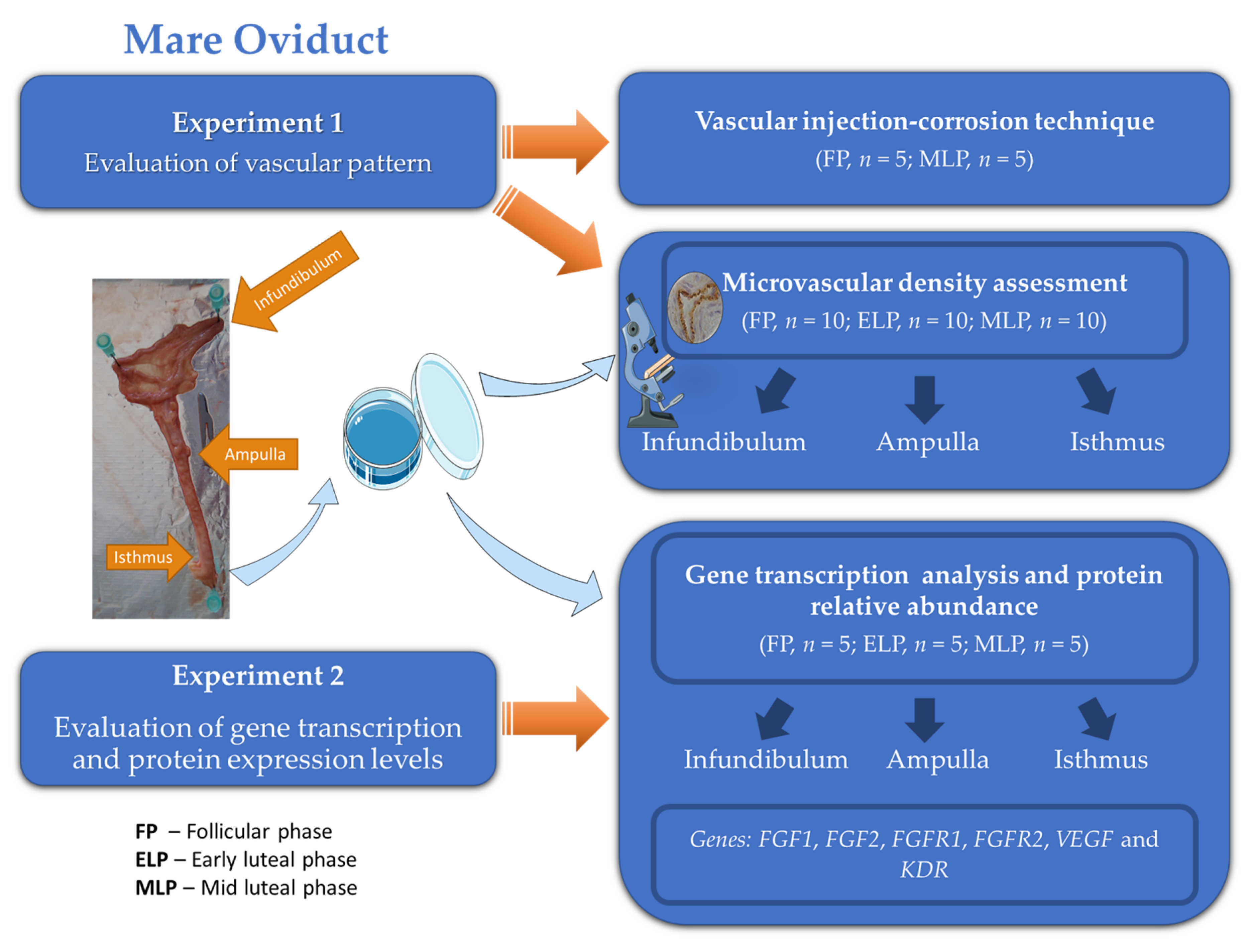
2. Materials and Methods
2.1. Collection of Mare Oviducts
2.2. Experiment 1: Evaluation of Vascular Pattern
2.2.1. Vascular Injection-Corrosion Technique
2.2.2. Microvascular Density Assessment
2.3. Experiment 2
2.3.1. Gene Transcription Analysis
2.3.2. Relative Protein Abundance
2.4. Statistical Analysis
3. Results
3.1. Experiment 1: Evaluation of Vascularization Pattern
3.1.1. Vascular Injection-Corrosion Technique
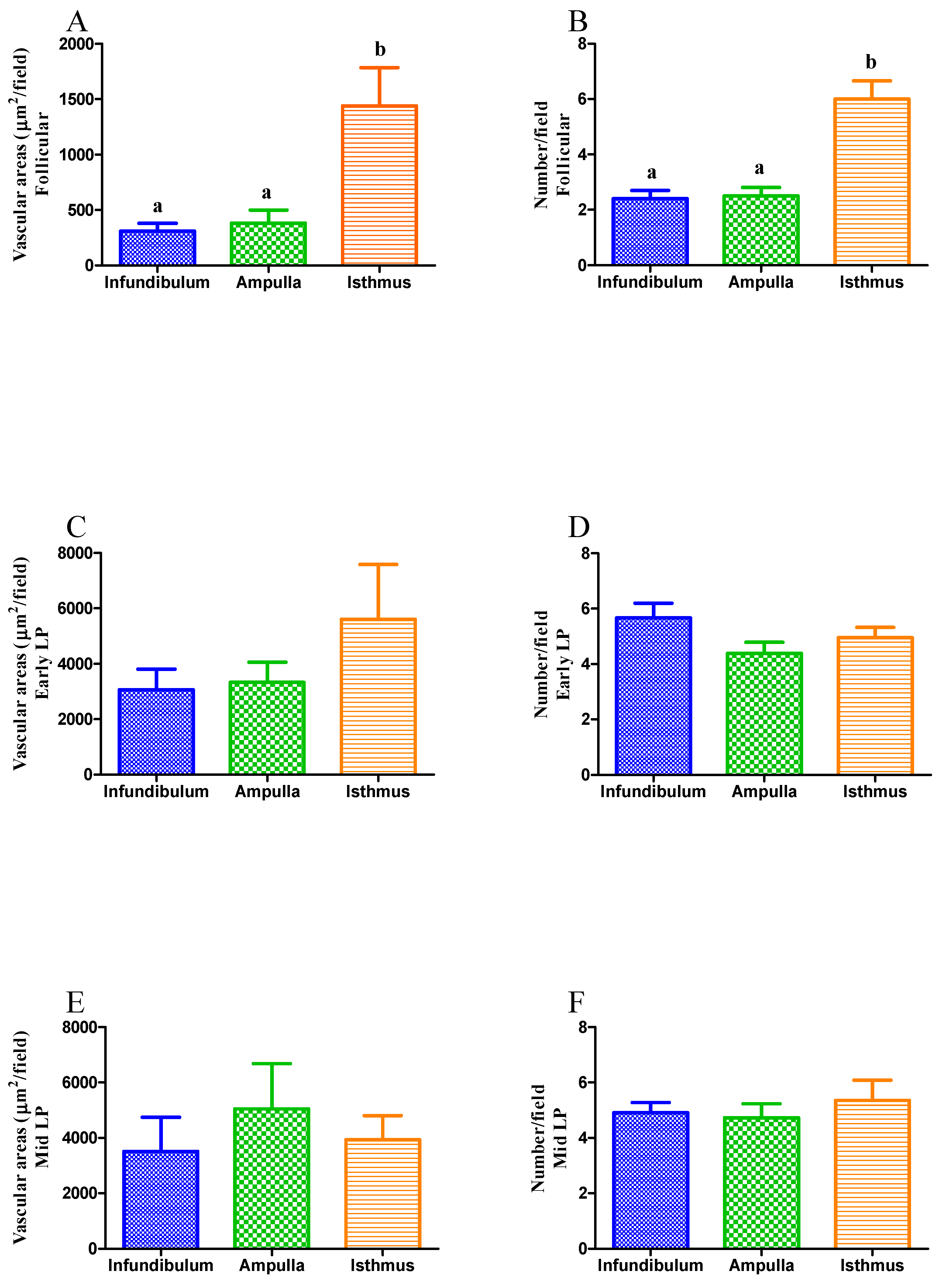
3.1.2. Microvascular Density
3.2. Experiment 2
3.2.1. Transcription of Angiogenic Growth Factors and Their Receptors
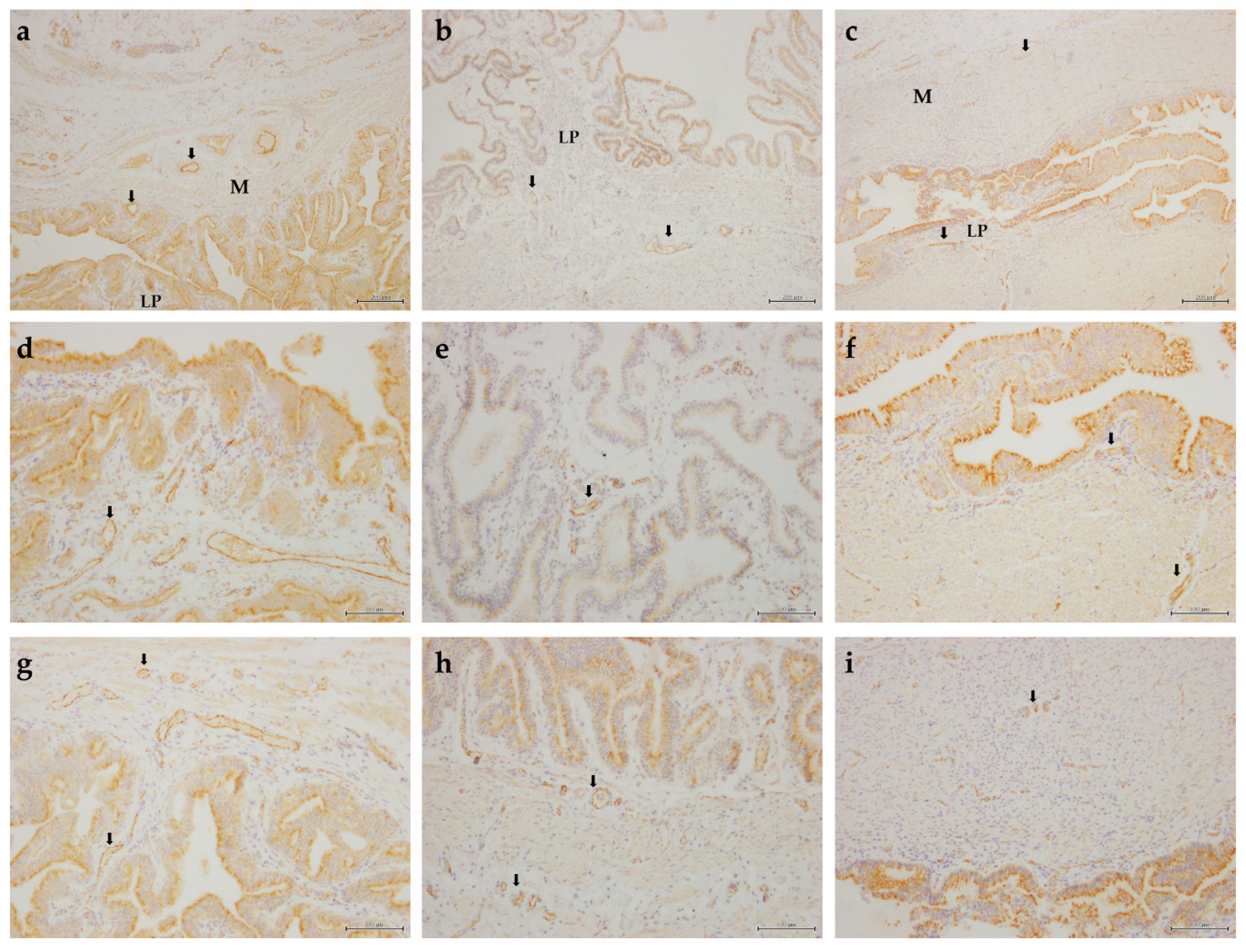
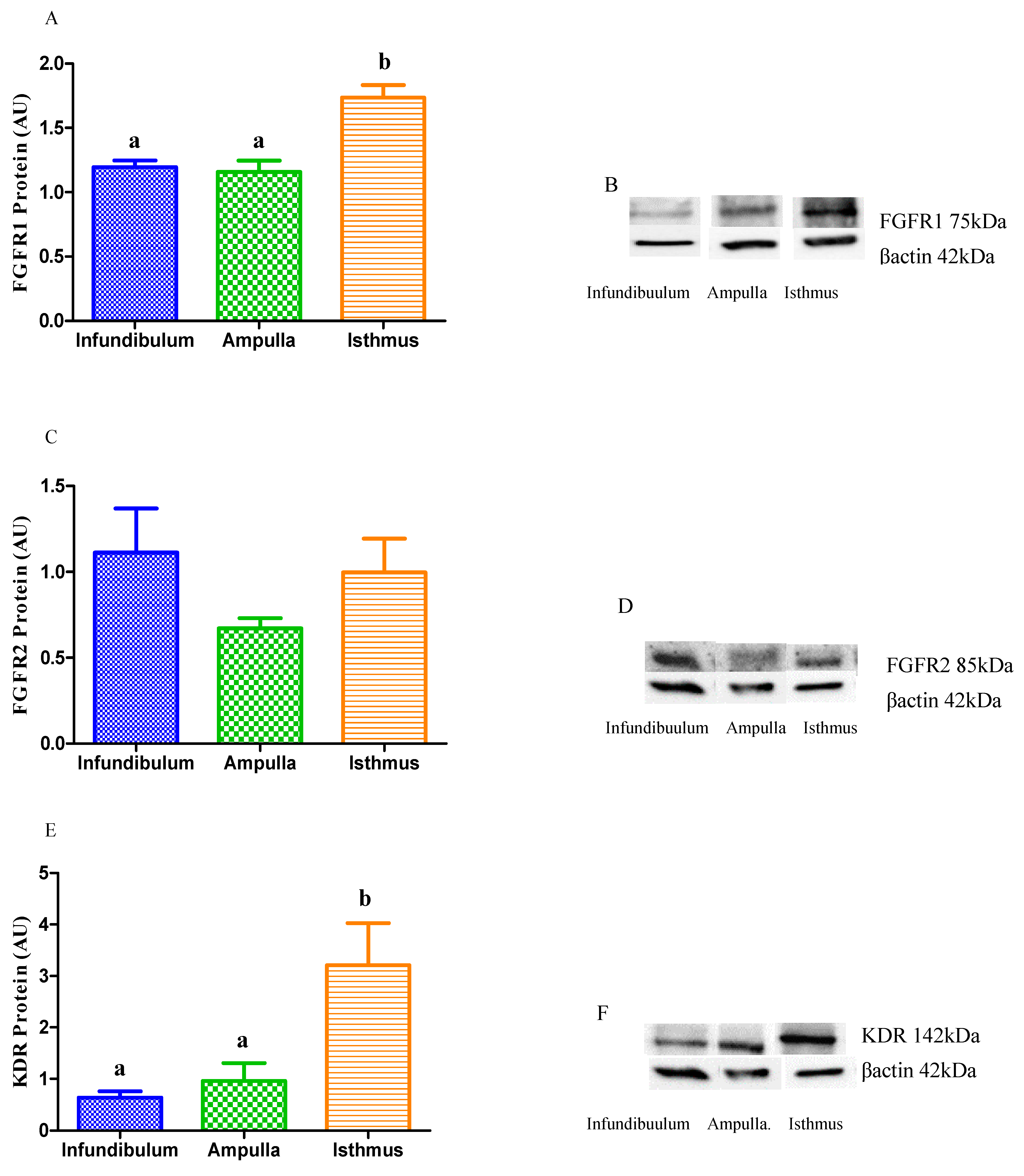
3.2.2. Relative Protein Abundance of Angiogenic Growth Factor Receptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gadella, B.M.; Rathi, R.; Brouwers, J.F.H.M.; Stout, T.A.E.; Colenbrander, B. Capacitation and the acrosome reaction in equine sperm. Anim. Reprod. Sci. 2001, 68, 249–265. [Google Scholar] [CrossRef]
- Goudet, G. Fertilisation in the horse and paracrine signalling in the oviduct. Reprod. Fertil. Dev. 2011, 23, 941–951. [Google Scholar] [CrossRef]
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; De Schauwer, C.; Nelis, H.; Hoogewijs, M.; Van Soom, A. Why doesn’t conventional IVF work in the horse? The equine oviduct as a microenvironment for capacitation/fertilization. Reproduction 2016, 152, R233–R245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cerezales, S.; Ramos-Ibeas, P.; Acuña, O.S.; Avilés, M.; Coy, P.; Rizos, D.; Gutiérrez-Adán, A. The oviduct: From sperm selection to the epigenetic landscape of the embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the early embryonic microenvironment: Physiology and regulation of oviductal secretions. Int. J. Mol. Sci. 2020, 21, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelis, H.; Vanden-Bussche, J.; Wojciechowicz, B.; Franczak, A.; Leemans, B.; Vanhaecke, L.; Cornillie, P.; Peelman, L.; Van Soom, A.; Smits, K. Steroids in the equine oviduct: Synthesis, local concentrations and receptor expression. Reprod. Fertil. Dev. 2015, 28, 1390–1404. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.P.; Killilea, S.D.; Redmer, D.A. Angiogenesis in the female reproductive system. FASEB J. 1992, 6, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Redmer, D.A.; Reynolds, L.P. Angiogenesis in the ovary. Rev. Reprod. 1996, 1, 182–192. [Google Scholar] [CrossRef]
- Yao, C.; Narumiya, S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br. J. Pharmacol. 2019, 176, 337–354. [Google Scholar] [CrossRef]
- López Albors, O.; Olsson, F.; Llinares, A.B.; Gutiérrez, H.; Latorre, R.; Candanosa, E.; Guillén-Martínez, A.; Izquierdo-Rico, M.J. Expression of the vascular endothelial growth factor system (VEGF) in the porcine oviduct during the estrous cycle. Theriogenology 2017, 93, 46–54. [Google Scholar] [CrossRef]
- Vailhé, B.; Feige, J.J. Thrombospondins as anti-angiogenic therapeutic agents. Curr. Pharm. Des. 2003, 9, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Brigati, C.; Ventura, A.; Lorusso, G.; Pinter, M.; Morini, M.; Mancino, A.; Sica, A.; Noonan, D.M. Angiostatin anti-angiogenesis requires IL-12: The innate immune system as a key target. J. Transl. Med. 2009, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Garside, S.A.; Harlow, C.R.; Hillier, S.G.; Fraser, H.M.; Thomas, F.H. Thrombospondin-1 inhibits angiogenesis and promotes follicular atresia in a novel in vitro angiogenesis assay. Endocrinology 2010, 151, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.M.; Briton-Jones, C.; Cheung, C.K.; Lok, I.H.; Yuen, P.M.; Cheung, L.P.; Haines, C. Vascular endothelial growth factor in the human oviduct: Localization and regulation of messenger RNA expression in vivo. Biol. Reprod. 2003, 68, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Wollenhaupt, K.; Welter, H.; Einspanier, R.; Manabe, N.; Brüssow, K.