Game-Changing Approaches in Sperm Sex-Sorting: Microfluidics and Nanotechnology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Overview of the Sex-Sorting Importance
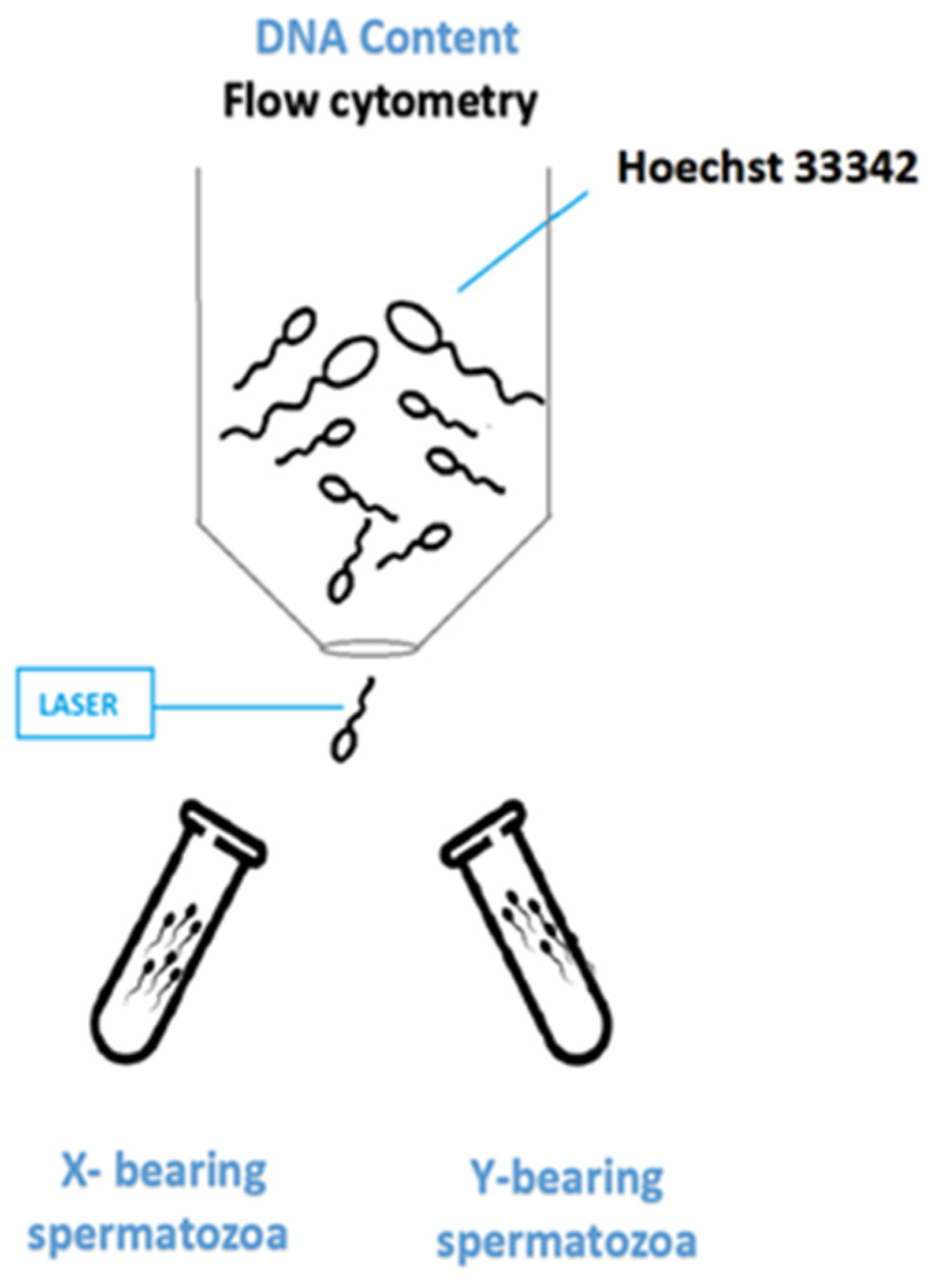
3. Conventional Approach—Flow Cytometry Sorting
4. Conventional and Sex-Sorted Semen Market—Present and Future Trends
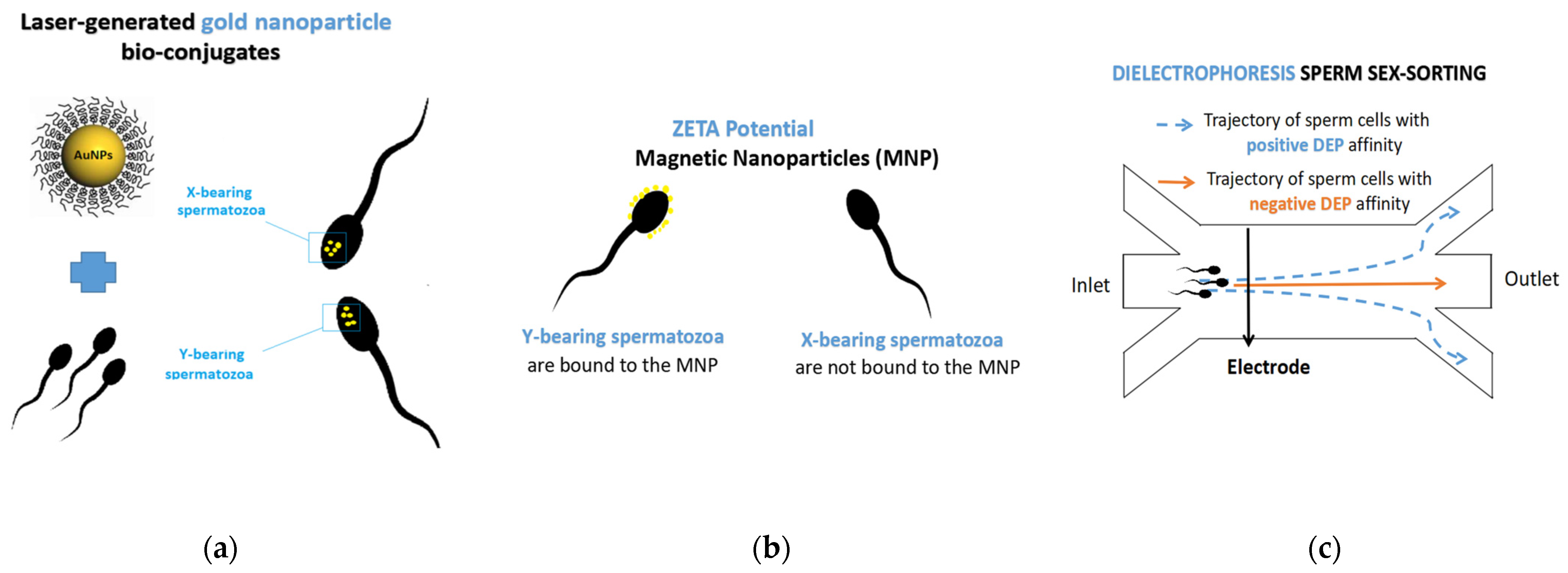
5. New Generation Technologies for Sperm Sex-Sorting: Microfluidics and Nanotechnology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef] [Green Version]
- Röös, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Garner, D.L.; Gledhill, B.L.; Pinkel, D.; Lake, S.; Stephenson, D.; Van Dilla, M.A.; Johnson, L.A. Quantification of the X-and Y-Chromosome-Bearing Spermatozoa of Domestic Animals by Flow Cytometry. Biol. Reprod. 1983, 28, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cran, D.G.; Johnson, L.A.; Miller, N.G.; Cochrane, D.; Polge, C. Production of bovine calves following separation of X-and Y-chromosome bearing sperm and in vitro fertilisation. Bov. Pract. 1993, 132, 143–144. [Google Scholar] [CrossRef]
- Maxwell, W.; Welch, G.R.; Johnson, L.A. Viability and membrane integrity of spermatozoa after dilution and flow cytometric sorting in the presence or absence of seminal plasma. Reprod. Fertil. Dev. 1996, 8, 1165–1178. [Google Scholar] [CrossRef]
- Cottle, D.; Wallace, M.; Lonergan, P.; Fahey, A. Bioeconomics of sexed semen utilization in a high-producing Holstein-Friesian dairy herd. J. Dairy Sci. 2018, 101, 4498–4512. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.A.; Butler, S.T. Review: Applications and benefits of sexed semen in dairy and beef herds. Animal 2018, 12, s97–s103. [Google Scholar] [CrossRef]
- Diers, S.; Heise, J.; Krebs, T.; Groenewold, J.; Tetens, J. Effect of sexed semen on different production and functional traits in German Holsteins. Vet. Anim. Sci. 2020, 9, 100101. [Google Scholar] [CrossRef] [PubMed]
- Bittante, G.; Negrini, R.; Bergamaschi, M.; Cecchinato, A.; Alvarado, H.T. Pure-breeding with sexed semen and crossbreeding with semen of double-muscled sires to improve beef production from dairy herds: Factors affecting heifer and cow fertility and the sex ratio. J. Dairy Sci. 2020, 103, 5246–5257. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.V.S.; Garai, S.; Maiti, S.; Meena, B.S.; Bhakat, M.; Kadian, K.S. Indian dairy farmers’ willingness to pay for sexed semen. J. Dairy Res. 2020, 87, 406–409. [Google Scholar] [CrossRef]
- Obuchi, T.; Osada, M.; Ozawa, T.; Nakagawa, H.; Hayashi, M.; Akiyama, K.; Sakagami, N.; Miura, R.; Geshi, M.; Ushijima, H. Comparative evaluation of the cost and efficiency of four types of sexing methods for the production of dairy female calves. J. Reprod. Dev. 2019, 65, 345–352. [Google Scholar] [CrossRef]
- McCullock, K.; Hoag, D.L.; Parsons, J.; Lacy, M.; Seidel, G.E.; Wailes, W. Factors affecting economics of using sexed semen in dairy cattle. J. Dairy Sci. 2013, 96, 6366–6377. [Google Scholar] [CrossRef]
