Protein and Amino Acid Metabolism in Poultry during and after Heat Stress: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Amino Acid and/or Protein Metabolism
3. Biosynthesis of Amino Acids
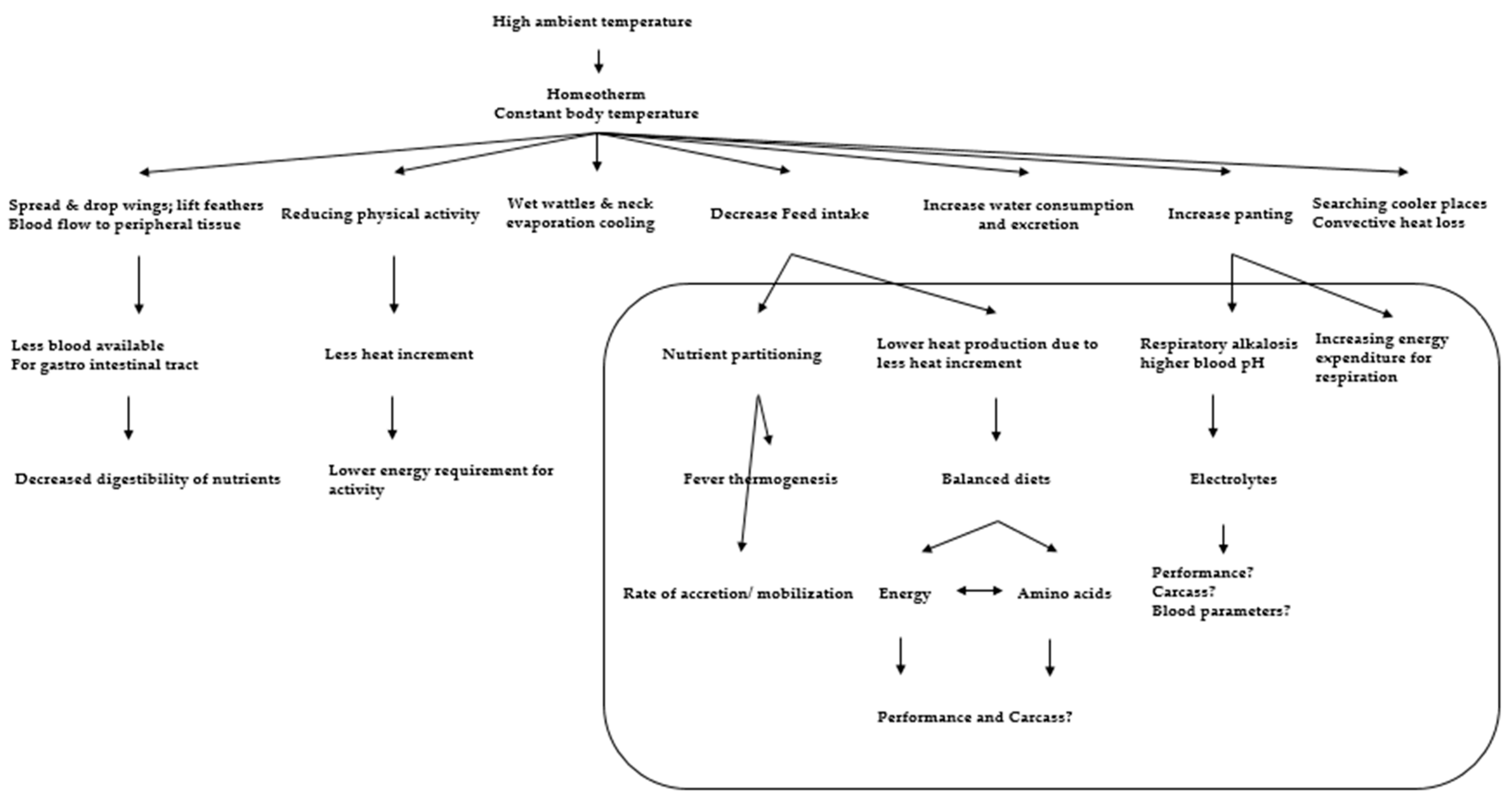
4. Effect of HS on Protein Metabolism or Turnover
5. Heat Shock Proteins
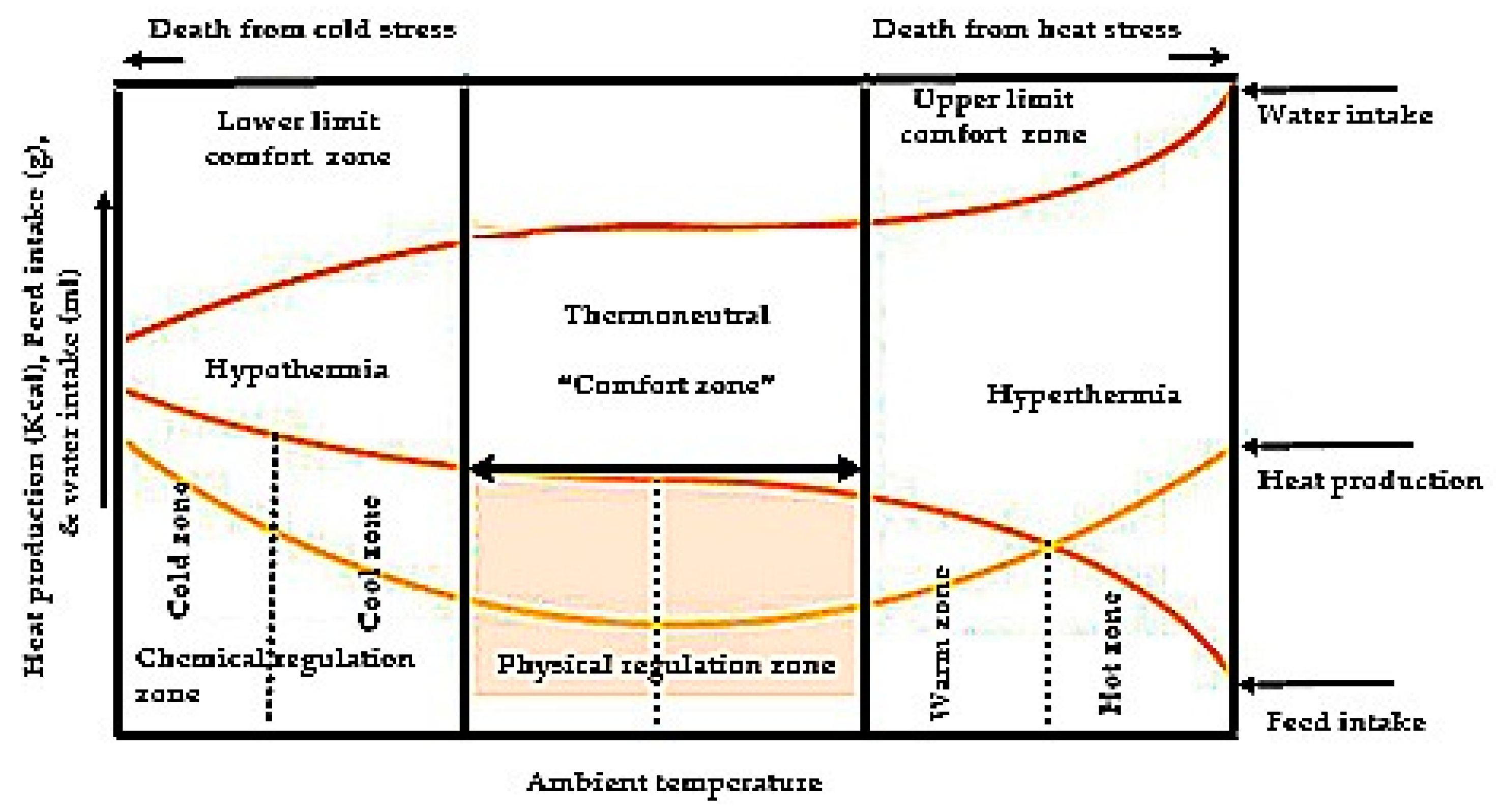
6. Physiological Mechanism of Stress Regulation in Poultry
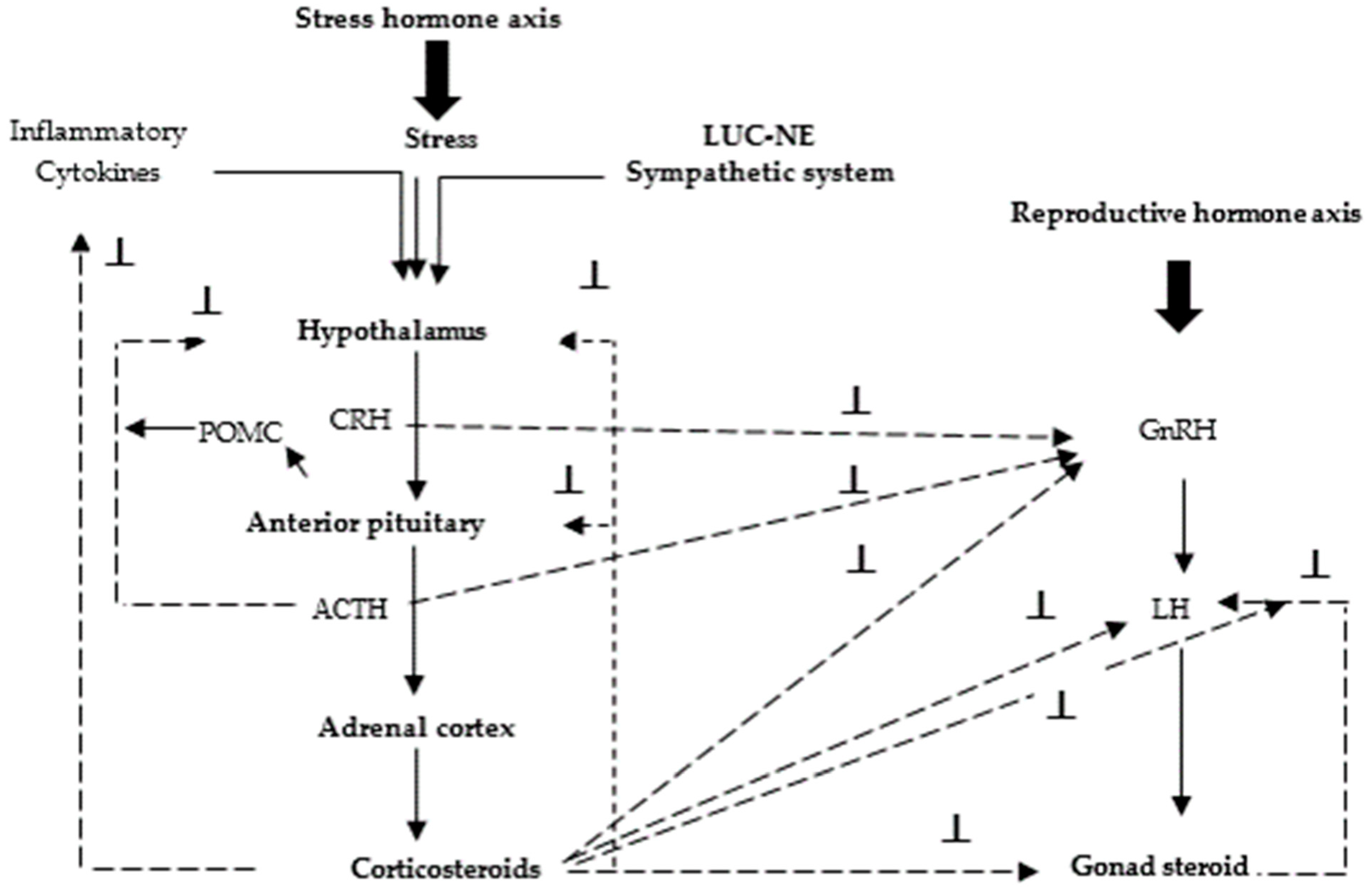
7. Hormonal Responses to Stress and the Hormonal Control of Protein Metabolism
8. Assessment of Stress
9. Nutritional Strategies for Preventing HS in Poultry
10. Effect of HS on Amino Acids
11. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perini, F.; Cendron, F.; Rovelli, G.; Castellini, C.; Cassandro, M.; Lasagna, E. Emerging genetic tools to investigate molecular pathways related to heat stress in chickens: A review. Animals 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat stress impacts on broiler performance: A systematic review and meta-analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef] [PubMed]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality? A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Toplu, H.D.O.; Oral, D.; Nazligul, A.; Karaarslan, S.; Kaya, M.; Yagin, O. Effects of heat conditioning and dietary ascorbic acid supplementation on growth performance, carcass and meat quality characteristics in heat-stressed broilers. Ankara Üniversitesi Veteriner Fakültesi Dergisi 2014, 61, 295–302. [Google Scholar] [CrossRef]
- Majekodunmi, B.; Ogunwole, O.; Sokunbi, O. Effect of supplemental electrolytes and ascorbic acid on the performance and carcass characteristics of broiler raised during high temperature period in Nigeria. Int. J. Poult. Sci. 2012, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Syafwan, W. Effects of Dietary Changes on Heat Stress in Broiler and Kampung Chickens. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2012. [Google Scholar]
- Wasti, S.; Sah, N.; Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; de Smet, S. Association between heat stress and oxidative stress in poultry; Mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maharjan, P.; Mullenix, G.; Hilton, K.; Beitia, A.; Weil, J.; Suesuttajit, N.; Martinez, D.; Umberson, C.; England, J.; Caldas, J. Effects of dietary amino acid levels and ambient temperature on mixed muscle protein turnover in Pectoralis major during finisher feeding period in two broiler lines. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Hassan, S.S. Broiler tolerance to heat stress at various dietary protein/energy levels. Europ. Poult. Sci. 2017, 81, 1–15. [Google Scholar]