-P. Expression of epidermal growth factor receptor (EGF-R), vascular endothelial growth factor receptor (VEGF-R) and fibroblast growth factor receptor (FGF-R) systems in porcine oviduct and endometrium during the time of implantation. J. Reprod. Dev. 2004, 50, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberto-da-Costa, R.P.; Ferreira-Dias, G.; Mateus, L.; Korzekwa, A.; Andronowska, A.; Platek, R.; Skarzynski, D.J. Endometrial nitric oxide production and nitric oxide synthases in the equine endometrium: Relationship with microvascular density during the estrous cycle. Domest. Anim. Endocrinol. 2007, 32, 287–302. [Google Scholar] [CrossRef]
- Roberto-da-Costa, R.P.; Costa, A.S.; Korzekwa, A.J.; Platek, R.; Siemieniuch, M.; Galvão, A.; Redmer, D.A.; Silva, J.R.; Skarzynski, D.J.; Ferreira-Dias, G. Actions of a nitric oxide donor on prostaglandin production and angiogenic activity in the equine endometrium. Reprod. Fertil. Dev. 2008, 20, 674–683. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhou, W.-J.; Luo, X.-Z.; Lao, Y.; Li, D.-J. Synergistic effect of regulatory T cells and proinflammatory cytokines in angiogenesis in the endometriotic milieu. Hum. Reprod. 2017, 32, 1304–1317. [Google Scholar] [CrossRef]
- Adair, T.H.; Montani, J.P. Regulation: Metabolic Factors. In Angiogenesis; Chapter 3; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- García-Martínez, S.; Sánchez Hurtado, M.A.; Gutiérrez, H.; Sánchez Margallo, F.M.; Romar, R.; Latorre, R.; Coy, P.; López Albors, O. Mimicking physiological O2 tension in the female reproductive tract improves assisted reproduction outcomes in pig. Mol. Hum. Reprod. 2018, 24, 260–270. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Godin, P.; Tsoi, M.; Paquet, M.; Boerboom, D. YAP and TAZ are required for the postnatal development and the maintenance of the structural integrity of the oviduct. Reproduction 2020, 160, 307–318. [Google Scholar] [CrossRef]