- Murphy, C.; Shalloo, L.; Hutchinson, I.; Butler, S. Expanding the dairy herd in pasture-based systems: The role of sexed semen within alternative breeding strategies. J. Dairy Sci. 2016, 99, 6680–6692. [Google Scholar] [CrossRef]
- Ettema, J.; Thomasen, J.; Hjortø, L.; Kargo, M.; Østergaard, S.; Sørensen, A. Economic opportunities for using sexed semen and semen of beef bulls in dairy herds. J. Dairy Sci. 2017, 100, 4161–4171. [Google Scholar] [CrossRef]
- Osada, M.; Iwabuchi, H.; Aoki, T.; Sasaki, K.; Ushijima, H.; Ozawa, T. Economic evaluation of artificial insemination of sex-sorted semen on a Brown Swiss dairy farm—A case study. Anim. Sci. J. 2019, 90, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Moreno, J.F. Review: Semen sexing–current state of the art with emphasis on bovine species. Animal 2018, 12, s85–s96. [Google Scholar] [CrossRef]
- Seidel, G. Economics of selecting for sex: The most important genetic trait. Theriogenology 2003, 59, 585–598. [Google Scholar] [CrossRef]
- Norman, H.; Hutchison, J.; Miller, R. Use of sexed semen and its effect on conception rate, calf sex, dystocia, and stillbirth of Holsteins in the United States. J. Dairy Sci. 2010, 93, 3880–3890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, D.; Seidel, G. History of commercializing sexed semen for cattle. Theriogenology 2008, 69, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Naniwa, Y.; Sakamoto, Y.; Toda, S.; Uchiyama, K. Bovine sperm sex-selection technology in Japan. Reprod. Med. Biol. 2018, 18, 17–26. [Google Scholar] [CrossRef]
- Hall, J.; Glaze, J. REVIEW: System application of sexed semen in beef cattle. Prof. Anim. Sci. 2014, 30, 279–284. [Google Scholar] [CrossRef]
- Squires, E. Current Reproductive Technologies Impacting Equine Embryo Production. J. Equine Vet. Sci. 2020, 89, 102981. [Google Scholar] [CrossRef]
- Cuervo-Arango, J. Choosing the sex of the offspring in a commercial equine embryo transfer center. Med. Weter. 2015, 71, 195–197. [Google Scholar]
- Spinaci, M.; Perteghella, S.; Chlapanidas, T.; Galeati, G.; Vigo, D.; Tamanini, C.; Bucci, D. Storage of sexed boar spermatozoa: Limits and perspectives. Theriogenology 2016, 85, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Altomonte, I.; Salari, F.; Caroli, A.M. Short communication: Monitoring nutritional quality of Amiata donkey milk: Effects of lactation and productive season. J. Dairy Sci. 2014, 97, 6819–6822. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Pucciarelli, S.; Santini, G.; Klimanova, Y.; Polzonetti, V.; Polidori, P. B-Vitamins Determination in Donkey Milk. Beverages 2020, 6, 46. [Google Scholar] [CrossRef]
- Bathgate, R.; Mace, N.; Heasman, K.; Evans, G.; Maxwell, W.; De Graaf, S. Birth of Kids after Artificial Insemination with Sex-Sorted, Frozen-Thawed Goat Spermatozoa. Reprod. Domest. Anim. 2013, 48, 893–898. [Google Scholar] [CrossRef]
