Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Function and Structure of FSH
3. The Molecular Basis of FSH Synthesis Regulation
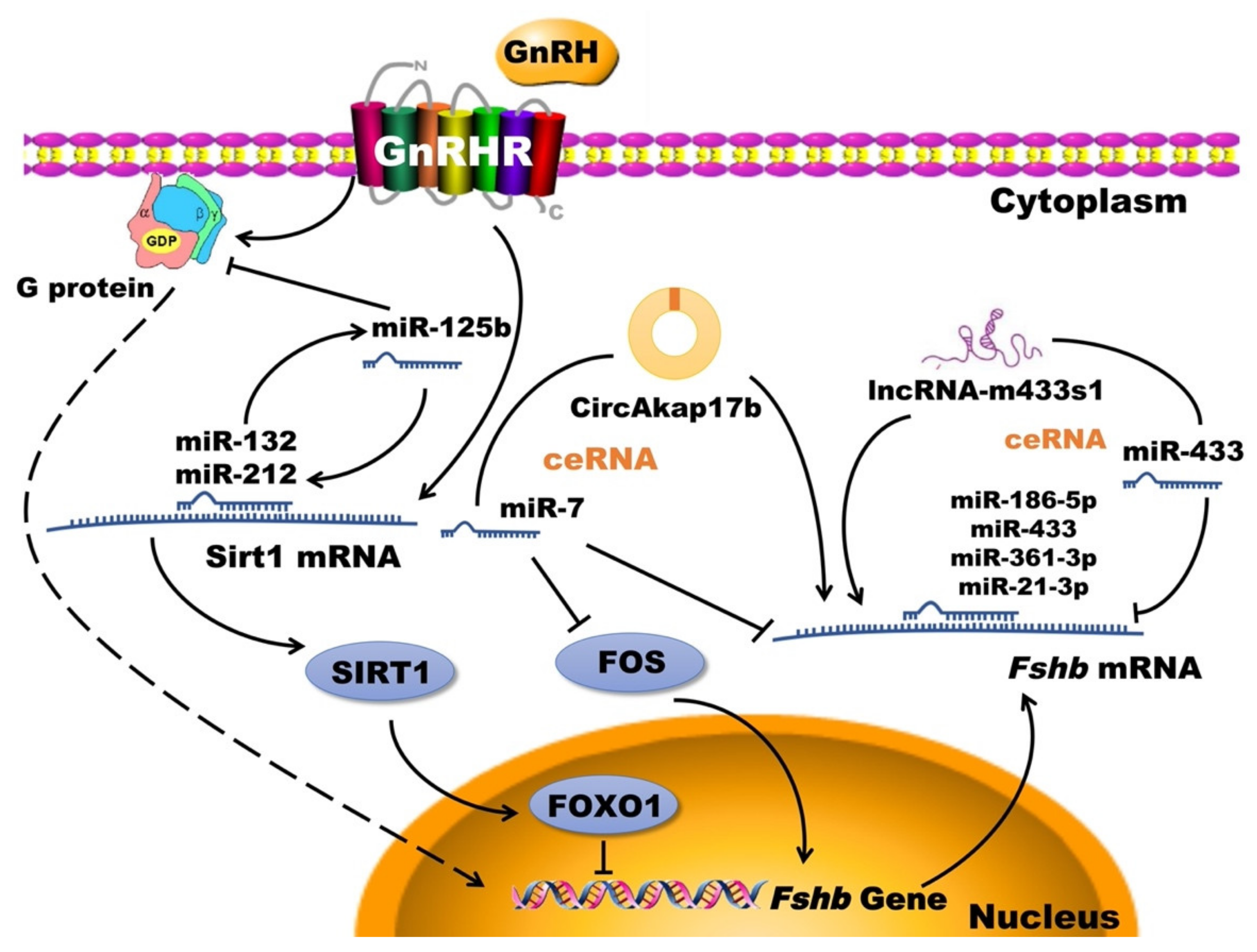
3.1. Gonadotropin-Releasing Hormone
3.2. Kisspeptin
3.3. Activin and Inhibin
3.4. Steroid Hormones
3.5. Pituitary Adenylate Cyclase Activating Polypeptide
3.6. Transcriptional Regulation of FSH Synthesis and Secretion
3.6.1. Activator Protein-1
3.6.2. FOXL2
3.6.3. Single Nucleotide Polymorphisms (SNPs) in the FSHR and FSHB Genes
3.7. Post-Transcriptional Regulation of FSH Synthesis and Secretion
3.7.1. Non-Coding RNA
3.7.2. Chromatin and Histone Modification
4. GnRH-Regulated FSH Synthesis and Secretion Signaling Pathways
4.1. cAMP/PKA/CREB Signaling Pathway
4.2. PKC/MAPK Signaling Pathway
4.3. Ca2+/CaMK II Signaling Pathway
5. Conclusions and Prospects
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AC | Adenylate cyclase |
| AP-1 | Activator protein-1 |
| ATP | Adeosine triphosphate |
| CaMK II | Calmodulin-dependent protein kinase II |
| cAMP | Cyclic adenosine monophosphate |
| ceRNA | Competing endogenous RNA |
| CHH | Congenital hypogonadotropic hypogonadism |
| CREs | cAMP responsive elements |
| CREB | cAMP response element binding |
| DAG | Diacylglycerol |
| DUOX | Dual oxidase |
| Dyn | Dynorphin |
| E2 | Estradiol |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| Egr1 | Early growth response protein 1 |
| ER | Endoplasmic reticulum |
| ERK | Extracellular regulated protein kinase |
| ERβ | Estrogen receptor β |
| FOXO1 | Forkhead box O1 |
| FSH | Follicle-stimulating hormone |
| FSHR | Follicle-stimulating hormone receptor |
| GABA | γ—aminobutyric acid |
| GnRH | Gonadotropin-releasing hormone |
| GnRHR | Gonadotropin-releasing hormone receptor |
| GPCR | G protein-coupled receptor |
| HPG | Hypothalamic-pituitary-gonadal |
| IHH | Idiopathic hypogonadotropic hypogonadism |
| IP3 | Inositol triphosphate |
| IP3R | Inositol triphosphate receptor |
| JNK | c-Jun N-terminal kinase |
| LH | Luteinizing hormone |
| MAPK | Mitogen-activated protein kinase |
| MEK | MAPK/ERK kinase |
| MKK | Mitogen-activated protein kinase kinase |
| NFAT | Nuclear factor of activated T-cells |
| NKB | Neurokinin B |
| NOX | NADPH oxidase |
| PACAP | Pituitary adenylate cyclase activating polypeptide |
| PCOS | Polycystic ovary syndrome |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| ROS | Reactive oxygen species |
| SAPK | Stress-activated protein kinase |
| SIRT1 | Silent information regulator 1 |
| SMAD2 | Drosophila mothers against decapentaplegic protein 2 |
| SMAD3 | Drosophila mothers against decapentaplegic protein 3 |
| SMAD4 | Drosophila mothers against decapentaplegic protein 4 |
| SNPs | Single nucleotide polymorphisms |
| TGF-β | Transforming growth factor-β |
| TSA | Trichostatin A |
References
- Hong, G.K.; Payne, S.C.; Jane, J.A., Jr. Anatomy, Physiology, and Laboratory Evaluation of the Pituitary Gland. Otolaryngol. Clin. N. Am. 2016, 49, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, R.B.; Mahesh, V.B. Pituitary-Ovarian Relationships. Metabolism 1965, 14, 320–326. [Google Scholar] [CrossRef]
- Baird, D.T.; Corker, C.S.; Davidson, D.W.; Hunter, W.M.; Michie, E.A.; Van Look, P.F. Pituitary-ovarian relationships in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1977, 45, 798–801. [Google Scholar] [CrossRef]
- Seibel, M.M.; Kamrava, M.M.; McArdle, C.; Taymor, M.L. Treatment of polycystic ovary disease with chronic low-dose follicle stimulating hormone: Biochemical changes and ultrasound correlation. Int. J. Fertil. 1984, 29, 39–43. [Google Scholar] [PubMed]
- Hamilton-Fairley, D.; Kiddy, D.; Watson, H.; Sagle, M.; Franks, S. Low-dose gonadotrophin therapy for induction of ovulation in 100 women with polycystic ovary syndrome. Hum. Reprod. 1991, 6, 1095–1099. [Google Scholar] [CrossRef]
- Rougier, C.; Hieronimus, S.; Panaia-Ferrari, P.; Lahlou, N.; Paris, F.; Fenichel, P. Isolated follicle-stimulating hormone (FSH) deficiency in two infertile men without FSH beta gene mutation: Case report and literature review. Ann. Endocrinol. 2019, 80, 234–239. [Google Scholar] [CrossRef]
- Attia, A.M.; Abou-Setta, A.M.; Al-Inany, H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst. Rev. 2013, CD005071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, M.; Brigante, G.; Rochira, V.; Santi, D.; Casarini, L. Prospects for FSH Treatment of Male Infertility. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Bern, H.A.; Geschwind, I.I.; Pierce, J.G.; Li, C.H. Pituitary hormones: International colloquium. Science 1969, 163, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Jia, J.; Pfeffer, R.L.; Sealfon, S.C. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J. Biol. Chem. 2012, 287, 21550–21560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.; Xiao, L.; Ge, W.; Yang, S.; Jiang, Y.; Lv, J.; Hu, J.; Zhang, Y.; Zhao, X.; Hua, Y. Follicle-stimulating hormone and luteinizing hormone regulate the synthesis mechanism of dihydrotestosterone in sheep granulosa cells. Reprod. Domest. Anim. 2021, 56, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Lazzaretti, C.; Paradiso, E.; Limoncella, S.; Riccetti, L.; Sperduti, S.; Melli, B.; Marcozzi, S.; Anzivino, C.; Sayers, N.S.; et al. Membrane Estrogen Receptor (GPER) and Follicle-Stimulating Hormone Receptor (FSHR) Heteromeric Complexes Promote Human Ovarian Follicle Survival. iScience 2020, 23, 101812. [Google Scholar] [CrossRef] [PubMed]