- Hooglugt, A.; van der Stoel, M.M.; Boon, R.A.; Huveneers, S. Endothelial YAP/TAZ Signaling in Angiogenesis and Tumor Vasculature. Front. Oncol. 2021, 10, 612802. [Google Scholar] [CrossRef]
- Folkman, J.; Klagsburn, M. Angiogenic factors. Science 1987, 235, 442–447. [Google Scholar] [CrossRef]
- Turner, N.; Lambros, M.B.; Horlings, H.M.; Pearson, A.; Sharpe, R.; Natrajan, R.; Geyer, F.C.; van Kouwenhove, M.; Kreike, B.; Mackay, A.; et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene 2010, 29, 2013–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvão, A.; Henriques, S.; Pestka, D.; Lukasik, K.; Skarzynski, D.J.; Mateus, L.; Ferreira-Dias, G. Equine luteal function regulation may depend on the interaction between cytokines and vascular endothelial growth factor: An in vitro study. Biol. Reprod. 2012, 86, 187. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.M.; Ferreira-Dias, G.; Skarzynski, D.J. Cytokines and angiogenesis in the corpus luteum. Mediat. Inflamm. 2013, 2013, 420186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvão, A.M.; Skarzynski, D.; Ferreira-Dias, G. Luteolysis and the auto-paracrine role of cytokines from tumor necrosis factor α and transforming growth factor β Superfamilies. Vitam. Horm. 2018, 107, 287–315. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.P.; Talbi, S.; Hamilton, A.E.; Baston-Buest, D.M.; Nyegaard, M.; Irwin, J.C.; Barragan, F.; Kruessel, J.S.; Germeyer, A.; Giudice, L.C. The human oviduct transcriptome reveals an anti-inflammatory, anti-angiogenic, secretory and matrix-stable environment during embryo transit. Reprod. Biomed. Online 2013, 27, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Stuttfeld, E.; Ballmer-Hofer, K. Structure and function of VEGF receptors. IUBMB Life 2009, 61, 915–922. [Google Scholar] [CrossRef]