- Hermes, R.; Behr, B.; Hildebrandt, T.B.; Blottner, S.; Sieg, B.; Frenzel, A.; Knieriem, A.; Saragusty, J.; Rath, D. Sperm sex-sorting in the Asian elephant (Elephas maximus). Anim. Reprod. Sci. 2009, 112, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.; Maxwell, W. A review on reproductive biotechnologies for conservation of endangered mammalian species. Anim. Reprod. Sci. 2007, 99, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Behr, B.; Rath, D.; Hildebrandt, T.; Goeritz, F.; Blottner, S.; Portas, T.; Bryant, B.; Sieg, B.; Knieriem, A.; De Graaf, S.; et al. Germany/Australia Index of Sperm Sex Sortability in Elephants and Rhinoceros. Reprod. Domest. Anim. 2009, 44, 273–277. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Roth, T.; Stoops, M.; Ball, R.; Steinman, K.; Montano, G.; Love, C.; Robeck, T. Sperm sex-sorting and preservation for managing the sex ratio and genetic diversity of the southern white rhinoceros (Ceratotherium simum simum). Anim. Reprod. Sci. 2015, 152, 137–153. [Google Scholar] [CrossRef]
- George, S.M. Millions of missing girls: From fetal sexing to high technology sex selection in India. Prenat. Diagn. 2006, 26, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kippen, R.; Gray, E.; Evans, A. High and growing disapproval of sex-selection technology in Australia. Reprod. Health 2018, 15, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, B. Upsetting the balance on sex selection. Bioethics 2019, 33, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Dejarnette, J.M.; Leach, M.A.; Nebel, R.L.; Marshall, C.E.; McCleary, C.R.; Moreno, J.F. Effects of sex-sorting and sperm dosage on conception rates of Holstein heifers: Is comparable fertility of sex-sorted and conventional semen plausible? J. Dairy Sci. 2011, 94, 3477–3483. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Locke, J.; Bonacker, R.; Knickmeyer, E.; Wilson, D.; Vishwanath, R.; Arnett, A.; Smith, M.; Patterson, D. Evaluation of SexedULTRA 4M™ sex-sorted semen in timed artificial insemination programs for mature beef cows. Theriogenology 2019, 123, 100–107. [Google Scholar] [CrossRef]
- Noguchi, M.; Yoshioka, K.; Hikono, H.; Iwagami, G.; Suzuki, C.; Kikuchi, K. Centrifugation on Percoll density gradient enhances motility, membrane integrity and in vitro fertilizing ability of frozen–thawed boar sperm. Zygote 2013, 23, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Andara, K.; Briones, E.; Felmer, R. Bovine sperm separation by Swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reprod. Biol. 2017, 17, 126–132. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Gloria, A.; Carluccio, A.; Wegher, L.; Robbe, D.; Befacchia, G.; Contri, A. Single and double layer centrifugation improve the quality of cryopreserved bovine sperm from poor quality ejaculates. J. Anim. Sci. Biotechnol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Wang, Y.; Cao, F.; Yu, C.; Gao, T.; Xia, X.; Wu, J.; Chen, L. Sperm enrichment from poor semen samples by double density gradient centrifugation in combination with swim-up for IVF cycles. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.Y.; Byskov, A.G. Enhanced separation of X and Y bearing sperm cells by a combined density gradient centrifugation evaluated by fluorescencein situhybridization of the Y-chromosome. Acta Obstet. Gynecol. Scand. 1997, 76, 131–134. [Google Scholar] [CrossRef]
- Underwood, S.; Bathgate, R.; Maxwell, W.; Evans, G. Development of Procedures for Sex-sorting Frozen-Thawed Bovine Spermatozoa. Reprod. Domest. Anim. 2009, 44, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Lucio, A.; Resende, M.; Dernowseck-Meirelles, J.; Perini, A.; Oliveira, L.; Miguel, M.; Carmo, A.; Tomita, S.; Alves, B.; Fazano, F.; et al. Assessment of swim-up and discontinuous density gradient in sperm sex preselection for bovine embryo production. Arq. Bras. Med. Vet. Zootec. 2012, 64, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Kanesharatnam, N.; Eswaramohan, T.; Balasubramaniam, K.A. Preliminary Study for Sperm Sexing by Using Sucrose Den-sity Gradients in Jersey Bull at Artificial Insemination Centre at Thirunelvely (Northern Province of Sri Lanka). In Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics, Chennai, India, 10–11 May 2012; Volume 31, pp. 37–40. [Google Scholar]
- Promthep, K.; Satitmanwiwat, S.; Kitiyanant, N.; Tantiwattanakul, P.; Jirajaroenrat, K.; Sitthigripong, R.; Singhapo, C. Practical use of percoll density gradient centrifugation on sperm sexdetermination in commercial dairy farm in Thailand. Indian J. Anim. Res. 2016, 50, 310–313. [Google Scholar]
- Lin, S.-P.; Lee, R.K.-K.; Tsai, Y.-J.; Hwu, Y.-M.; Lin, M.-H. Separating X-Bearing Human Spermatozoa Through a Discontinuous Percoll Density Gradient Proved to Be Inefficient by Double-Label Fluorescent In Situ Hybridization. J. Assist. Reprod. Genet. 1998, 15, 565–569. [Google Scholar] [CrossRef]
- Wolf, C.A.; Brass, K.E.; Rubin, M.I.B.; Pozzobon, S.E.; Mozzaquatro, F.D.; De La Corte, F.D. The effect of sperm selection by Percoll or swim-up on the sex ratio of in vitro produced bovine embryos. Anim. Reprod. 2008, 5, 110–115. [Google Scholar]
- Esmaeilpour, T.; Elyasi, L.; Bahmanpour, S.; Ghannadi, A.; Monabbati, A.; Dehghani, F.; Kazerooni, M. Effect of combined density gradient centrifugation on X-and Y-sperm separation and chromatin integrity. Iran. J. Reprod. Med. 2012, 10, 435–440. [Google Scholar] [PubMed]
- Javed, M.; Laqwer, R.; Mahouk, B.; Kannachath, A.; Najashi, S.; Sufyan, H. Does gradients or swim up procedure produce baby of desired gender? Obstet. Gynecol. Int. J. 2019, 10, 56–59. [Google Scholar] [CrossRef]
- Hadi, I.H.; Mossa, H.L.S. Gender selection by Percoll® gradients by using intra-peritoneal insemination in mice: As a model of human. EurAsian J. BioSci. 2020, 14, 2385–2390. [Google Scholar]
