Clinical Cytogenetics of the Dog: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Sex Chromosome Aneuploidies
3. Leukocyte Chimerism XX/XY
| Proportion of XX and XY Cell Lines [%] | Breed | Phenotypic Sex Considered by Owners | Characteristic Feature of Phenotype | Reference |
|---|---|---|---|---|
| Lack of information | Schipperke | female | Enlarged clitoris, testis and ovotestis, uterus, | [34] |
| 43/57 | Pug | female | Enlarged clitoris, hypospadias, no signs of estrus, testis and ovotestis | [23] |
| 45/55 | Dachshund | male | Abnormal urogenital tract, hematuria, ovaries | [35] |
| Lack of information | Spaniel × Papillon | unknown | Ovaries | [36] |
| Lack of information | American Eskimo | female | Normal vulva and clitoris, ovotestis | [37] |
| Lack of information | Spaniel | unknown | Small penis, empty rudimentary scrotum, uterus, ovaries with reduced number of follicles | [38] |
| 85/15 | Belgium Shepherd | male | Aggressive behavior, intersexuality, abdominal testes, underdeveloped penis, urethra ended under the anus, vas deferens connected to an oviduct, blind uterus | [29] |
| Lack of information | Fila Brasileiro | male | Prepuce-like structure located closer to the anus, testicles with an immature epididymides | [39] |
| 43/57 | Border Terrier | male | Undeveloped penis, ovarian-like structure | [30] |
| 70/30 | Wirehaired Pointing Griffon | female | Primary anestrus, juvenile vulva, enlarged clitoris, testis | [28] |
| 78/22 | Shih Tzu | ambiguous | Residual penis with a prepuce located in a position typical of a male, prostate, gonads remained undetectable | [31] |
| 20/80 | French Bulldog | female | Enlarge clitoris, ovotestes | [32] |
| 30/70 | Great Dane | female | Underdeveloped internal reproductive organs, rudimentary testicles | [40] |
| 54/46 | Great Dane | female | Undeveloped foreskin | [14] |
4. Structural Chromosome Rearrangements
| Chromosome Involved in the Fusion | Breed (Number of Cases) | Characteristic Feature of Phenotype | Reference |
|---|---|---|---|
| Not identified | Mixed terrier (1) | Cardiac defect | [48] |
| Not identified | Miniature Poodle (1) | Bone chondrodysplasia | [49] |
| Not identified | Setter–Retriever cross (1) | Phenotypically and clinically normal female | [50] |
| 13 and 17 | Golden Retriever cross (1) | Normal, fertile female | [51] |
| 13 and 23 | Golden Retriever-type (1 + 11 offspring) | Normal phenotype with the exception of congenital inguinal hernia in two female homozygotes in progeny | [52] |
| 1 and 31 | Poodle (6, including 1 homozygote male) | Normal phenotype | [53] |
| 21 and 33 | Walker Hound (1 + sister and 4 offspring) | Narrow vulva, absence of estrus | [43] |
| Not identified | Mixed breed (1) | Infertile female | [44] |
| 8 and 14 | West Highland White Terrier (1) | Infertile female, normal reproductive organs | [45] |
| 5 and 23 | Bernese Mountain Dog (1) | XX DSD, SRY-negative enlarged clitoris, testicle, ovotestis, uterus | [42] |
| Not identified | Miniature Schnauzer (1) | XY DSD, PMDS (Persistent Mullerian Duct Syndrome) | [46] |
| 1 and unidentified | Miniature Schnauzer (1) | XY DSD, PMDS | [47] |
| Not identified | American Staffordshire Terrier (1) | XX DSD, SRY-negative (Sex Determining Region Y) enlarged clitoris, ovotestis, | [14] |
5. Cytogenetic Characterization of Other Forms of DSD Cases