- Widayati, D.T.; Pangestu, M. Effect of follicle-stimulating hormone on Bligon goat oocyte maturation and embryonic development post in vitro fertilization. Vet. World 2020, 13, 2443–2446. [Google Scholar] [CrossRef] [PubMed]
- El-Hayek, S.; Demeestere, I.; Clarke, H.J. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc. Natl. Acad. Sci. USA 2014, 111, 16778–16783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, L.; Sun, Y.; Wu, D.; Li, J.; Zhu, L.; Jiang, S.; Pan, X. The optimized research of the in vitro culture of preantral follicles in mice. J. Clin. Lab. Anal. 2020, 34, e23498. [Google Scholar] [CrossRef] [PubMed]
- Smitz, J.; Andersen, A.N.; Devroey, P.; Arce, J.C.; Group, M. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum. Reprod. 2007, 22, 676–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, T.R.; Wang, Y.; Lu, N.; Matzuk, M.M. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997, 15, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Rahimova, A.; Kim, S.M.; Atabiekov, I.; Javaid, S.; Alamoush, B.; Taneja, C.; Khan, A.; Sun, L.; Azziz, R.; et al. FSH Beyond Fertility. Front. Endocrinol. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Bhartiya, D. Direct action of FSH on testicular stem cells. Stem Cell Res. Ther. 2019, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Boeri, L.; Capogrosso, P.; Salonia, A. Gonadotropin Treatment for the Male Hypogonadotropic Hypogonadism. Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Bhartiya, D. Testicular Stem Cells Express Follicle-Stimulating Hormone Receptors and Are Directly Modulated by FSH. Reprod. Sci. 2016, 23, 1493–1508. [Google Scholar] [CrossRef]
- Orth, J.M. The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology 1984, 115, 1248–1255. [Google Scholar] [CrossRef]
- Gautam, M.; Bhattacharya, I.; Rai, U.; Majumdar, S.S. Hormone induced differential transcriptome analysis of Sertoli cells during postnatal maturation of rat testes. PLoS ONE 2018, 13, e0191201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.; Xu, B.; Huang, W. A study on expression of FSH and its effects on the secretion of insulin and glucagon in rat pancreas. Tissue Cell 2010, 42, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Peng, Y.; Sharrow, A.C.; Iqbal, J.; Zhang, Z.; Papachristou, D.J.; Zaidi, S.; Zhu, L.L.; Yaroslavskiy, B.B.; Zhou, H.; et al. FSH directly regulates bone mass. Cell 2006, 125, 247–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.M.; Chan, H.C.; Ding, G.L.; Cai, J.; Song, Y.; Wang, T.T.; Zhang, D.; Chen, H.; Yu, M.K.; Wu, Y.T.; et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell 2015, 14, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Terado, Y.; Sakamaki, K.; Inoue, M.; Nakajima, A.; Lu, Y.; Hisasue, S.; Yamaguchi, R.; Muto, S.; Horie, S. Serum level of follicle-stimulating hormone is associated with extraprostatic extension of prostate cancer. Prostate Int. 2013, 1, 109–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousfield, G.R.; Dias, J.A. Synthesis and secretion of gonadotropins including structure-function correlates. Rev. Endocr. Metab. Disord. 2011, 12, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Das, N.; Kumar, T.R. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J. Mol. Endocrinol. 2018, 60, R131–R155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stilley, J.A.; Christensen, D.E.; Dahlem, K.B.; Guan, R.; Santillan, D.A.; England, S.K.; Al-Hendy, A.; Kirby, P.A.; Segaloff, D.L. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol. Reprod. 2014, 91, 74. [Google Scholar] [CrossRef] [Green Version]
- Belchetz, P.E.; Plant, T.M.; Nakai, Y.; Keogh, E.J.; Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 1978, 202, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.R.; Lainez, N.M.; Boehm, U.; Coss, D. GnRH Receptor Expression and Reproductive Function Depend on JUN in GnRH ReceptorExpressing Cells. Endocrinology 2018, 159, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Nakada, T.; Murata, K.; Ohkura, S.; Mogi, K.; Navarro, V.M.; Clifton, D.K.; Mori, Y.; Tsukamura, H.; Maeda, K.; et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010, 30, 3124–3132. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.N.; Di Giorgio, N.; Bonaventura, M.M.; Bettler, B.; Libertun, C.; Lux-Lantos, V.A. Lack of functional GABA(B) receptors alters GnRH physiology and sexual dimorphic expression of GnRH and GAD-67 in the brain. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E683–E696. [Google Scholar] [CrossRef]
- Hu, L.; Gustofson, R.L.; Feng, H.; Leung, P.K.; Mores, N.; Krsmanovic, L.Z.; Catt, K.J. Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Mol. Endocrinol. 2008, 22, 2250–2259. [Google Scholar] [CrossRef] [Green Version]
- Dalkin, A.C.; Haisenleder, D.J.; Ortolano, G.A.; Ellis, T.R.; Marshall, J.C. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology 1989, 125, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Wildt, L.; Hausler, A.; Marshall, G.; Hutchison, J.S.; Plant, T.M.; Belchetz, P.E.; Knobil, E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 1981, 109, 376–385. [Google Scholar] [CrossRef]
- Savoy-Moore, R.T.; Swartz, K.H. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Adv. Exp. Med. Biol. 1987, 219, 641–645. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell. Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef]