- Ma, B.; Zhang, L.; Li, J.; Xing, T.; Jiang, Y.; Gao, F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021, 100, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.; Hassan, R.; Tag El-Din, A.; Abou-Shehema, B. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J. Anim. Physiol. Anim. Nutr. 2011, 95, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.; Zulkifli, I.; Soleimani, A.; Law, F.; Ramiah, S.; Mohamed-Yousif, I.; Hussein, E.; Khalil, E. Response of broilers to reduced-protein diets under heat stress conditions. World Poult. Sci. J. 2019, 75, 583–598. [Google Scholar] [CrossRef]
- Sahin, N.; Onderci, M.; Sahin, K.; Gursu, M.; Smith, M. Ascorbic acid and melatonin reduce heat-induced performance inhibition and oxidative stress in Japanese quails. Br. Poult. Sci. 2004, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Selle, P.; Liu, S.Y. The relevance of starch and protein digestive dynamics in poultry. J. Appl. Poult. Res. 2019, 28, 531–545. [Google Scholar] [CrossRef]
- Dublecz, K. Poultry Nutrition; Pannon Egyetem: Egyetem, Hungary, 2011; pp. 18–22. [Google Scholar]
- Qaid, M.M.; Abdelrahman, M.M. Role of insulin and other related hormones in energy metabolism—A review. Cogent Food Agric. 2016, 2, 1267691. [Google Scholar] [CrossRef]
- Squires, E.J. Applied Animal Endocrinology; CABI: Wallingford, UK, 2010. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Sanz Fernandez, M. Effects of heat stress on post-absorptive metabolism. In Proceedings of the ADSA-ASAS Midwest Meeting, Des Moines, IA, USA, 17–19 March 2014. [Google Scholar]
- Wheelock, J.; Rhoads, R.; Vanbaale, M.; Sanders, S.; Baumgard, L. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Virden, W.; Kidd, M. Physiological stress in broilers: Ramifications on nutrient digestibility and responses. J. Appl. Poult. Res. 2009, 18, 338–347. [Google Scholar] [CrossRef]
- Ognik, K.; Sembratowicz, I. Stress as a factor modifying the metabolism in poultry. A review. Annales UMCS Zootech. 2012, 30, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, M.; Kamiya, Y.; Tanaka, M.; Oki, T.; Nishiba, Y.; Shioya, S. Effects of high ambient temperature and restricted feed intake on urinary and plasma 3-methylhistidine in lactating Holstein cows. Anim. Sci. J. 2006, 77, 201–207. [Google Scholar] [CrossRef]