- Reinsalu, O.; Scheler, O.; Mikelsaar, R.; Mikelsaar, A.-V.; Hallap, T.; Jaakma, Ü.; Padrik, P.; Kavak, A.; Salumets, A.; Kurg, A. A dual colour FISH method for routine validation of sexed Bos taurus semen. BMC Vet. Res. 2019, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Flook, J.P.; Look, M.V. Flow cytometry of X and Y chromosome-bearing sperm for DNA using an improved preparation method and staining with Hoechst 33342. Gamete Res. 1987, 17, 203–212. [Google Scholar] [CrossRef]
- Corral-Baqués, M.I.; Rivera, M.M.; Rigau, T.; Rodríguez-Gil, J.E.; Rigau, J. The effect of low-level laser irradiation on dog spermatozoa motility is dependent on laser output power. Lasers Med. Sci. 2008, 24, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.B.; Ma, Y.; Li, J.; Wu, G.Q.; Li, D.J.; Na Ni, Y.; Lv, C.R.; Zhu, L.; Hong, Q.H. Effects of Hoechst33342 staining on the viability and flow cytometric sex-sorting of frozen-thawed ram sperm. Cryobiology 2015, 70, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Domingues, W.B.; Da Silveira, T.L.R.; Komninou, E.R.; Monte, L.G.; Remião, M.H.; Dellagostin, O.A.; Corcini, C.D.; Junior, A.S.V.; Seixas, F.K.; Collares, T.; et al. Flow cytometric sex sorting affects CD4 membrane distribution and binding of exogenous DNA on bovine sperm cells. Zygote 2017, 25, 519–528. [Google Scholar] [CrossRef]
- Balao da Silva, C.M.; Ortega-Ferrusola, C.; Morrell, J.M.; Rodriguez-Martinez, H.; Pena, F.J. Flow Cytometric Chromoso-mal Sex Sorting of Stallion Spermatozoa Induces Oxidative Stress on Mitochondria and Genomic DNA. Reprod. Domest. Anim. 2016, 51, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, N.; Namyari, M.; Rasmi, Y.; Pourjabali, M.; Chodari, L. Protective effect of curcumin on fertility of rats after expo-sure to compact fluorescent lamps: An experimental study. Int. J. Reprod. BioMed. 2018, 16, 447–454. [Google Scholar]
- Dararatana, N.; Tuantranont, A.; Wongtawan, T.; Oonkhanond, B. The dielectrophoresis microfluidic chip for cell separation: Case study of separation of floating cell and moving cells. In Proceedings of the 2015 8th Biomedical Engineering International Conference (BMEiCON), Pattaya, Thailand, 25–27 November 2015; pp. 1–5. [Google Scholar]
- Carvalho, J.; Sartori, R.; Machado, G.; Mourão, G.; Dode, M. Quality assessment of bovine cryopreserved sperm after sexing by flow cytometry and their use in in vitro embryo production. Theriogenology 2010, 74, 1521–1530. [Google Scholar] [CrossRef]
- Macedo, G.G.; de Sá Filho, M.F.; Vasconcellos Sala, R.; Ferreira Mendanha, M.; Pires de Campos Filho, M.; Sampaio Baruselli, P. The Use Of Sex-Sorted Sperm For Reproductive Programs In cattle, Success in Artificial Insemination. In Quality of Semen and Diagnostics Employed; Lemma, A., Ed.; IntechOpen: London, UK, 2013; pp. 39–61. [Google Scholar]