- Sperduti, S.; Limoncella, S.; Lazzaretti, C.; Paradiso, E.; Riccetti, L.; Turchi, S.; Ferrigno, I.; Bertacchini, J.; Palumbo, C.; Poti, F.; et al. GnRH Antagonists Produce Differential Modulation of the Signaling Pathways Mediated by GnRH Receptors. Int. J. Mol. Sci. 2019, 20, 5548. [Google Scholar] [CrossRef] [Green Version]
- Reame, N.E.; Sauder, S.E.; Case, G.D.; Kelch, R.P.; Marshall, J.C. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: Evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J. Clin. Endocrinol. Metab. 1985, 61, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mao, J.; Wu, X.; Nie, M.; Huang, B.; Xu, H.; Wang, X.; Zheng, J. Effectiveness and safety of pulsatile GnRH pump therapy on female patients with IHH. Zhonghua Yi Xue Za Zhi 2015, 95, 3432–3435. [Google Scholar] [PubMed]
- Dwyer, A.A.; Sykiotis, G.P.; Hayes, F.J.; Boepple, P.A.; Lee, H.; Loughlin, K.R.; Dym, M.; Sluss, P.M.; Crowley, W.F., Jr.; Pitteloud, N. Trial of recombinant follicle-stimulating hormone pretreatment for GnRH-induced fertility in patients with congenital hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 2013, 98, E1790–E1795. [Google Scholar] [CrossRef] [Green Version]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brezillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [Green Version]
- Shahab, M.; Mastronardi, C.; Seminara, S.B.; Crowley, W.F.; Ojeda, S.R.; Plant, T.M. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 2129–2134. [Google Scholar] [CrossRef] [Green Version]
- Castellano, J.M.; Navarro, V.M.; Fernandez-Fernandez, R.; Castano, J.P.; Malagon, M.M.; Aguilar, E.; Dieguez, C.; Magni, P.; Pinilla, L.; Tena-Sempere, M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol. Cell. Endocrinol. 2006, 257–258, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, K.; Herbison, A.E. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 2008, 149, 4605–4614. [Google Scholar] [CrossRef] [Green Version]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef]
- Mishra, G.K.; Patra, M.K.; Singh, L.K.; Sheikh, P.A.; Upmanyu, V.; Chakravarti, S.; Karikalan, M.; Sonwane, A.; Singh, S.K.; Das, G.K.; et al. Expression of Kisspeptin and its receptor in the hypothalamus of cyclic and acyclic buffalo (Bubalus bubalis). Theriogenology 2019, 139, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Cunningham, M.J.; Rissman, E.F.; Clifton, D.K.; Steiner, R.A. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005, 146, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Dungan, H.M.; Stoll, E.A.; Gottsch, M.L.; Braun, R.E.; Eacker, S.M.; Clifton, D.K.; Steiner, R.A. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005, 146, 2976–2984. [Google Scholar] [CrossRef]
- Smith, J.T.; Clay, C.M.; Caraty, A.; Clarke, I.J. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007, 148, 1150–1157. [Google Scholar] [CrossRef]
- Roa, J.; Vigo, E.; Castellano, J.M.; Gaytan, F.; Navarro, V.M.; Aguilar, E.; Dijcks, F.A.; Ederveen, A.G.; Pinilla, L.; van Noort, P.I.; et al. Opposite roles of estrogen receptor (ER)-alpha and ERbeta in the modulation of luteinizing hormone responses to kisspeptin in the female rat: Implications for the generation of the preovulatory surge. Endocrinology 2008, 149, 1627–1637. [Google Scholar] [CrossRef]
- Uenoyama, Y.; Nakamura, S.; Hayakawa, Y.; Ikegami, K.; Watanabe, Y.; Deura, C.; Minabe, S.; Tomikawa, J.; Goto, T.; Ieda, N.; et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J. Neuroendocrinol. 2015, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Guerriero, K.A.; Keen, K.L.; Kenealy, B.P.; Seminara, S.B.; Terasawa, E. Kisspeptin and Neurokinin B Signaling Network Underlies the Pubertal Increase in GnRH Release in Female Rhesus Monkeys. Endocrinology 2017, 158, 3269–3280. [Google Scholar] [CrossRef]
- Ayturk, N.; Firat, T.; Kukner, A.; Ozogul, C.; Tore, F.; Kandirali, I.E.; Yilmaz, B. The effect of kisspeptin on spermatogenesis and apoptosis in rats. Turk. J. Med. Sci. 2017, 47, 334–342. [Google Scholar] [CrossRef] [PubMed]
- El-Sherry, T.M.; Abdel-Ghani, M.A.; Mahmoud, G.B.; Ezzat, A.A. Kisspeptin injection improved the semen characteristics and sperm rheotaxis in Ossimi ram. Reprod. Domest. Anim. 2020, 55, 240–247. [Google Scholar] [CrossRef]
- Silveira, L.G.; Latronico, A.C.; Seminara, S.B. Kisspeptin and clinical disorders. Adv. Exp. Med. Biol. 2013, 784, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Ruiz, A.; Skorupskaite, K.; Gaytan, F.; Torres, E.; Perdices-Lopez, C.; Mannaerts, B.M.; Qi, S.; Leon, S.; Manfredi-Lozano, M.; Lopez-Rodriguez, C.; et al. Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum. Reprod. 2019, 34, 2495–2512. [Google Scholar] [CrossRef]
- George, J.T.; Veldhuis, J.D.; Roseweir, A.K.; Newton, C.L.; Faccenda, E.; Millar, R.P.; Anderson, R.A. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J. Clin. Endocrinol. Metab. 2011, 96, E1228–E1236. [Google Scholar] [CrossRef] [Green Version]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Barreiro, M.L.; Casanueva, F.F.; Aguilar, E.; Dieguez, C.; et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 2005, 146, 1689–1697. [Google Scholar] [CrossRef]