6. Sperm Cytogenetics
7. Cancer Cytogenetics
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, C.; Pörtl, D. How Old Are (Pet) Dog Breeds? Pet Behav. Sci. 2019, 29–37. [Google Scholar] [CrossRef]
- Breen, M. Canine Cytogenetics — from Band to Basepair. Cytogenet. Genome Res. 2008, 120, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Świtoński, M.; Reimann, N.; Bosma, A.A.; Long, S.; Bartnitzke, S.; Pieńkowska, A.; Moreno-Milan, M.M.; Fischer, P. Report on the Progress of Standardization of the G-Banded Canine (Canis Familiaris) Karyotype. Chromosome Res. 1996, 4, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Bullerdiek, J.; Langford, C.F. The DAPI banded karyotype of the domestic dog (Canis familiaris) generated using chromosome-specific paint probes. Chromosome Res. 1999, 7, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Hitte, C.; Lorentzen, T.D.; Thomas, R.; Cadieu, E.; Sabacan, L.; Scott, A.; Evanno, G.; Parker, H.G.; Kirkness, E.F.; et al. An Integrated 4249 Marker FISH/RH Map of the Canine Genome. BMC Genom. 2004, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Smith, K.C.; Ostrander, E.A.; Galibert, F.; Breen, M. Chromosome Aberrations in Canine Multicentric Lymphomas Detected with Comparative Genomic Hybridisation and a Panel of Single Locus Probes. Br. J. Cancer 2003, 89, 1530–1537. [Google Scholar] [CrossRef] [Green Version]
- Reimann-Berg, N.; Bullerdiek, J.; Escobar, H.; Nolte, I. Chromosome analyses in dogs. Tierärztl. Prax. Ausg. K Kleintiere Heimtiere 2012, 40, 191–196. [Google Scholar] [CrossRef]
- Smith, F.W.K.; Buoen, L.C.; Weber, A.F.; Johnston, S.D.; Randolph, J.F.; Waters, D.J. X-Chromosomal Monosomy (77, XO) in a Doberman Pinscher With Gonadal Dysgenesis. J. Vet. Intern. Med. 1989, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Löfstedt, R.M.; Buoen, L.C.; Weber, A.F.; Johnston, S.D.; Huntington, A.; Concannon, P.W. Prolonged Proestrus in a Bitch with X Chromosomal Monosomy (77,XO). J. Am. Vet. Med. Assoc. 1992, 200, 1104–1106. [Google Scholar]
- Switonski, M. Two Cases of Infertile Bitches with 78,XX/77,X Mosaic Karyotype: A Need for Cytogenetic Evaluation of Dogs With Reproductive Disorders. J. Hered. 2003, 94, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Mayenco-Aguirre, A.M.; Padilla, J.A.; Flores, J.M.; Daza, M.A. Canine Gonadal Dysgenesis Syndrome: A Case of Mosaicism (77,XO-78,XX). Vet. Rec. 1999, 145, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, T.; Das, P.J.; Avila, F.; Chowdhary, B.P. The Pseudoautosomal Region and Sex Chromosome Aneuploidies in Domestic Species. Sex. Dev. 2012, 6, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, T.; Chowdhary, B.P. The Eutherian Pseudoautosomal Region. Cytogenet. Genome Res. 2015, 147, 81–94. [Google Scholar] [CrossRef]
- Szczerbal, I.; Nizanski, W.; Dzimira, S.; Nowacka-Woszuk, J.; Stachecka, J.; Biezynski, J.; Ligocka, Z.; Jagodka, D.; Fabian-Kurzok, H.; Switonski, M. Chromosome Abnormalities in Dogs with Disorders of Sex Development (DSD). Anim. Reprod. Sci. 2021. submitted. [Google Scholar]
- Johnston, S.D.; Buoen, L.C.; Weber, A.F.; Madl, J.E. X Trisomy in an Airedale Bitch with Ovarian Dysplasia and Primary Anestrus. Theriogenology 1985, 24, 597–607. [Google Scholar] [CrossRef]