- Pearce, S. The Effects of Heat Stress and Nutritional Status on Metabolism and Intestinal Integrity in Growing Pigs. Master’s Thesis, Iowa State University, Ames, IA, USA, 2011. [Google Scholar]
- Santiago, L.Â.M.; Lima Neto, L.G.; Pereira, G.B.; Leite, R.D.; Mostarda, C.T.; Navarro, F. Influence of creatine kinase on C-reactive protein in muscle adaptation. Rev. Bras. Med. Esporte 2019, 25, 413–417. [Google Scholar] [CrossRef]
- Hara, T.; Ohtsuka, A.; Hayashi, K. Role of Ca2+ in corticosterone-induced muscle growth retardation. Anim. Sci. J. 2002, 73, 383–387. [Google Scholar]
- Yaman, M.A.; Kita, K.; Okumura, J.-I. Various macronutrient intakes additively stimulate protein synthesis in liver and muscle of food-deprived chicks. J. Nutr. 2000, 130, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski-Meissner, H.T. The physiological and biochemical responses of broilers exposed to short-term thermal stress. Comp. Biochem. Physiol. Part A Physiol. 1981, 70, 1–8. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Khan, M.; Shim, K. Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Temim, S.; Chagneau, A.-M.; Peresson, R.; Tesseraud, S. Chronic heat exposure alters protein turnover of three different skeletal muscles in finishing broiler chickens fed 20 or 25% protein diets. J. Nutr. 2000, 130, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Esquerra, R.; Leeson, S. Effects of acute versus chronic heat stress on broiler response to dietary protein. Poult. Sci. 2005, 84, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.A.; Kim, E.Y. Hyponatremia caused by excessive intake of water as a form of child abuse. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiyabutr, N. Physiological reactions of poultry to heat stress and methods to reduce its effects on poultry production. Thai J. Vet. Med. 2004, 34, 17–30. [Google Scholar]
- Tsahar, E.; Arad, Z.; Izhaki, I.; Guglielmo, C.G. The relationship between uric acid and its oxidative product allantoin: A potential indicator for the evaluation of oxidative stress in birds. J. Comp. Physiol. B 2006, 176, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Pijarska, I.; Czech, A.; Malec, H.; Tymczyna, L. Effect of road transportation of chicks on blood biochemical indices and productive results of broilers. Vet. Med. 2006, 62, 408–410. [Google Scholar]