- Chu, Y.L.; Xu, Y.R.; Yang, W.X.; Sun, Y. The role of FSH and TGF-beta superfamily in follicle atresia. Aging 2018, 10, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bernard, D.J. Activin A induction of murine and ovine follicle-stimulating hormone beta transcription is SMAD-dependent and TAK1 (MAP3K7)/p38 MAPK-independent in gonadotrope-like cells. Cell Signal. 2012, 24, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Kojima, I.; Ogata, E. Activin-A: A modulator of multiple types of anterior pituitary cells. Biochem. Biophys. Res. Commun. 1988, 157, 48–54. [Google Scholar] [CrossRef]
- Suszko, M.I.; Balkin, D.M.; Chen, Y.; Woodruff, T.K. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Mol. Endocrinol. 2005, 19, 1849–1858. [Google Scholar] [CrossRef] [Green Version]
- Bernard, D.J. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol. Endocrinol. 2004, 18, 606–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, S.J.; Lacza, C.T.; Detz, A.A.; Xu, S.; Petrillo, L.A.; Kaiser, U.B. Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-beta gene. Mol. Endocrinol. 2005, 19, 237–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, R.S.; Corrigan, A.Z.; Vale, W.; Chin, W.W. Activin stabilizes follicle-stimulating hormone-beta messenger ribonucleic acid levels. Endocrinology 1991, 129, 1721–1726. [Google Scholar] [CrossRef]
- Rivier, C.; Rivier, J.; Vale, W. Inhibin-mediated feedback control of follicle-stimulating hormone secretion in the female rat. Science 1986, 234, 205–208. [Google Scholar] [CrossRef]
- Bernard, D.J.; Tran, S. Mechanisms of activin-stimulated FSH synthesis: The story of a pig and a FOX. Biol. Reprod. 2013, 88, 78. [Google Scholar] [CrossRef] [PubMed]
- Pierik, F.H.; Vreeburg, J.T.; Stijnen, T.; De Jong, F.H.; Weber, R.F. Serum inhibin B as a marker of spermatogenesis. J. Clin. Endocrinol. Metab. 1998, 83, 3110–3114. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Andersson, A.M.; Hjollund, N.H.; Scheike, T.; Kolstad, H.; Giwercman, A.; Henriksen, T.B.; Ernst, E.; Bonde, J.P.; Olsen, J.; et al. Inhibin B as a serum marker of spermatogenesis: Correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J. Clin. Endocrinol. Metab. 1997, 82, 4059–4063. [Google Scholar] [CrossRef] [Green Version]
- Hayes, F.J.; Pitteloud, N.; DeCruz, S.; Crowley, W.F., Jr.; Boepple, P.A. Importance of inhibin B in the regulation of FSH secretion in the human male. J. Clin. Endocrinol. Metab. 2001, 86, 5541–5546. [Google Scholar] [CrossRef]
- Fuller, P.J.; Chu, S.; Jobling, T.; Mamers, P.; Healy, D.L.; Burger, H.G. Inhibin subunit gene expression in ovarian cancer. Gynecol. Oncol. 1999, 73, 273–279. [Google Scholar] [CrossRef]
- Dean, M.; Davis, D.A.; Burdette, J.E. Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer Lett. 2017, 391, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Szabo, M.; Kilen, S.M.; Saberi, S.; Ringstrom, S.J.; Schwartz, N.B. Antiprogestins suppress basal and activin-stimulated follicle-stimulating hormone secretion in an estrogen-dependent manner. Endocrinology 1998, 139, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.A.; Padmanabhan, V.; Roche, J.F.; Crowe, M.A. Alterations in the ability of the bovine pituitary gland to secrete gonadotropins in vitro during the first follicle-stimulating hormone increase of the estrous cycle and in response to exogenous steroids. Domest. Anim. Endocrinol. 2005, 28, 190–201. [Google Scholar] [CrossRef]
- Herbison, A.E.; Pape, J.R. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 2001, 22, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.; Wu, S. Estrogen receptor-beta in the gonadotropin-releasing hormone neuron. Semin. Reprod. Med. 2012, 30, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cheong, R.Y.; Kwakowsky, A.; Barad, Z.; Porteous, R.; Herbison, A.E.; Abraham, I.M. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology 2012, 153, 3792–3803. [Google Scholar] [CrossRef]
- Kanasaki, H.; Purwana, I.N.; Mijiddorj, T.; Sukhbaatar, U.; Oride, A.; Miyazaki, K. Effects of estradiol and progesterone on gonadotropin LHbeta- and FSHbeta-subunit promoter activities in gonadotroph LbetaT2 cells. Neuro Endocrinol. Lett. 2012, 33, 608–613. [Google Scholar]
- Schleicher, G.; Khan, S.; Nieschlag, E. Differentiation between androgen and estrogen receptor mediated effects of testosterone on FSH using androgen receptor deficient (Tfm) and normal mice. J. Steroid Biochem. 1989, 33, 49–51. [Google Scholar] [CrossRef]
- Ringstrom, S.J.; McAndrews, J.M.; Rahal, J.O.; Schwartz, N.B. Cortisol in vivo increases FSH beta mRNA selectively in pituitaries of male rats. Endocrinology 1991, 129, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.M.; Blount, A.L.; Donaldson, C.J.; Bilezikjian, L.M.; Vale, W.W. Regulation of follicle-stimulating hormone secretion by the interactions of activin-A, dexamethasone and testosterone in anterior pituitary cell cultures of male rats. Neuroendocrinology 2003, 77, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Yeh, D.M.; Coss, D. PACAP induces FSHbeta gene expression via EPAC. Mol. Cell. Endocrinol. 2019, 492, 110438. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Okada, Y.; Moore, J.P., Jr.; Dalkin, A.C.; Winters, S.J. Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-beta mRNA levels by stimulating follistatin gene expression: Effects on folliculostellate cells, gonadotrophs and LbetaT2 gonadotroph cells. Mol. Cell. Endocrinol. 2002, 192, 55–64. [Google Scholar] [CrossRef]
- Kanasaki, H.; Purwana, I.N.; Mijiddorj, T.; Oride, A.; Miyazaki, K. Possible involvement of PACAP and PACAP type 1 receptor in GnRH-induced FSH beta-subunit gene expression. Regul Pept 2011, 167, 227–232. [Google Scholar] [CrossRef]