- Switonski, M.; Godynicki, S.; Jackowiak, H.; Piekowska, A.; Turczuk-Bierla, I.; Szymas, J.; Golinski, P.; Bereszynski, A. Brief Communication. X Trisomy in an Infertile Bitch: Cytogenetic, Anatomic, and Histologic Studies. J. Hered. 2000, 91, 149–150. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, B.; Paulino, F.O.; Sauza, L.M.; Gomes, H.F. Infertility Related to X-Trisomy in a Labrador Retriever Bitch. J. Israeli Vet. Med. Assoc. 2003, 58, 123–124. [Google Scholar]
- O’Connor, C.L.; Schweizer, C.; Gradil, C.; Schlafer, D.; Lopate, C.; Prociuk, U.; Meyers-Wallen, V.N.; Casal, M.L. Trisomy-X with Estrous Cycle Anomalies in Two Female Dogs. Theriogenology 2011, 76, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Clough, E.; Pyle, R.L.; Hare, W.C.D.; Kelly, D.F.; Patterson, D.F. An XXY Sex-Chromosome Constitution in a Dog with Testicular Hypoplasia and Congenital Heart Disease. Cytogenet. Genome Res. 1970, 9, 71–77. [Google Scholar] [CrossRef]
- Marshall, L.S.; Oehlert, M.L.; Haskins, M.E.; Selden, J.R.; Patterson, D.F. Persistent Müllerian Duct Syndrome in Miniature Schnauzers. J. Am. Vet. Med. Assoc. 1982, 181, 798–801. [Google Scholar]
- Goldschmidt, B.; El-Jaick, K.B.; Souza, L.M.; Carvalho, E.C.Q.; Moura, V.L.S.; Benevides Filho, I.M. Cryptorchidism Associated with 78,XY/79,XXY Mosaicism in Dog. Israel J. Vet. Med. 2001, 56, 56e8. [Google Scholar]
- Reimann-Berg, N.; Escobar, H.M.; Nolte, I.; Bullerdiek, J. Testicular Tumor in an XXY Dog. Cancer Genet. Cytogenet. 2008, 183, 114–116. [Google Scholar] [CrossRef]
- Bosu, W.T.; Chick, B.F.; Basrur, P.K. Clinical, Pathologic and Cytogenetic Observations on Two Intersex Dogs. Cornell Vet. 1978, 68, 375–390. [Google Scholar]
- Nie, G.J.; Johnston, S.D.; Hayden, D.W.; Buoen, L.C.; Stephens, M. Theriogenology Question of the Month. Azoospermia Associated with 79,XXY Chromosome Complement (Canine Klinefelter’s Syndrome). J. Am. Vet. Med. Assoc. 1998, 212, 1545–1547. [Google Scholar]
- Biason-Lauber, A. Control of Sex Development. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 163–186. [Google Scholar] [CrossRef] [Green Version]
- Esteves, A.; Bage, R.; Payan-Carreira, R. Freemartinism in cattle. In Ruminants: Anatomy, Behavior and Diseases; Mendes, R.E., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 99–120. [Google Scholar]
- Szczerbal, I.; Nowacka-Woszuk, J.; Dzimira, S.; Matuszczyk, A.; Iskrzak, P.; Switonski, M. Elevated Incidence of Freemartinism in Pigs Detected by Droplet Digital PCR and Cytogenetic Techniques. Livest. Sci. 2019, 219, 52–56. [Google Scholar] [CrossRef]
- Beccaglia, M.; Ronchese, M.; Grieco, V.; Parma, P.; Luvoni, G.C. XX/XY Chimaerism/Mosaicism in a Phenotypically Female Wirehaired Pointing Griffon Dog. In Proceedings of the 7th International Symposium on Canine and Feline Reproduction, Whistler, BC, Canada, 26–29 July 2012. [Google Scholar]
- Genero, E.R.; Moreno-Millán, M.; Ocaña-Quero, J.M. XX/XY Chromosome Chimaerism in an Intersex Dog. Vet. Rec. 1998, 142, 340. [Google Scholar] [CrossRef]
- Kuiper, H.; Distl, O. Intersexuality in dogs: Causes and genetics. DTW Dtsch. Tierarztl. Wochenschr. 2004, 111, 251–258. [Google Scholar]