- Wijayagunawardane, M.P.B.; Kodithuwakku, S.P.; Yamamoto, D.; Miyamoto, A. Vascular endothelial growth factor system in the cow oviduct: A possible involvement in the regulation of oviductal motility and embryo transport. Mol. Reprod. Dev. 2005, 72, 511–520. [Google Scholar] [CrossRef]
- Małysz-Cymborska, I.; Andronowska, A. Expression of the vascular endothelial growth factor receptor system in porcine oviducts after induction of ovulation and superovulation. Domest. Anim. Endocrinol. 2014, 49, 86–95. [Google Scholar] [CrossRef]
- Javerzat, S.; Auguste, P.; Bikfalvi, A. The role of fibroblast growth factors in vascular development. Trends Mol. Med. 2002, 8, 483–489. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesso-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Berisha, B.; Sinowatz, F.; Schams, D. Expression and localization of fibroblast growth factor (FGF) family members during the final growth of bovine ovarian follicles. Mol. Reprod. Dev. 2004, 67, 162–171. [Google Scholar] [CrossRef]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/ fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Dias, G.; Serrão, P.M.; Costa Durão, J.; Robalo Silva, J. Microvascular development and growth of the uterus during the estrous cycle in the mare. Am. J. Vet. Res. 2001, 62, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Dias, G.; Pinto Bravo, P.; Mateus, L.; Redmer, D.A.; Medeiros, J.A. Microvascularization and angiogenic activity of equine corpora lutea throughout the estrous cycle. Domest. Anim. Endocrinol. 2006, 30, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of anti-fibrotic PGE2 pathway may influence neutrophil extracellular traps-induced fibrosis in the mare endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Alexandre-Pires, G. Vascularization of rabbit term placenta (Oryctolagus cuniculus). Braz. J. Morphol. Sci. 1998, 15, 95–106. [Google Scholar]