- Kanasaki, H.; Purwana, I.N.; Miyazaki, K. Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation. Biol. Reprod. 2013, 88, 35. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, V.; Dalkin, A.; Yasin, M.; Haisenleder, D.J.; Marshall, J.C.; Landefeld, T.D. Are immediate early genes involved in gonadotropin-releasing hormone receptor gene regulation? Characterization of changes in GnRH receptor (GnRH-R), c-fos, and c-jun messenger ribonucleic acids during the ovine estrous cycle. Biol. Reprod. 1995, 53, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Wurmbach, E.; Yuen, T.; Ebersole, B.J.; Sealfon, S.C. Gonadotropin-releasing hormone receptor-coupled gene network organization. J. Biol. Chem. 2001, 276, 47195–47201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strahl, B.D.; Huang, H.J.; Pedersen, N.R.; Wu, J.C.; Ghosh, B.R.; Miller, W.L. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-beta gene. Endocrinology 1997, 138, 2621–2631. [Google Scholar] [CrossRef]
- Liu, F.; Austin, D.A.; Mellon, P.L.; Olefsky, J.M.; Webster, N.J. GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells. Mol. Endocrinol. 2002, 16, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Coss, D.; Jacobs, S.B.; Bender, C.E.; Mellon, P.L. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J. Biol. Chem. 2004, 279, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.J.; Sebastian, J.; Strahl, B.D.; Wu, J.C.; Miller, W.L. Transcriptional regulation of the ovine follicle-stimulating hormone-beta gene by activin and gonadotropin-releasing hormone (GnRH): Involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology 2001, 142, 2267–2274. [Google Scholar] [CrossRef]
- Jonak, C.R.; Lainez, N.M.; Roybal, L.L.; Williamson, A.D.; Coss, D. c-JUN Dimerization Protein 2 (JDP2) Is a Transcriptional Repressor of Follicle-stimulating Hormone beta (FSHbeta) and Is Required for Preventing Premature Reproductive Senescence in Female Mice. J. Biol. Chem. 2017, 292, 2646–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.; Ovitt, C.E.; Anlag, K.; Fehsenfeld, S.; Gredsted, L.; Treier, A.C.; Treier, M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004, 131, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.C.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009, 139, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzechocinska, B.; Warzecha, D.; Wypchlo, M.; Ploski, R.; Wielgos, M. Premature ovarian insufficiency as a variable feature of blepharophimosis, ptosis, and epicanthus inversus syndrome associated with c.223C > T p.(Leu75Phe) FOXL2 mutation: A case report. BMC Med. Genet. 2019, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Crisponi, L.; Deiana, M.; Loi, A.; Chiappe, F.; Uda, M.; Amati, P.; Bisceglia, L.; Zelante, L.; Nagaraja, R.; Porcu, S.; et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 2001, 27, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, J.; Yang, W.; Wang, D.; Zhang, T.; Cheng, M. New STAT3-FOXL2 pathway and its function in cancer cells. BMC Mol. Cell Biol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Hu, J.; Ke, H.; Luo, W.; Yang, Y.; Liu, H.; Li, G.; Qin, Y.; Ma, J.; Zhao, S. A novel FOXL2 mutation in two infertile patients with blepharophimosis-ptosis-epicanthus inversus syndrome. J. Assist. Reprod. Genet. 2020, 37, 223–229. [Google Scholar] [CrossRef]
- Corpuz, P.S.; Lindaman, L.L.; Mellon, P.L.; Coss, D. FoxL2 Is required for activin induction of the mouse and human follicle-stimulating hormone beta-subunit genes. Mol. Endocrinol. 2010, 24, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Schang, G.; Boehm, U.; Deng, C.X.; Graff, J.; Bernard, D.J. SMAD3 Regulates Follicle-stimulating Hormone Synthesis by Pituitary Gonadotrope Cells in Vivo. J. Biol. Chem. 2017, 292, 2301–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roybal, L.L.; Hambarchyan, A.; Meadows, J.D.; Barakat, N.H.; Pepa, P.A.; Breen, K.M.; Mellon, P.L.; Coss, D. Roles of binding elements, FOXL2 domains, and interactions with cJUN and SMADs in regulation of FSHbeta. Mol. Endocrinol. 2014, 28, 1640–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongaro, L.; Schang, G.; Zhou, Z.; Kumar, T.R.; Treier, M.; Deng, C.X.; Boehm, U.; Bernard, D.J. Human Follicle-Stimulating Hormone ss Subunit Expression Depends on FOXL2 and SMAD4. Endocrinology 2020, 161. [Google Scholar] [CrossRef] [PubMed]
- Carles, A.; Trigo-Gonzalez, G.; Cao, Q.; Cheng, S.G.; Moksa, M.; Bilenky, M.; Huntsman, D.G.; Morin, G.B.; Hirst, M. The Pathognomonic FOXL2 C134W Mutation Alters DNA-Binding Specificity. Cancer Res. 2020, 80, 3480–3491. [Google Scholar] [CrossRef]
- Weis-Banke, S.E.; Lerdrup, M.; Kleine-Kohlbrecher, D.; Mohammad, F.; Sidoli, S.; Jensen, O.N.; Yanase, T.; Nakamura, T.; Iwase, A.; Stylianou, A.; et al. Mutant FOXL2(C134W) Hijacks SMAD4 and SMAD2/3 to Drive Adult Granulosa Cell Tumors. Cancer Res. 2020, 80, 3466–3479. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Sadovsky, Y.; Swirnoff, A.H.; Polish, J.A.; Goda, P.; Gavrilina, G.; Milbrandt, J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 1996, 273, 1219–1221. [Google Scholar] [CrossRef]
- Achrekar, S.K.; Modi, D.N.; Desai, S.K.; Mangoli, V.S.; Mangoli, R.V.; Mahale, S.D. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil. Steril. 2009, 91, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Mohiyiddeen, L.; Nardo, L.G. Single-nucleotide polymorphisms in the FSH receptor gene and ovarian performance: Future role in IVF. Hum. Fertil. 2010, 13, 72–78. [Google Scholar] [CrossRef]