- Szczerbal, I.; Nowacka-Woszuk, J.; Nizanski, W.; Salamon, S.; Ochota, M.; Dzimira, S.; Atamaniuk, W.; Switonski, M. A Case of Leucocyte Chimerism (78,XX/78,XY) in a Dog with a Disorder of Sexual Development. Reprod. Domest. Anim. 2014, 49, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Szczerbal, I.; Nowacka-Woszuk, J.; Nizanski, W.; Dzimira, S.; Ligocka, Z.; Jastrzebska, A.; Kabala, B.; Biernacik, M.; Przadka, P.; Switonski, M. Disorders of Sex Development Are an Emerging Problem in French Bulldogs: A Description of Six New Cases and a Review of the Literature. Sex. Dev. 2019, 13, 205–211. [Google Scholar] [CrossRef]
- Poth, T.; Breuer, W.; Walter, B.; Hecht, W.; Hermanns, W. Disorders of Sex Development in the Dog—Adoption of a New Nomenclature and Reclassification of Reported Cases. Anim. Reprod. Sci. 2010, 121, 197–207. [Google Scholar] [CrossRef]
- Hare, W.C. Intersexuality in the Dog. Can. Vet. J. Rev. Veterinaire Can. 1976, 17, 7–15. [Google Scholar]
- Weaver, A.D.; Harvey, M.J.; Munro, C.D.; Rogerson, P.; McDonald, M. Phenotypic Intersex (Female Pseudohermaphroditism) in a Dachshund Dog. Vet. Rec. 1979, 105, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.E.; Long, S.E.; Gibbs, C. Disorders of Urination Associated with Canine Intersexuality. J. Small Anim. Pract. 1983, 24, 475–487. [Google Scholar] [CrossRef]
- Johnston, S.D. Premature Gonadal Failure in Female Dogs and Cats. J. Reprod. Fertil. Suppl. 1989, 39, 65–72. [Google Scholar] [PubMed]
- Chaffaux, S.; Cribiu, E. Clinical, Histological and Cytogenetic Observations on Nine Intersex Dogs. Genet. Sel. Evol. 1991, 23, S81. [Google Scholar] [CrossRef]
- Meyers-Wallen, V.N. Inherited abnormalities of sexual development in dogs and cats. In Recent Advances in Small Animal Reproduction; Concannon, P.W., England, G., Ver-stegen, J., Eds.; International Veterinary Information Service, USA, 2001; Available online: https://www.ivis.org/library/recent-advances-small-animal-reproduction/inherited-abnormalities-of-sexual-development (accessed on 10 January 2021).
- Sumner, S.M.; Case, J.B.; Regier, P.J.; Oliveira, L.; Abbott, J.R. Laparoscopic Gonadectomy in a Dog with 78,XX/78,XY Chimerism and Underdeveloped Reproductive Organs. J. Am. Vet. Med. Assoc. 2021, 258, 80–84. [Google Scholar] [CrossRef]
- Szczerbal, I.; Nowacka-Woszuk, J.; Albarella, S.; Switonski, M. Technical Note: Droplet Digital PCR as a New Molecular Method for a Simple and Reliable Diagnosis of Freemartinism in Cattle. J. Dairy Sci. 2019, 102, 10100–10104. [Google Scholar] [CrossRef] [PubMed]
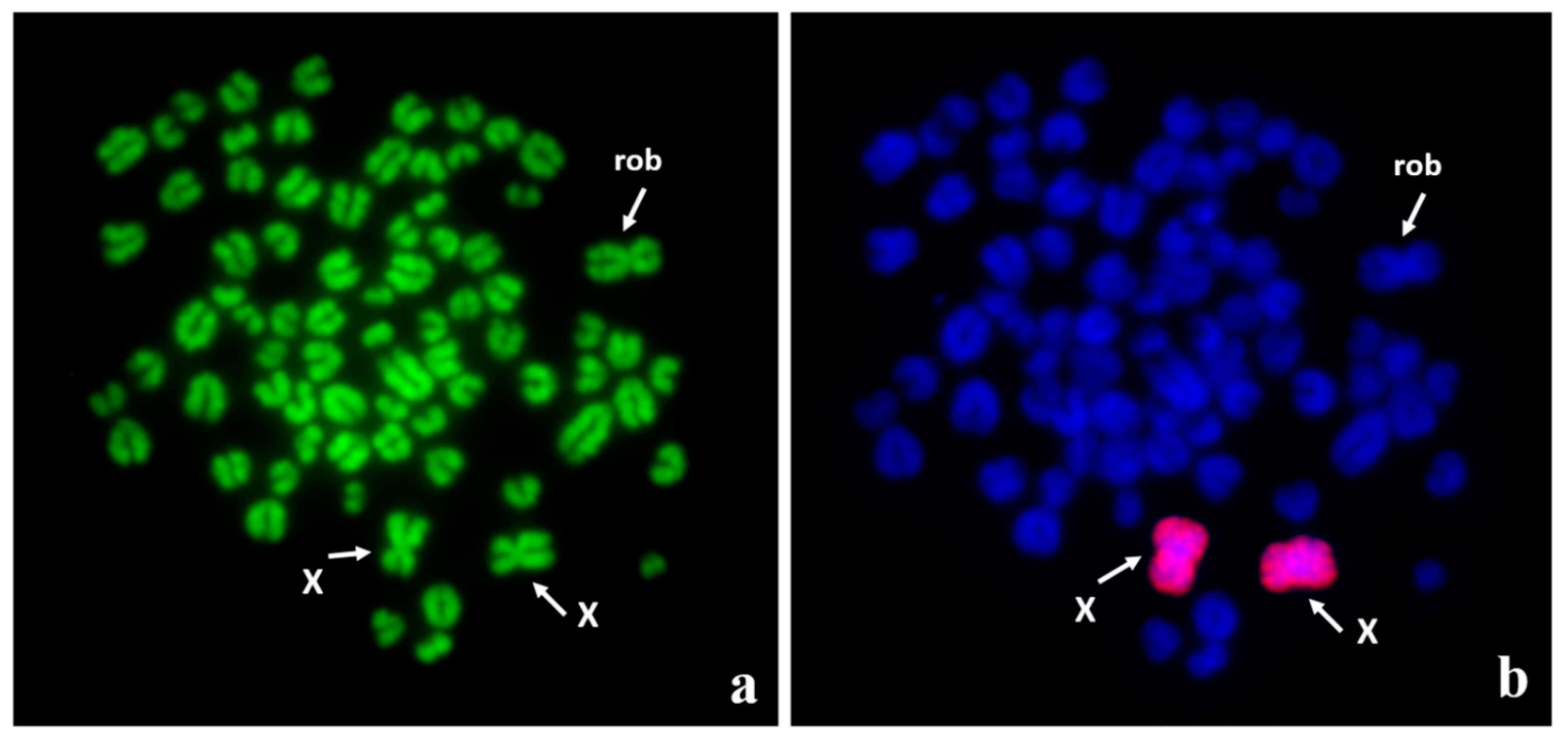
- Switonski, M.; Szczerbal, I.; Nizanski, W.; Kociucka, B.; Bartz, M.; Dzimira, S.; Mikolajewska, N. Robertsonian Translocation in a Sex Reversal Dog (XX, SRY Negative) May Indicate That the Causative Mutation for This Intersexuality Syndrome Resides on Canine Chromosome 23 (CFA23). Sex. Dev. 2011, 5, 141–146. [Google Scholar] [CrossRef]
- Stone, D.M.; Mickelsen, W.D.; Jacky, P.B.; Prieur, D.J. A Novel Robertsonian Translocation in a Family of Walker Hounds. Genome 1991, 34, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Switonski, M.; Slota, E.; Pietrzak, A.; Klukowska, J. Chimerism 78,XX/77,XX, Rb in a bitch revealed by chromosome and microsatellite studies. Vet. Med. Czech. 2000, 45, 296–298. [Google Scholar]
- Switonski, M.; Szczerbal, I.; Skorczyk, A.; Yang, F.; Antosik, P. Robertsonian Translocation (8;14) in an Infertile Bitch (Canis Familiaris). J. Appl. Genet. 2003, 44, 525–527. [Google Scholar] [PubMed]