- Seidel, G. Update on sexed semen technology in cattle. Animal 2014, 8, 160–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.; Locke, J.; Vishwanath, R.; Hall, J.; Ellersieck, M.; Smith, M.; Patterson, D. Effective use of SexedULTRA™ sex-sorted semen for timed artificial insemination of beef heifers. Theriogenology 2017, 98, 88–93. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, H.; Makri, D.; Maalouf, W.E.; Reese, S.; Kölle, S. Bovine Sperm Sexing Alters Sperm Morphokinetics and Subsequent Early Embryonic Development. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostek, A.; Janta, A.; Ciereszko, A. Proteomic comparison of non-sexed and sexed (X-bearing) cryopreserved bull semen. Anim. Reprod. Sci. 2020, 221, 106552. [Google Scholar] [CrossRef]
- Magata, F.; Urakawa, M.; Matsuda, F.; Oono, Y. Developmental kinetics and viability of bovine embryos produced in vitro with sex-sorted semen. Theriogenology 2021, 161, 243–251. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Agricultural Outlook for Markets and Income 2019–2030; European Commission: Luxembourg, 2019. [Google Scholar]
- Bayram, M. Future of Meat Industry. MOJ Food Process. Technol. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Veterinary Artificial Insemination Market Size, Share & Trends Analysis Report By Animal Type (Cattle, Swine, Sheep, Canine, Equine), By Product (Normal Semen, Sexed Semen), by End Use, and Segment Forecasts, 2021–2028. Report ID:GVR-3-68038-570-0. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/veterinary-artificial-insemination-market (accessed on 1 April 2021).
- Maxwell, W.; Evans, G.; Hollinshead, F.; Bathgate, R.; de Graaf, S.; Eriksson, B.; Gillan, L.; Morton, K.; O’Brien, J. Integration of sperm sexing technology into the ART toolbox. Anim. Reprod. Sci. 2004, 82, 79–95. [Google Scholar] [CrossRef]
- De Vries, A.; Overton, M.; Fetrow, J.; Leslie, K.; Eicker, S.; Rogers, G. Exploring the Impact of Sexed Semen on the Structure of the Dairy Industry. J. Dairy Sci. 2008, 91, 847–856. [Google Scholar] [CrossRef]
- Chebel, R.C.; Guagnini, F.S.; Santos, J.E.P.; Fetrow, J.P.; Lima, J.R. Sex-sorted semen for dairy heifers: Effects on reproductive and lactational performances. J. Dairy Sci. 2010, 93, 2496–2507. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.B.; Rutledge, J.J.; Fischer-Brown, A.; VanEtten, T.; Malusky, S.; Beebe, D.J. Application of sexed semen technology to in vitro embryo production in cattle. Theriogenology 2006, 65, 219–227. [Google Scholar] [CrossRef]
- Peippo, J.; Vartia, K.; Kananen-Anttila, K.; Räty, M.; Korhonen, K.; Hurme, T.; Myllymaki, H.; Sairanen, A.; Mäki-Tanila, A. Embryo production from superovulated Holstein-Friesian dairy heifers and cows after insemination with frozen-thawed sex-sorted X spermatozoa or unsorted semen. Anim. Reprod. Sci. 2009, 111, 80–92. [Google Scholar] [CrossRef]