- Pinto-Bravo, P.; Galvão, A.; Rebordão, M.R.; Amaral, A.; Ramilo, D.; Silva, E.; Szóstek- Mioduchowska, A.; Alexandre-Pires, G.; Roberto-da-Costa, R.; Skarzynski, D.J.; et al. Ovarian steroids, oxytocin, and tumor necrosis factor modulate equine oviduct function. Domest. Anim. Endocrinol. 2017, 61, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Verco, C.J.; Gannon, B.J.; Jones, W.R. Variations in rabbit oviduct microvascular architecture after ovulation induced by hCG. J. Reprod. Fertil. 1984, 72, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verco, C.J.; Gannon, B.J.; Jones, W.R. Microvascular architecture of the pregnant rabbit oviduct. Acta Anat. 1984, 118, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.K.; Gerhardt, H. Angiogenesis: A team effort coordinated by notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Gabler, C.; Einspanier, A.; Schams, D.; Einspanier, R. Expression of vascular endothelial growth factor (VEGF) and its corresponding receptors (flt-1 and flk-1) in the bovine oviduct. Mol. Reprod. Dev. 1999, 34, 733–745. [Google Scholar] [CrossRef]
- Lam, P.M.; Briton-Jones, C.; Cheung, C.K.; Lok, I.H.; Cheung, L.P.; Haines, C. In vivo regulation of mRNA expression of vascular endothelial growth factor receptors (KDR and flt-1) in the human oviduct. Fertil. Steril. 2004, 81, 416–423. [Google Scholar] [CrossRef]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Baffert, F.; Le, T.; Sennino, B.; Thurston, G.; Kuo, C.; Hu-Lowe, D.; McDonald, M. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H547–H559. [Google Scholar] [CrossRef] [Green Version]
- Sia, D.; Alsinet, C.; Newell, P.; Villanueva, A. VEGF signaling in cancer treatment. Curr. Pharm. Des. 2014, 20, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, Y.; Nishimura, R.; Shibaya, M.; Lee, H.; Acosta, T.; Okuda, K. Expression of VEGF and its receptors in the bovine endometrium throughout the estrous cycle: Effects of VEGF on prostaglandin production in endometrial cells. J. Reprod. Dev. 2010, 56, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thisse, B.; Thisse, C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 2005, 287, 390–402. [Google Scholar] [CrossRef]