- Dzimira, S.; Wydooghe, E.; Van Soom, A.; Van Brantegem, L.; Nowacka-Woszuk, J.; Szczerbal, I.; Switonski, M. Sertoli Cell Tumour and Uterine Leiomyoma in Miniature Schnauzer Dogs with Persistent Müllerian Duct Syndrome Caused by Mutation in the AMHR2 Gene. J. Comp. Pathol. 2018, 161, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.M.; Armada, J.L.A.; Penedo, D.M.; Tannouz, V.G.S.; Meyers-Wallen, V.N. Persistent Mullerian Duct Syndrome in a Brazilian Miniature Schnauzer Dog. An. Acad. Bras. Ciênc. 2019, 91, e20180752. [Google Scholar] [CrossRef]
- Shive, R.J.; Hare, W.C.D.; Patterson, D.F. Chromosome Studies in Dogs with Congenital Cardiac Defects. Cytogenet. Genome Res. 1965, 4, 340–348. [Google Scholar] [CrossRef]
- Hare, W.C.; Wilkinson, J.S.; McFeely, R.A.; Riser, W.H. Bone Chondroplasia and a Chromosome Abnormality in the Same Dog. Am. J. Vet. Res. 1967, 28, 583–587. [Google Scholar]
- Ma, N.S.F.; Gilmore, C.E. Chromosomal Abnormality in a Phenotypically and Clinically Normal Dog. Cytogenet. Genome Res. 1971, 10, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.E.; Dias, E.; Cervenka, J. Centric Fusion of Autosomal Chromosomes in a Bitch and Offspring. Am. J. Vet. Res. 1978, 39, 861–864. [Google Scholar]
- Larsen, R.E.; Dias, E.; Flores, G.; Selden, J.R. Breeding Studies Reveal Segregation of a Canine Robertsonian Translocation along Mendelian Proportions. Cytogenet. Cell Genet. 1979, 24, 95–101. [Google Scholar] [CrossRef]
- Mayr, B.; Krutzler, J.; Schleger, W.; Auer, H. A New Type of Robertsonian Translocation in the Domestic Dog. J. Hered. 1986, 77, 127. [Google Scholar] [CrossRef]
- Schelling, C.; Pieńkowska, A.; Arnold, S.; Hauser, B.; Switoński, M. A Male to Female Sex-Reversed Dog with a Reciprocal Translocation. J. Reprod. Fertil. Suppl. 2001, 57, 435–438. [Google Scholar] [PubMed]
- O’Connor, R.E.; Fonseka, G.; Frodsham, R.; Archibald, A.L.; Lawrie, M.; Walling, G.A.; Griffin, D.K. Isolation of Subtelomeric Sequences of Porcine Chromosomes for Translocation Screening Reveals Errors in the Pig Genome Assembly. Anim. Genet. 2017, 48, 395–403. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.E.; Kiazim, L.G.; Rathje, C.C.; Jennings, R.L.; Griffin, D.K. Rapid Multi-Hybridisation FISH Screening for Balanced Porcine Reciprocal Translocations Suggests a Much Higher Abnormality Rate Than Previously Appreciated. Cells 2021, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.L.; Griffin, D.K.; O’Connor, R.E. A New Approach for Accurate Detection of Chromosome Rearrangements That Affect Fertility in Cattle. Animals 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers-Wallen, V.N. Gonadal and Sex Differentiation Abnormalities of Dogs and Cats. Sex. Dev. 2012, 6, 46–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Switonski, M.; Payan-Carreira, R.; Bartz, M.; Nowacka-Woszuk, J.; Szczerbal, I.; Colaço, B.; Pires, M.A.; Ochota, M.; Nizanski, W. Hypospadias in a Male (78,XY.; SRY-Positive) Dog and Sex Reversal Female (78,XX.; SRY-Negative) Dogs: Clinical, Histological and Genetic Studies. Sex. Dev. 2012, 6, 128–134. [Google Scholar] [CrossRef]
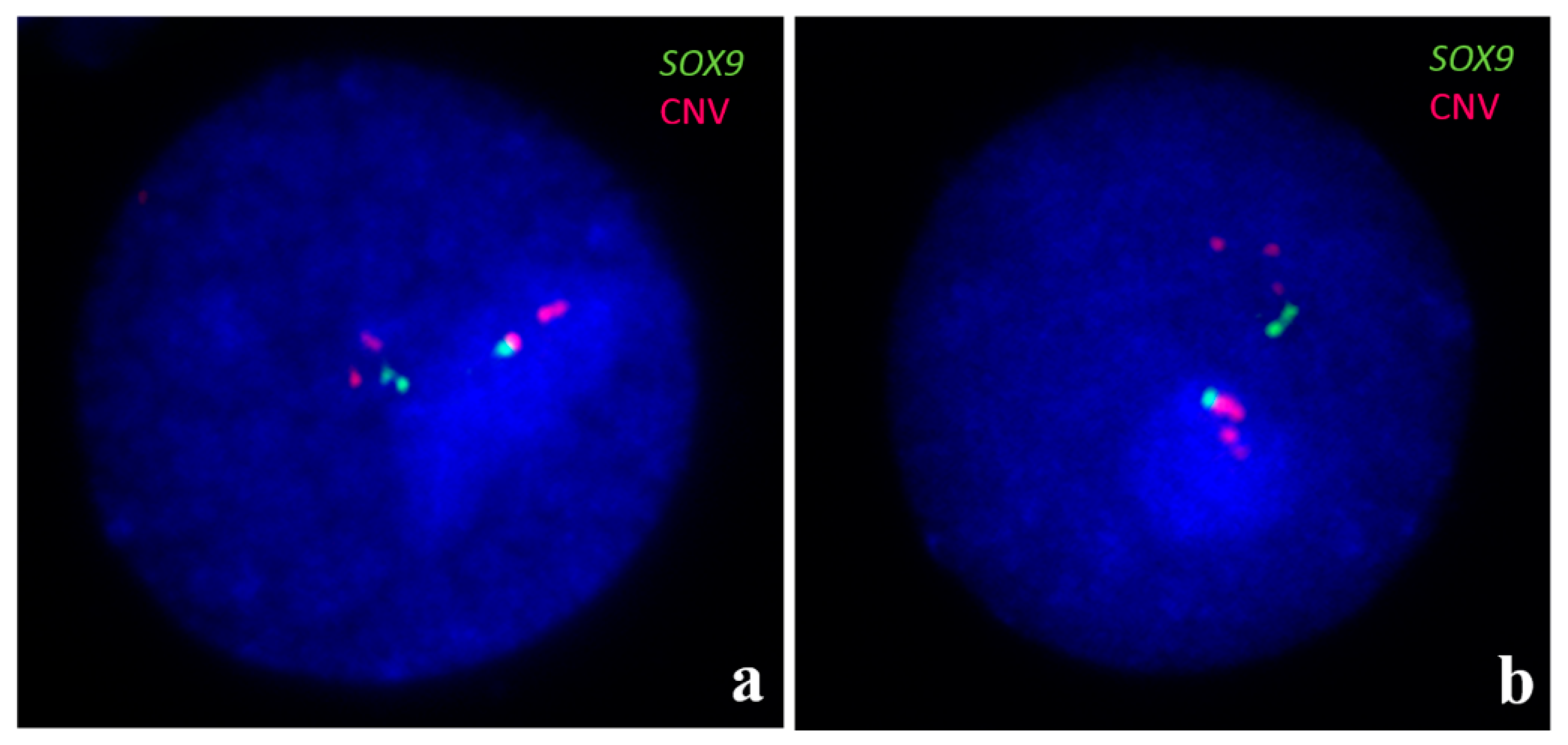
- Rossi, E.; Radi, O.; De Lorenzi, L.; Vetro, A.; Groppetti, D.; Bigliardi, E.; Luvoni, G.C.; Rota, A.; Camerino, G.; Zuffardi, O.; et al. Sox9 Duplications Are a Relevant Cause of Sry-Negative XX Sex Reversal Dogs. PLoS ONE 2014, 9, e101244. [Google Scholar] [CrossRef] [Green Version]