- Cannarella, R.; Musso, N.; Condorelli, R.A.; Musmeci, M.; Stefani, S.; La Vignera, S.; Calogero, A.E. Combined Effects of the FSHR 2039 A/G and FSHR -29 G/A Polymorphisms on Male Reproductive Parameters. World J. Mens Health 2020. [Google Scholar] [CrossRef]
- Anagnostou, E.; Kafkoutsou, A.; Mavrogianni, D.; Domali, E.; Dimitroulia, E.; Mathiopoulos, D.; Drakakis, P.; Loutradis, D. Individual and Combined Assessment of Ser680Asn FSH Receptor and FSHbeta -211 G>T Gene Polymorphisms in Ovarian Response in IVF/ICSI Program. Curr. Pharm. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Casarini, L.; Moriondo, V.; Marino, M.; Adversi, F.; Capodanno, F.; Grisolia, C.; La Marca, A.; La Sala, G.B.; Simoni, M. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol. Cell. Endocrinol. 2014, 393, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, M.; Punab, M.; Ausmees, K.; Laan, M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum. Reprod. 2008, 23, 2160–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Zhao, Z.; Zhao, R.; Xiao, S.; Jiang, H.; Yue, X.; Li, X.; Gao, Y.; Liu, J.; Zhang, J. Effects of novel single nucleotide polymorphisms of the FSH beta-subunit gene on semen quality and fertility in bulls. Anim. Reprod. Sci. 2009, 114, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, Z.; Kuang, Y.; Zhou, X.; Jin, L.; He, L.; Sun, X.; Tao, T.; Wang, L. Genetic variations in the 3′-untranslated region of SLC18A2 are associated with serum FSH concentration in polycystic ovary syndrome patients and regulate gene expression in vitro. Hum. Reprod. 2016, 31, 2150–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, C.A.; Kurz, T.L.; Thackray, V.G. A human FSHB promoter SNP associated with low FSH levels in men impairs LHX3 binding and basal FSHB transcription. Endocrinology 2013, 154, 3016–3021. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Perez Lanuza, L.; Gromoll, J. Pharmacogenetics of FSH Action in the Male. Front. Endocrinol. 2019, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Gharesi-Fard, B.; Ghasemi, Z.; Shakeri, S.; Behdin, S.; Aghaei, F.; Malek-Hosseini, Z. The frequency of follicle stimulating hormone receptor gene polymorphisms in Iranian infertile men with azoospermia. Iran. J. Reprod. Med. 2015, 13, 673–678. [Google Scholar]
- Tsitlakidis, D.; Katopodi, T.; Goulis, D.G.; Papadimas, I.; Kritis, A. Association of follicle-stimulating hormone receptor single nucleotide polymorphisms with fertility in Greek men. J. Endocrinol. Investig. 2017, 40, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, C.; Koh, E.; Yamamoto, K.; Matsui, F.; Sugimoto, K.; Sin, H.S.; Maeda, Y.; Kanaya, J.; Yoshida, A.; Namiki, M. Single nucleotide polymorphism analysis of the follicle-stimulating hormone (FSH) receptor in Japanese with male infertility: Identification of codon combination with heterozygous variations of the two discrete FSH receptor gene. Endocr. J. 2009, 56, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Ding, Y.; Kong, X.; Feng, G.; Xiang, W.; Chen, L.; Yang, F.; Zhang, K.; Chu, M.; Wang, P.; et al. Reproductive role of miRNA in the hypothalamic-pituitary axis. Mol. Cell. Neurosci. 2018, 88, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Han, D.X.; Sun, X.L.; Xu, M.Q.; Chen, C.Z.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Roles of differential expression of microRNA-21-3p and microRNA-433 in FSH regulation in rat anterior pituitary cells. Oncotarget 2017, 8, 36553–36565. [Google Scholar] [CrossRef] [PubMed]
- Han, D.X.; Xiao, Y.; Wang, C.J.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Regulation of FSH expression by differentially expressed miR-186-5p in rat anterior adenohypophyseal cells. PLoS ONE 2018, 13, e0194300. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.J.; Guo, H.X.; Han, D.X.; Yu, Z.W.; Zheng, Y.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Pituitary tissue-specific miR-7a-5p regulates FSH expression in rat anterior adenohypophyseal cells. PeerJ 2019, 7, e6458. [Google Scholar] [CrossRef] [PubMed]
- Han, D.X.; Sun, X.L.; Fu, Y.; Wang, C.J.; Liu, J.B.; Jiang, H.; Gao, Y.; Chen, C.Z.; Yuan, B.; Zhang, J.B. Identification of long non-coding RNAs in the immature and mature rat anterior pituitary. Sci. Rep. 2017, 7, 17780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.X.; Wang, C.J.; Sun, X.L.; Liu, J.B.; Jiang, H.; Gao, Y.; Chen, C.Z.; Yuan, B.; Zhang, J.B. Identification of circular RNAs in the immature and mature rat anterior pituitary. J. Endocrinol. 2019, 240, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Han, D.X.; Sun, X.L.; Wang, C.J.; Yu, Z.W.; Zheng, Y.; Huang, Y.J.; Wang, W.H.; Jiang, H.; Gao, Y.; Yuan, B.; et al. Differentially expressed lncRNA-m433s1 regulates FSH secretion by functioning as a miRNA sponge in male rat anterior pituitary cellsdagger. Biol. Reprod. 2019, 101, 416–425. [Google Scholar] [CrossRef]
- Lannes, J.; L’Hote, D.; Fernandez-Vega, A.; Garrel, G.; Laverriere, J.N.; Cohen-Tannoudji, J.; Querat, B. A regulatory loop between miR-132 and miR-125b involved in gonadotrope cells desensitization to GnRH. Sci. Rep. 2016, 6, 31563. [Google Scholar] [CrossRef]
- Godoy, J.; Nishimura, M.; Webster, N.J. Gonadotropin-releasing hormone induces miR-132 and miR-212 to regulate cellular morphology and migration in immortalized LbetaT2 pituitary gonadotrope cells. Mol. Endocrinol. 2011, 25, 810–820. [Google Scholar] [CrossRef]