- Katsahambas, S.; Hearn, M.T. Localization of basic fibroblast growth factor mRNA (FGF-2 mRNA) in the uterus of mated and unmated gilts. J. Histochem. Cytochem. 1996, 44, 1289–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Bazer, F.W.; Jaeger, L.A. Immunolocalization of acidic and basic fibroblast growth factors in porcine uterine and conceptus tissues. Biol. Reprod. 1997, 56, 1527–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazul-Bilska, A.T.; Redmer, D.A.; Jablonka-Shariff, A.; Biondini, M.E.; Reynolds, L.P. Proliferation and progesterone production of ovine luteal cells from several stages of the estrous cycle: Effects of fibroblast growth factors (FGF) and luteinizing hormone (LH). Can. J. Physiol. Pharmacol. 1995, 73, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Gabler, C.; Lauer, B.; Einspanier, A.; Schams, D.; Einspanier, R. Detection of mRNA and immunoreactive proteins for acidic and basic fibroblast growth factor and expression of the fibroblast growth factor receptors in the bovine oviduct. J. Reprod. Fertil. 1979, 109, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saucedo, L.; Sobarzo, C.; Brukman, N.G.; Guidobaldi, H.A.; Lustig, L.; Giojalas, L.C.; Buffone, M.G.; Vazquez-Levin, M.H.; Marín-Briggiler, C. Involvement of fibroblast growth factor 2 (FGF2) and its receptors in the regulation of mouse sperm physiology. Reproduction 2018, 156, 163–172. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Redmer, D.A.; Reynolds, L.P. Growth factors during ovarian angiogenesis. In Vascular Morphogenesis in the Female Reproductive System; Augustin, H.G., Iruela-Arispe, M.L., Roger, P.A.W., Smith, S.K., Eds.; Birkhauser: Boston, MA, USA, 2001; pp. 131–147. [Google Scholar]



| Gene (Accession Number) | Sequence 5′–3′ | Tm (°C) a | Amplicon (Base Pairs) |
|---|---|---|---|
| FGF1 (XM_005599133) | Forward: GTGGATGGGACAAGGGACAG | 58.8 | 187 |