- Albarella, S.; Lorenzi, L.D.; Rossi, E.; Prisco, F.; Riccardi, M.G.; Restucci, B.; Ciotola, F.; Parma, P. Analysis of XX SRY-Negative Sex Reversal Dogs. Animals 2020, 10, 1667. [Google Scholar] [CrossRef]
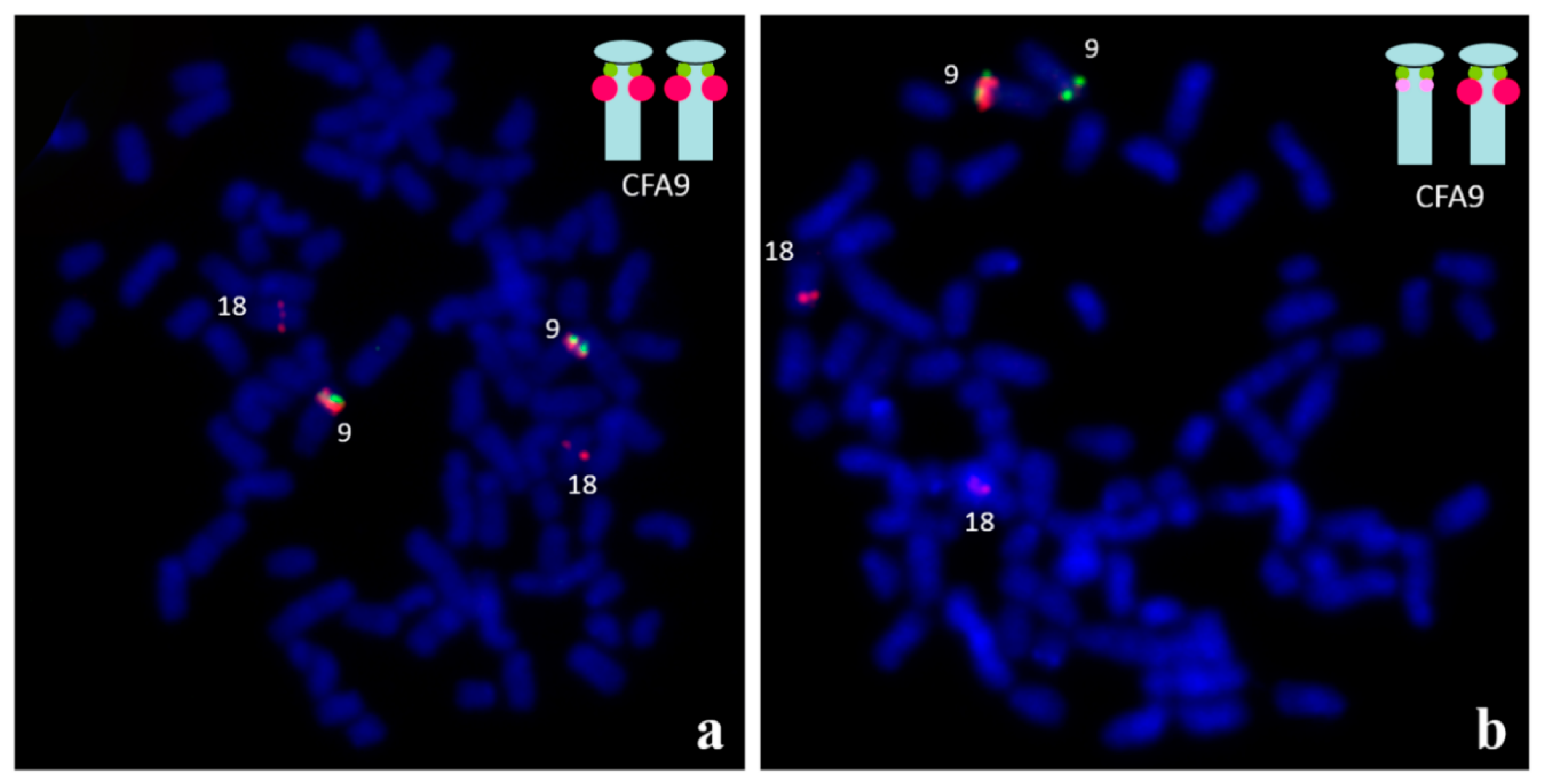
- Marcinkowska-Swojak, M.; Szczerbal, I.; Pausch, H.; Nowacka-Woszuk, J.; Flisikowski, K.; Dzimira, S.; Nizanski, W.; Payan-Carreira, R.; Fries, R.; Kozlowski, P.; et al. Copy Number Variation in the Region Harboring SOX9 Gene in Dogs with Testicular/Ovotesticular Disorder of Sex Development (78,XX.; SRY-Negative). Sci. Rep. 2015, 5, 14696. [Google Scholar] [CrossRef] [Green Version]
- Szczerbal, I.; Nowacka-Woszuk, J.; Dzimira, S.; Atamaniuk, W.; Nizanski, W.; Switonski, M. A Rare Case of Testicular Disorder of Sex Development in a Dog (78,XX.; SRY-Negative) with Male External Genitalia and Detection of Copy Number Variation in the Region Upstream of the SOX9 Gene. Sex. Dev. 2016, 10, 74–78. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J.; Szczerbal, I.; Stachowiak, M.; Szydlowski, M.; Nizanski, W.; Dzimira, S.; Maslak, A.; Payan-Carreira, R.; Wydooghe, E.; Nowak, T.; et al. Association between Polymorphisms in the SOX9 Region and Canine Disorder of Sex Development (78,XX.; SRY-Negative) Revisited in a Multibreed Case-Control Study. PLoS ONE 2019, 14, e0218565. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.J. Spotlight on reproduction in domestic dogs as a model for human reproduction. In Animals Model and Human Reproduction; Schatten, H., Constantinescu, G.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 247–358. [Google Scholar]
- Oi, M.; Yamada, K.; Hayakawa, H.; Suzuki, H. Sexing of Dog Sperm by Fluorescence In Situ Hybridization. J. Reprod. Dev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Komaki, H.; Oi, M.; Suzuki, H. Detection of Sex Chromosome Aneuploidy in Dog Spermatozoa by Triple Color Fluorescence in Situ Hybridization. Theriogenology 2014, 82, 652–656. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Travis, A.J.; Songsasen, N. The Domestic Dog Embryo: In Vitro Fertilization, Culture, and Transfer. In Comparative Embryo Culture; Herrick, J.R., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 2006, pp. 247–267. ISBN 978-1-4939-9565-3. [Google Scholar]
- Hyttel, P.; Pessôa, L.V.d.F.; Secher, J.B.-M.; Dittlau, K.S.; Freude, K.; Hall, V.J.; Fair, T.; Assey, R.J.; Laurincik, J.; Callesen, H.; et al. Oocytes, Embryos and Pluripotent Stem Cells from a Biomedical Perspective. Anim. Reprod. 2019, 16, 508–523. [Google Scholar] [CrossRef]
- Flisikowski, K.; Flisikowska, T.; Sikorska, A.; Perkowska, A.; Kind, A.; Schnieke, A.; Switonski, M. Germline Gene Polymorphisms Predisposing Domestic Mammals to Carcinogenesis: Gene Polymorphisms Predisposing to Carcinogenesis. Vet. Comp. Oncol. 2017, 15, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Ostrander, E.A.; Dreger, D.L.; Evans, J.M. Canine Cancer Genomics: Lessons for Canine and Human Health. Annu. Rev. Anim. Biosci. 2019, 7, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Makino, S. Some epidemiologic aspects of venereal tumors of dogs as revealed by chromosome and DNA studies. Ann. N. Y. Acad. Sci. 2006, 108, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Reimann, N.; Nolte, I.; Bonk, U.; Werner, M.; Bullerdiek, J.; Bartnitzke, S. Trisomy 18 in a Canine Thyroid Adenoma. Cancer Genet. Cytogenet. 1996, 90, 154–156. [Google Scholar] [CrossRef]