- Temim, S.; Chagneau, A.-M.; Guillaumin, S.; Michel, J.; Peresson, R.; Geraert, P.-A.; Tesseraud, S. Effects of chronic heat exposure and protein intake on growth performance, nitrogen retention and muscle development in broiler chickens. Reprod. Nutr. Dev. 1999, 39, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Taniguchi, N.; Kinjou, T.; Ohtsuka, A. Heat stress causes death via glucose metabolism in broiler. In Proceedings of the 13th European Symposium on Poultry Nutrition and Ascites Workshop, Blankenberge, Belgium, 30 September–4 October 2001. [Google Scholar]
- Samuels, S.; McAllister, T.; Thompson, J. Skeletal and heart muscle protein turnover during long-term exposure to high environmental temperatures in young rats. Can. J. Physiol. Pharmacol. 2000, 78, 557–564. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yenari, M.A. The immune modulating properties of the heat shock proteins after brain injury. Anat. Cell Biol. 2013, 46, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Aqil, A.; Zulkifli, I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009, 88, 1358–1364. [Google Scholar] [CrossRef]
- Youssef, S.; Selim, N.; Galal, M. Increasing the activity of antioxidant system in broiler chicks during summer season: 1-effect of some anti-heat stress agents on broiler performance, immune response and heat shock protein 70. Egypt. Poult. Sci. J. 2016, 36. [Google Scholar] [CrossRef]
- Frier, B.C.; Locke, M. Heat stress inhibits skeletal muscle hypertrophy. Cell Stress Chaperones 2007, 12, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. Glutamine and heat shock protein expression. Nutrition 2002, 18, 225–228. [Google Scholar] [CrossRef]
- Khan, R.; Naz, S.; Nikousefat, Z.; Selvaggi, M.; Laudadio, V.; Tufarelli, V. Effect of ascorbic acid in heat-stressed poultry. World Poult. Sci. J. 2012, 68, 477. [Google Scholar] [CrossRef]
- Ming, J.; Xie, J.; Xu, P.; Liu, W.; Ge, X.; Liu, B.; He, Y.; Cheng, Y.; Zhou, Q.; Pan, L. Molecular cloning and expression of two HSP70 genes in the Wuchang bream (Megalobrama amblycephala Yih). Fish Shellfish Immunol. 2010, 28, 407–418. [Google Scholar] [CrossRef]
- Mohan, J. Physiology of Stress in Poultry; Central Avian Research Intitute: Bareilly, India, 2005; Volume 11. [Google Scholar]
- Yunianto, V.D.; Hayashit, K.; Kaiwda, S.; Ohtsuka, A.; Tomita, Y. Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br. J. Nutr. 1997, 77, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.V.; van Kampen, M. Energy relationships in growing chickens given daily injections of corticosterone. Br. Poult. Sci. 1984, 25, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Naga Raja Kumari, K.; Narendra Nath, D. Ameliorative measures to counter heat stress in poultry. World Poult. Sci. J. 2018, 74, 117–130. [Google Scholar] [CrossRef]