- Lannes, J.; L’Hote, D.; Garrel, G.; Laverriere, J.N.; Cohen-Tannoudji, J.; Querat, B. Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol. Endocrinol. 2015, 29, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.S.; Li, M.; Li, C.Y.; Qi, Q.E.; Chen, T.; Cheng, X.; Wang, S.B.; Shu, G.; Wang, L.N.; Zhu, X.T.; et al. miR-361-3p regulates FSH by targeting FSHB in a porcine anterior pituitary cell model. Reproduction 2017, 153, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.J.; Gao, F.; Huang, Y.J.; Han, D.X.; Zheng, Y.; Wang, W.H.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. circAkap17b acts as a miR-7 family molecular sponge to regulate FSH secretion in rat pituitary cells. J. Mol. Endocrinol. 2020, 65, 135–148. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, N.A.; Lacza, C.T.; Hou, M.Y.; Gregory, S.J.; Kam, K.Y.; Xu, S.; Kaiser, U.B. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol. Endocrinol. 2008, 22, 1908–1923. [Google Scholar] [CrossRef]
- Lim, S.; Luo, M.; Koh, M.; Yang, M.; bin Abdul Kadir, M.N.; Tan, J.H.; Ye, Z.; Wang, W.; Melamed, P. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol. Cell. Biol. 2007, 27, 4105–4120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Hoffmann, H.M.; Iyer, A.K.; Brayman, M.J.; Ngo, C.; Sunshine, M.J.; Mellon, P.L. Chromatin status and transcription factor binding to gonadotropin promoters in gonadotrope cell lines. Reprod. Biol. Endocrinol. 2017, 15, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oride, A.; Kanasaki, H.; Mijiddorj, T.; Sukhbaatar, U.; Miyazaki, K. Trichostatin A specifically stimulates gonadotropin FSHbeta gene expression in gonadotroph LbetaT2 cells. Endocr. J. 2014, 61, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Edwards, B.S.; Muth, A.; Thompson, P.R.; Cherrington, B.D.; Navratil, A.M. GnRH Stimulates Peptidylarginine Deiminase Catalyzed Histone Citrullination in Gonadotrope Cells. Mol. Endocrinol. 2016, 30, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Haj, M.; Wijeweera, A.; Rudnizky, S.; Taunton, J.; Pnueli, L.; Melamed, P. Mitogen- and stress-activated protein kinase 1 is required for gonadotropin-releasing hormone-mediated activation of gonadotropin alpha-subunit expression. J. Biol. Chem. 2017, 292, 20720–20731. [Google Scholar] [CrossRef] [Green Version]
- Yosefzon, Y.; David, C.; Tsukerman, A.; Pnueli, L.; Qiao, S.; Boehm, U.; Melamed, P. An epigenetic switch repressing Tet1 in gonadotropes activates the reproductive axis. Proc. Natl. Acad. Sci. USA 2017, 114, 10131–10136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Usui, I.; Evans, L.G.; Austin, D.A.; Mellon, P.L.; Olefsky, J.M.; Webster, N.J. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J. Biol. Chem. 2002, 277, 32099–32108. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, R.; Mistry, D.; Webster, N.J. Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells. J. Biol. Chem. 2010, 285, 20262–20272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugami, S.; Dobkin-Bekman, M.; Rahamim-Ben Navi, L.; Naor, Z. Differential roles of PKC isoforms (PKCs) in GnRH stimulation of MAPK phosphorylation in gonadotrope derived cells. Mol. Cell. Endocrinol. 2018, 463, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Haisenleder, D.J.; Burger, L.L.; Walsh, H.E.; Stevens, J.; Aylor, K.W.; Shupnik, M.A.; Marshall, J.C. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: Evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology 2008, 149, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Burger, L.L.; Haisenleder, D.J.; Aylor, K.W.; Marshall, J.C. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: Roles for CaMK II, ERK, and JNK activation. Biol. Reprod. 2008, 79, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.H.; Farshori, M.P.; Jambusaria, A.; Catt, K.J. Roles of Src and epidermal growth factor receptor transactivation in transient and sustained ERK1/2 responses to gonadotropin-releasing hormone receptor activation. J. Biol. Chem. 2003, 278, 19118–19126. [Google Scholar] [CrossRef] [Green Version]
- Okitsu-Sakurayama, S.; Higa-Nakamine, S.; Torihara, H.; Higashiyama, S.; Yamamoto, H. Roles of Pyk2 in signal transduction after gonadotropin-releasing hormone receptor stimulation. J. Cell. Physiol. 2021, 236, 3033–3043. [Google Scholar] [CrossRef]
- Reddy, G.R.; Xie, C.; Lindaman, L.L.; Coss, D. GnRH increases c-Fos half-life contributing to higher FSHbeta induction. Mol. Endocrinol. 2013, 27, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, I.R.; Kaiser, U.B. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol. Cell. Endocrinol. 2014, 385, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, I.R.; Ciccone, N.A.; Xu, S.; Zaytseva, S.; Carroll, R.S.; Kaiser, U.B. GnRH pulse frequency-dependent stimulation of FSHbeta transcription is mediated via activation of PKA and CREB. Mol. Endocrinol. 2013, 27, 606–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafer, C.M.; Thomas, R.; Lambrakos, L.; Montoya, I.; White, S.; Halvorson, L.M. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol. Endocrinol. 2009, 23, 1022–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrel, G.; Simon, V.; Thieulant, M.L.; Cayla, X.; Garcia, A.; Counis, R.; Cohen-Tannoudji, J. Sustained gonadotropin-releasing hormone stimulation mobilizes the cAMP/PKA pathway to induce nitric oxide synthase type 1 expression in rat pituitary cells in vitro and in vivo at proestrus. Biol. Reprod. 2010, 82, 1170–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.M.; Chen, J.; Chen, H.; Diao, R.Y.; Fok, K.L.; Dong, J.D.; Sun, T.T.; Chen, W.Y.; Yu, M.K.; Zhang, X.H.; et al. Defective CFTR-dependent CREB activation results in impaired spermatogenesis and azoospermia. PLoS ONE 2011, 6, e19120. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal. Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Mordret, G. MAP kinase kinase: A node connecting multiple pathways. Biol. Cell 1993, 79, 193–207. [Google Scholar] [CrossRef]