- Reimann, N.; Bartnitzke, S.; Bullerdiek, J.; Mischke, R.; Nolte, I. Trisomy 1 in a Canine Acute Leukemia Indicating the Pathogenetic Importance of Polysomy 1 in Leukemias of the Dog. Cancer Genet. Cytogenet. 1998, 101, 49–52. [Google Scholar] [CrossRef]
- Winkler, S.; Reimann-Berg, N.; Escobar, H.M.; Loeschke, S.; Eberle, N.; Höinghaus, R.; Nolte, I.; Bullerdiek, J. Polysomy 13 in a Canine Prostate Carcinoma Underlining Its Significance in the Development of Prostate Cancer. Cancer Genet. Cytogenet. 2006, 169, 154–158. [Google Scholar] [CrossRef]
- Reimann-Berg, N.; Willenbrock, S.; Murua Escobar, H.; Eberle, N.; Gerhauser, I.; Mischke, R.; Bullerdiek, J.; Nolte, I. Two New Cases of Polysomy 13 in Canine Prostate Cancer. Cytogenet. Genome Res. 2011, 132, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Bridge, W.; Benke, K.; Breen, M. Isolation and Chromosomal Assignment of Canine Genomic BAC Clones Representing 25 Cancer-Related Genes. Cytogenet. Genome Res. 2003, 102, 249–253. [Google Scholar] [CrossRef]
- Breen, M.; Modiano, J.F. Evolutionarily Conserved Cytogenetic Changes in Hematological Malignancies of Dogs and Humans—Man and His Best Friend Share More than Companionship. Chromosome Res. 2008, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Cruz Cardona, J.A.; Milner, R.; Alleman, A.R.; Williams, C.; Vernau, W.; Breen, M.; Tompkins, M. BCR-ABL Translocation in a Dog with Chronic Monocytic Leukemia: BCR-ABL Translocation in Canine CMoL. Vet. Clin. Pathol. 2011, 40, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.F.; Culver, S.; Behling-Kelly, E.; Breen, M.; Friedrichs, K.R. Acute Myeloblastic Leukemia with Associated BCR-ABL Translocation in a Dog. Vet. Clin. Pathol. 2012, 41, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Vozdova, M.; Kubickova, S.; Cernohorska, H.; Fröhlich, J.; Fictum, P.; Rubes, J. Structural and Copy Number Chromosome Abnormalities in Canine Cutaneous Mast Cell Tumours. J. Appl. Genet. 2019, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.A.; Thomas, R.; Binns, M.M.; Breen, M. Comparative Genomic Hybridization (CGH) in Dogs—Application to the Study of a Canine Glial Tumour Cell Line. Vet. J. 2000, 160, 77–82. [Google Scholar] [CrossRef]
- Thomas, R.; Fiegler, H.; Ostrander, E.A.; Galibert, F.; Carter, N.P.; Breen, M. A Canine Cancer-Gene Microarray for CGH Analysis of Tumors. Cytogenet. Genome Res. 2003, 102, 254–260. [Google Scholar] [CrossRef]
- Thomas, R. Construction of a 2-Mb Resolution BAC Microarray for CGH Analysis of Canine Tumors. Genome Res. 2005, 15, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Duke, S.E.; Bloom, S.K.; Breen, T.E.; Young, A.C.; Feiste, E.; Seiser, E.L.; Tsai, P.-C.; Langford, C.F.; Ellis, P.; et al. A Cytogenetically Characterized, Genome-Anchored 10-Mb BAC Set and CGH Array for the Domestic Dog. J. Hered. 2007, 98, 474–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, D.; Voith von Voithenberg, L.; Kaigala, G.V. Fluorescence in Situ Hybridization (FISH): History, Limitations and What to Expect from Micro-Scale FISH? Micro Nano Eng. 2018, 1, 15–24. [Google Scholar] [CrossRef]