- Donkoh, A.; Atuahene, C. Management of environmental temperature and rations for poultry production in the hot and humid tropics. Int. J. Biometeorol. 1988, 32, 247–253. [Google Scholar] [CrossRef]
- Shakeri, M.; Oskoueian, E.; Le, H.H.; Shakeri, M. Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Vet. Sci. 2020, 7, 71. [Google Scholar] [CrossRef]
- Anderson, P. Further explorations into the avian-human bond. In Proceedings of the 14th Annual International Society for Anthrozoology Conference, Niagara Falls, NY, USA, 11–12 July 2005. [Google Scholar]
- Veldkamp, T. Heat Stress and Diet Utilization in Male Turkeys: The Role of Dietary Energy and Amino Acids. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- Chowdhury, V.S. Heat stress biomarker amino acids and neuropeptide afford thermotolerance in chicks. J. Poult. Sci. 2019, 56, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yahav, S. Domestic fowl-strategies to confront environmental conditions. Poult. Avian Biol. Rev. 2000, 11, 81–95. [Google Scholar]
- Gonzalez-Esquerra, R.; Leeson, S. Physiological and metabolic responses of broilers to heat stress-implications for protein and amino acid nutrition. World Poult. Sci. J. 2006, 62, 282–295. [Google Scholar] [CrossRef]
- Singh, P.; Kesharwani, R.K.; Keservani, R.K. Protein, carbohydrates, and fats: Energy metabolism. In Sustained Energy for Enhanced Human Functions and Activity; Elsevier: Amsterdam, The Netherlands, 2017; pp. 103–115. [Google Scholar]
- Cheng, T.K.; Hamre, M.L.; Coon, C.N. Responses of broilers to dietary protein levels and amino acid supplementation to low protein diets at various environmental temperatures. J. Appl. Poult. Res. 1997, 6, 18–33. [Google Scholar] [CrossRef]
- Teeter, R.G.; Belay, T. Broiler management during acute heat stress. Anim. Feed Sci. Technol. 1996, 58, 127–142. [Google Scholar] [CrossRef]
- Furlan, R.L.; de Faria Filho, D.; Rosa, P.; Macari, M. Does low-protein diet improve broiler performance under heat stress conditions? Rev. Bras. Ciência Avícola 2004, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Van der Klis, J.; de Lange, L. Water intake in poultry. In Proceedings of the 19th European Symposium on Poultry Nutrition, Postdam, Germany, 26–29 August 2013; pp. 102–107. [Google Scholar]