- Stewart, B.; Block, J.; Morelli, P.; Navarette, A.; Amstalden, M.; Bonilla, L.; Hansen, P.; Bilby, T. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J. Dairy Sci. 2011, 94, 3437–3445. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, C.; Morotti, F.; Untura, R.; Pontes, J.; Pellegrino, M.; Campolina, J.; Seneda, M.; Barbosa, F.; Henry, M. Use of sexed sorted semen for fixed-time artificial insemination or fixed-time embryo transfer of in vitro–produced embryos in cattle. Theriogenology 2016, 86, 888–893. [Google Scholar] [CrossRef]
- Lopes, J.S.; Alcázar-Triviño, E.; Soriano-Úbeda, C.; Hamdi, M.; Cánovas, S.; Rizos, D.; Coy, P. Reproductive Outcomes and Endocrine Profile in Artificially Inseminated versus Embryo Transferred Cows. Animals 2020, 10, 1359. [Google Scholar] [CrossRef]
- Schenk, J.; Suh, T.; Seidel, G. Embryo production from superovulated cattle following insemination of sexed sperm. Theriogenology 2006, 65, 299–307. [Google Scholar] [CrossRef] [PubMed]
- DeJarnette, J.; Nebel, R.; Marshall, C. Evaluating the success of sex-sorted semen in US dairy herds from on farm records. Theriogenology 2009, 71, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dawod, A.; Elbaz, H.T. Effect of sexed semen, puberty and breeding ages on fertility of Holstein dairy heifers treated with double Ovsynch protocol. Trop. Anim. Health Prod. 2020, 52, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Borchersen, S.; Peacock, M.; Danish, A.I. 2009 field data with sexed semen. Theriogenology 2009, 71, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Seidel, G.; Schenk, J. Pregnancy rates in cattle with cryopreserved sexed sperm: Effects of sperm numbers per inseminate and site of sperm deposition. Anim. Reprod. Sci. 2008, 105, 129–138. [Google Scholar] [CrossRef]
- Mikkola, M.; Andersson, M.; Taponen, J. Transfer of cattle embryos produced with sex-sorted semen results in impaired pregnancy rate and increased male calf mortality. Theriogenology 2015, 84, 1118–1122. [Google Scholar] [CrossRef]
- Joezy-Shekalgorabi, S.; Maghsoudi, A.; Mansourian, M.R. Reproductive performance of sexed versus conventional semen in Holstein heifers in various semiarid regions of Iran. Ital. J. Anim. Sci. 2017, 16, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Drake, E.; Holden, S.; Aublet, V.; Doyle, R.; Millar, C.; Moore, S.; Maicas, C.; Randi, F.; Cromie, A.; Lonergan, P.; et al. Evaluation of delayed timing of artificial insemination with sex-sorted sperm on pregnancy per artificial insemination in seasonal-calving, pasture-based lactating dairy cows. J. Dairy Sci. 2020, 103, 12059–12068. [Google Scholar] [CrossRef]
- Research and Markets. Sperm Bank Market Size Analysis Report by Service Type (Sperm Storage, Semen Analysis, Genetic Consultation), by Do-nor Type (Known, Anonymous), by End Use, and Segment Forecasts, 2019–2025. Report ID:978-1-68038-423-9. 2019. Available online: https://www.researchandmarkets.com/reports/4209726/sperm-bank-market-size-analysis-report-by-service (accessed on 6 April 2021).