| Reverse: GGTTTTCCTCCAGCCTTTCC | 58.6 | ||
| FGF2 (NM_001195221) | Forward: GGAGAAGAGCGACCCTCACA | 59 | 234 |
| Reverse: ATACTGCCCCGTTCGTTTCA | 59 | ||
| FGFR1 (XM_014736560) | Forward: ACCCAACCGTGTGACCAAAG | 59.3 | 260 |
| Reverse: GGTTGTGGCTGGGGTTGTAA | 59.3 | ||
| FGFR2 (XM_014732956) | Forward: CCAGCTCCTCCATGAACTCC | 58.3 | 237 |
| Reverse: TGACTGCTTCCTTGGGCTTC | 59 | ||
| VEGF (NM_001081821) | Forward: ATGCGGATCAAACCTCACCA | 59.9 | 117 |
| Reverse: AGGCCCACAGGGATTTTCTT | 58.5 | ||
| KDR (XM_014738773) | Forward: CTTCCAGTGGGCTGATGACC | 59.1 | 100 |
| Reverse: AGCTTCCACCGAAGATTCCA | 58.3 | ||
| β2M | Forward: CGGGCTACTCTCCCTGACTG | 58.5 | 92 |
| (X69083) | Reverse: TTGGCTTTCCATTCTCTGCTG | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Bravo, P.; Rebordão, M.R.; Amaral, A.; Fernandes, C.; Galvão, A.; Silva, E.; Pessa-Santos, P.; Alexandre-Pires, G.; Roberto da Costa, R.P.; Skarzynski, D.J.; et al. Microvascularization and Expression of Fibroblast Growth Factor and Vascular Endothelial Growth Factor and Their Receptors in the Mare Oviduct. Animals 2021, 11, 1099. https://doi.org/10.3390/ani11041099
Pinto-Bravo P, Rebordão MR, Amaral A, Fernandes C, Galvão A, Silva E, Pessa-Santos P, Alexandre-Pires G, Roberto da Costa RP, Skarzynski DJ, et al. Microvascularization and Expression of Fibroblast Growth Factor and Vascular Endothelial Growth Factor and Their Receptors in the Mare Oviduct. Animals. 2021; 11(4):1099. https://doi.org/10.3390/ani11041099
Chicago/Turabian StylePinto-Bravo, Pedro, Maria Rosa Rebordão, Ana Amaral, Carina Fernandes, António Galvão, Elisabete Silva, Pedro Pessa-Santos, Graça Alexandre-Pires, Rosário P. Roberto da Costa, Dariusz J. Skarzynski, and et al. 2021. "Microvascularization and Expression of Fibroblast Growth Factor and Vascular Endothelial Growth Factor and Their Receptors in the Mare Oviduct" Animals 11, no. 4: 1099. https://doi.org/10.3390/ani11041099