- Harris, C.; Milne, G.; McDiarmid, R. The retention and metabolism of Nτ-methylhistidine by cockerels: Implications for the measurement of muscle protein breakdown determined from the excretion of Nτ methylhistidine in excreta. Br. J. Nutr. 1987, 57, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Wang, L.; Wen, A.; Wang, L. Protection of glutamine supplementation to performance, intestinal enzyme activity and morphosis in broiler under heat stress. J. Chin. Cereals Oils Assoc. 2009, 24, 103–107. [Google Scholar]
- Porto, M.; Givisiez, P.; Saraiva, E.; Costa, F.; Moreira Filho, A.; Andrade, M.; Brandão, P.; Guerra, R. Glutamic acid improves body weight gain and intestinal morphology of broiler chickens submitted to heat stress. Revista Brasileira de Ciência Avícola 2015, 17, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Olubodun, J.O.; Zulkifli, I.; Farjam, A.S.; Hair-Bejo, M.; Kasim, A. Glutamine and glutamic acid supplementation enhances performance of broiler chickens under the hot and humid tropical condition. Ital. J. Anim. Sci. 2015, 14, 3263. [Google Scholar] [CrossRef]
- Firman, J.D.; Boling, S. Lysine: Ideal protein in turkeys. Poult. Sci. 1998, 77, 105–110. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Elnaggar, A.S. Productive, physiological and immunological responses of two broiler strains fed different dietary regimens and exposed to heat stress. Ital. J. Anim. Sci. 2018, 17, 686–697. [Google Scholar] [CrossRef] [Green Version]
- Syafwan, S.; Kwakkel, R.; Verstegen, M. Heat stress and feeding strategies in meat-type chickens. World Poult. Sci. J. 2011, 67, 653–674. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.K.; Hamre, M.L.; Coon, C.N. Effect of environmental temperature, dietary protein, and energy levels on broiler performance. J. Appl. Poult. Res. 1997, 6, 1–17. [Google Scholar] [CrossRef]
- Alleman, F.; Leclercq, B. Effect of dietary protein and environmental temperature on growth performance and water consumption of male broiler chickens. Br. Poult. Sci. 1997, 38, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S.; Han, G.; Eltahan, H.M.; Haraguchi, S.; Gilbert, E.R.; Cline, M.A.; Cockrem, J.F.; Bungo, T.; Furuse, M. Potential role of amino acids in the adaptation of chicks and market-age broilers to heat stress. Front. Vet. Sci. 2020, 7, 610541. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Zarate, A.; Moran, E.; Burnham, D. Exceeding essential amino acid requirements and improving their balance as a means to minimize heat stress in broilers. J. Appl. Poult. Res. 2003, 12, 37–44. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; Wang, J.; Al-Harthi, M.A.; Kim, W.K. Multiple amino acid supplementations to low-protein diets: Effect on performance, carcass yield, meat quality and nitrogen excretion of finishing broilers under hot climate conditions. Animals 2020, 10, 973. [Google Scholar] [CrossRef] [PubMed]