- Salama, M.; Isachenko, V.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Westphal, L.M.; Inhorn, M.C.; Patrizio, P. Cross border reproductive care (CBRC): A growing global phenomenon with multidimensional implications (a systematic and critical review). J. Assist. Reprod. Genet. 2018, 35, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Burinaru, T.A.; Avram, M.; Avram, A.; Mărculescu, C.; Tincu, B.; Ţucureanu, V.; Matei, A.; Militaru, M. Detection of Circulating Tumor Cells Using Microfluidics. ACS Comb. Sci. 2018, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Turcan, I.; Olariu, M.A. Dielectrophoretic Manipulation of Cancer Cells and Their Electrical Characterization. ACS Comb. Sci. 2020, 22, 554–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X. Dielectrophoretic microfluidic device for separation of red blood cells and platelets: A model-based study. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Knowlton, S.M.; Sadasivam, M.; Tasoglu, S. Microfluidics for sperm research. Trends Biotechnol. 2015, 33, 221–229. [Google Scholar] [CrossRef]
- Lee, D.; Hwang, B.; Kim, B. The potential of a dielectrophoresis activated cell sorter (DACS) as a next generation cell sorter. Micro Nano Syst. Lett. 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.B.; Rubessa, M. Integration of microfluidics and mammalian IVF. Mol. Hum. Reprod. 2016, 23, 248–256. [Google Scholar] [CrossRef]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef] [Green Version]
- Phiphattanaphiphop, C.; Leksakul, K.; Phatthanakun, R.; Khamlor, T. A novel microfluidic chip-based sperm-sorting device constructed using design of experiment method. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Comizzoli, P.; Holt, W.V. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol. Reprod. 2019, 101, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gac, S.; Ferraz, M.; Venzac, B.; Comizzoli, P. Understanding and Assisting Reproduction in Wildlife Species Using Microfluidics. Trends Biotechnol. 2020, 20, 30234–30241. [Google Scholar] [CrossRef]
- Barchanski, A.; Taylor, U.; Klein, S.; Petersen, S.; Rath, D.; Barcikowski, S. Golden Perspective: Application of Laser-Generated Gold Nanoparticle Conjugates in Reproductive Biology. Reprod. Domest. Anim. 2011, 46, 42–52. [Google Scholar] [CrossRef]
- Gamrad, L.; Mancini, R.; Werner, D.; Tiedemann, D.; Taylor, U.; Ziefuß, A.; Rehbock, C.; Klein, S.; Kues, W.; Barcikowski, S.; et al. Triplex-hybridizing bioconjugated gold nanoparticles for specific Y-chromosome sequence targeting of bull spermatozoa. Analyst 2017, 142, 2020–2028. [Google Scholar] [CrossRef]
- Mancini, R. Detection of Y-Chromosome Bearing Bovine Sperm Using Laser-Generated Gold Nanoparticle BIO-Conjugates. Ph.D. Thesis, Hamburg University, Hamburg, Germany, 2015. Available online: https://elib.tiho-hannover.de/servlets/MCRFileNodeServlet/etd_derivate_00000472/mancinir_ss15.pdf (accessed on 14 February 2021).
- Dominguez, E.; Moreno-Irusta, A.; Castex, H.R.; Bragulat, A.F.; Ugaz, C.; Clemente, H.; Giojalas, L.; Losinno, L. Sperm Sexing Mediated by Magnetic Nanoparticles in Donkeys, a Preliminary In Vitro Study. J. Equine Vet. Sci. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Ramírez Castex, H.; Domínguez, E.; Moreno Irusta, A.; Ugaz, C.; Clemente, H.; Ayarza, E. Nano-partículas magnéticas para separación de espermatozoides X en semen equino-Resultados preliminares. Reprod. Asis. Equi. 2017, 2, 85–100. [Google Scholar]
- Wongtawan, T.; Dararatana, N.; Thongkittidilok, C.; Kornmatitsuk, S.; Oonkhanond, B. Enrichment of bovine X-sperm using microfluidic dielectrophoretic chip: A proof-of- concept study. Heliyon 2020, 6, e05483. [Google Scholar] [CrossRef]
- Nakao, S.; Takeo, T.; Watanabe, H.; Kondoh, G.; Nakagata, N. Successful selection of mouse sperm with high viability and fertility using microfluidics chip cell sorter. Sci. Rep. 2020, 10, 8862. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Feng, H.; Jafek, A.; DesPain, D.; Jenkins, T.; Gale, B. Microfluidic—Based sperm sorting & analysis for treatment of male infertility. Transl. Androl. Urol. 2018, 7, S336–S347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neculai-Valeanu, A.-S.; Ariton, A.M. Game-Changing Approaches in Sperm Sex-Sorting: Microfluidics and Nanotechnology. Animals 2021, 11, 1182. https://doi.org/10.3390/ani11041182
Neculai-Valeanu A-S, Ariton AM. Game-Changing Approaches in Sperm Sex-Sorting: Microfluidics and Nanotechnology. Animals. 2021; 11(4):1182. https://doi.org/10.3390/ani11041182
Chicago/Turabian StyleNeculai-Valeanu, Andra-Sabina, and Adina Mirela Ariton. 2021. "Game-Changing Approaches in Sperm Sex-Sorting: Microfluidics and Nanotechnology" Animals 11, no. 4: 1182. https://doi.org/10.3390/ani11041182