- Binder, A.K.; Grammer, J.C.; Herndon, M.K.; Stanton, J.D.; Nilson, J.H. GnRH regulation of Jun and Atf3 requires calcium, calcineurin, and NFAT. Mol. Endocrinol. 2012, 26, 873–886. [Google Scholar] [CrossRef] [Green Version]
- Kanasaki, H.; Purwana, I.; Oride, A.; Mijiddorj, T.; Miyazaki, K. Extracellular Signal-Regulated Kinase (ERK) Activation and Mitogen-Activated Protein Kinase Phosphatase 1 Induction by Pulsatile Gonadotropin-Releasing Hormone in Pituitary Gonadotrophs. J. Signal. Transduct. 2012, 2012, 198527. [Google Scholar] [CrossRef] [Green Version]
- Kanasaki, H.; Bedecarrats, G.Y.; Kam, K.Y.; Xu, S.; Kaiser, U.B. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology 2005, 146, 5503–5513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliss, S.P.; Miller, A.; Navratil, A.M.; Xie, J.; McDonough, S.P.; Fisher, P.J.; Landreth, G.E.; Roberson, M.S. ERK signaling in the pituitary is required for female but not male fertility. Mol. Endocrinol. 2009, 23, 1092–1101. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Lawson, M.A. GnRH Regulates Gonadotropin Gene Expression Through NADPH/Dual Oxidase-Derived Reactive Oxygen Species. Endocrinology 2015, 156, 2185–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK during porcine oocyte maturation. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.Q.; Han, C.S.; Yang, W.; Jin, X.; Hu, Z.Y.; Liu, Y.X. Activation of the p38 MAPK pathway by follicle-stimulating hormone regulates steroidogenesis in granulosa cells differentially. J. Endocrinol. 2005, 186, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Gan, X.; Liu, K.; Lv, X.; Shen, H.; Dai, G.; Xu, H. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J. Ethnopharmacol. 2016, 194, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.K.; Murtazina, D.A.; Magee, C.; Navratil, A.M.; Clay, C.M.; Amberg, G.C. GnRH evokes localized subplasmalemmal calcium signaling in gonadotropes. Mol. Endocrinol. 2014, 28, 2049–2059. [Google Scholar] [CrossRef] [Green Version]
- Duran-Pasten, M.L.; Fiordelisio, T. GnRH-Induced Ca(2+) Signaling Patterns and Gonadotropin Secretion in Pituitary Gonadotrophs. Functional Adaptations to Both Ordinary and Extraordinary Physiological Demands. Front. Endocrinol. 2013, 4, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhanvantari, S.; Wiebe, J.P. Suppression of follicle-stimulating hormone by the gonadal- and neurosteroid 3 alpha-hydroxy-4-pregnen-20-one involves actions at the level of the gonadotrope membrane/calcium channel. Endocrinology 1994, 134, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Haisenleder, D.J.; Yasin, M.; Marshall, J.C. Gonadotropin subunit and gonadotropin-releasing hormone receptor gene expression are regulated by alterations in the frequency of calcium pulsatile signals. Endocrinology 1997, 138, 5227–5230. [Google Scholar] [CrossRef]
- Haisenleder, D.J.; Workman, L.J.; Burger, L.L.; Aylor, K.W.; Dalkin, A.C.; Marshall, J.C. Gonadotropin subunit transcriptional responses to calcium signals in the rat: Evidence for regulation by pulse frequency. Biol. Reprod. 2001, 65, 1789–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haisenleder, D.J.; Ferris, H.A.; Shupnik, M.A. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology 2003, 144, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Roberson, M.S.; Bliss, S.P.; Xie, J.; Navratil, A.M.; Farmerie, T.A.; Wolfe, M.W.; Clay, C.M. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol. Endocrinol. 2005, 19, 2412–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Yuan, Y.; Xu, Z.; Pu, M.; Wang, C.; Zhang, Y.; Liu, Z.; Wang, C.; Li, L.; Zhang, Z. Genetic variation in the calcium/calmodulin-dependent protein kinase (CaMK) pathway is associated with antidepressant response in females. J. Affect. Disord. 2012, 136, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, S.; Liu, Z.; Wu, L.; Li, M.; Yang, J.; Chen, R.; Liu, X.; Xu, H.; Cai, S.; et al. CAMK2N1 inhibits prostate cancer progression through androgen receptor-dependent signaling. Oncotarget 2014, 5, 10293–10306. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-Q.; Zhang, W.-D.; Yuan, B.; Zhang, J.-B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals 2021, 11, 1134. https://doi.org/10.3390/ani11041134
Wang H-Q, Zhang W-D, Yuan B, Zhang J-B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals. 2021; 11(4):1134. https://doi.org/10.3390/ani11041134
Chicago/Turabian StyleWang, Hao-Qi, Wei-Di Zhang, Bao Yuan, and Jia-Bao Zhang. 2021. "Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion" Animals 11, no. 4: 1134. https://doi.org/10.3390/ani11041134
APA StyleWang, H.-Q., Zhang, W.-D., Yuan, B., & Zhang, J.-B. (2021). Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals, 11(4), 1134. https://doi.org/10.3390/ani11041134