| Hormones | Protein Synthesis | Proteolysis |
|---|---|---|
| Insulin | Stimulated | Inhibited |
| Glucagon | Inhibited | Stimulated |
| Epinephrine | Inhibited | Stimulated |
| Glucocorticoids: ACTH *, CS, and Cortisol | Inhibited | Stimulated (gluconeogenesis) |
| Thyroid hormones T4 and T3 | Accelerated skeletal muscle protein turnover and heat production under the hot conditions | |
| Growth hormone | Stimulated | Inhibited |
| Potential Methods for Assessing Stress | ||
| Behavioral/Physiological | Endocrine | Metabolic Systems |
| Activity/sleep patterns | Catecholamines | Immune function |
| Posture/stereotypes | ACTH/CRH, glucocorticoids | Disease state |
| Feed and water intake | Gonadotrophin/sex steroids | Growth performance |
| Heart rate and blood pressure | Endorphin (β), renin and prolactin | Reproductive performance |
| Nutrients | Calorific Value (kJ/g) | ATP Production (%) | Lipid Synthesis (%) |
|---|---|---|---|
| Starch | 17.7 | 68 | 74 |
| Protein | 23.8 | 58 | 53 |
| Fatty acids | 39.8 | 66 | 90 |
| Amino Acid | Hen Turkeys | Broiler Chicken | Pigs |
|---|---|---|---|
| Lysine | 100 | 100 | 100 |
| Methionine + Cystine | 59 | 72 | 60 |
| Threonine | 55 | 67 | 65 |
| Valine | 76 | 77 | 68 |
| Arginine | 105 | 105 | NA1 |
| Histidine | 36 | 31 | 32 |
| Isoleucine | 69 | 67 | 60 |
| Leucine | 124 | 100 | 111 |
| Phenylalanine + Tyrosine | 105 | 105 | 95 |
| Tryptophan | 16 | 16 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaid, M.M.; Al-Garadi, M.A. Protein and Amino Acid Metabolism in Poultry during and after Heat Stress: A Review. Animals 2021, 11, 1167. https://doi.org/10.3390/ani11041167
Qaid MM, Al-Garadi MA. Protein and Amino Acid Metabolism in Poultry during and after Heat Stress: A Review. Animals. 2021; 11(4):1167. https://doi.org/10.3390/ani11041167
Chicago/Turabian StyleQaid, Mohammed M., and Maged A. Al-Garadi. 2021. "Protein and Amino Acid Metabolism in Poultry during and after Heat Stress: A Review" Animals 11, no. 4: 1167. https://doi.org/10.3390/ani11041167
APA StyleQaid, M. M., & Al-Garadi, M. A. (2021). Protein and Amino Acid Metabolism in Poultry during and after Heat Stress: A Review. Animals, 11(4), 1167. https://doi.org/10.3390/ani11041167