- Beliveau, B.J.; Kishi, J.Y.; Nir, G.; Sasaki, H.M.; Saka, S.K.; Nguyen, S.C.; Wu, C.; Yin, P. OligoMiner Provides a Rapid, Flexible Environment for the Design of Genome-Scale Oligonucleotide in Situ Hybridization Probes. Proc. Natl. Acad. Sci. USA 2018, 115, E2183–E2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beliveau, B.J.; Joyce, E.F.; Apostolopoulos, N.; Yilmaz, F.; Fonseka, C.Y.; McCole, R.B.; Chang, Y.; Li, J.B.; Senaratne, T.N.; Williams, B.R.; et al. Versatile Design and Synthesis Platform for Visualizing Genomes with Oligopaint FISH Probes. Proc. Natl. Acad. Sci. USA 2012, 109, 21301–21306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Wang, L.; Wang, H.; Ma, M.; Wang, S.; Liu, Z.; Xu, G.; Zhang, J.; Cram, D.S.; Yao, Y. The Clinical Utility of Next-Generation Sequencing for Identifying Chromosome Disease Syndromes in Human Embryos. Reprod. Biomed. Online 2015, 31, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Liang, F.; Cheng, D.; Zhang, Z.; Yu, G.; Zha, J.; Wang, Y.; Xia, Q.; Yuan, D.; Tan, Y.; et al. Location of Balanced Chromosome-Translocation Breakpoints by Long-Read Sequencing on the Oxford Nanopore Platform. Front. Genet. 2020, 10, 1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahajpal, N.S.; Barseghyan, H.; Kolhe, R.; Hastie, A.; Chaubey, A. Optical Genome Mapping as a Next-Generation Cytogenomic Tool for Detection of Structural and Copy Number Variations for Prenatal Genomic Analyses. Genes 2021, 12, 398. [Google Scholar] [CrossRef]
- Wang, C.; Wallerman, O.; Arendt, M.-L.; Sundström, E.; Karlsson, Å.; Nordin, J.; Mäkeläinen, S.; Pielberg, G.R.; Hanson, J.; Ohlsson, Å.; et al. A Novel Canine Reference Genome Resolves Genomic Architecture and Uncovers Transcript Complexity. Commun. Biol. 2021, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach, R.; Liehr, T.; Hastings, R.J. Chromosomes in the Genomic Age. Preserving Cytogenomic Competence of Diagnostic Genome Laboratories. Eur. J. Hum. Genet. 2020. [Google Scholar] [CrossRef]


| Karyotype | No. of Cells Analyzed | Breed | Characteristic Feature of Phenotype | Reference |
|---|---|---|---|---|
| 77,X | Lack of information | Doberman Pinscher | Small stature, excessive skin in the ventrum of the neck, no signs of estrus, small ovaries consisting primarily of interstitial-type cells and solid epithelial cords | [8] |
| 77,X | 60 | Miniature American Eskimo | Juvenile appearance, signs of proestrus, small and fibrous ovaries, no evidence of ovarian follicle development or corpora lutea | [9] |
| 77,X[95%]/78,XX[5%] | 40 | Toy Poodle | Abnormal estrus cycle and apparently persistent follicles, gonadal dysgenesis | [11] |
| 77,X[5%]/78,XX[95%] | 220 | Munsterlander | Infertility, vertical septum in vagina | [10] |
| 77,X[6%]/78,XX[94%] | 473 | Bearded Collie | Infertility, irregular and poorly manifested estrus cycles | [10] |
| Karyotype | Breed | Characteristic Feature of Phenotype | Reference |
|---|---|---|---|
| 79,XXX | Airedale Terrier | Primary anestrus, ovaries with solid epithelial cords and large masses of interstitial cells, lack of follicles and corpora lutea | [15] |
| 79,XXX | Mixed breed | Infertility, normal reproductive organs, ovaries with primary follicles and corpora lutea, dental anomalies, abnormal behavior (lack of barking and fearfulness) | [16] |
| 79,XXX | Labrador Retriever | Primary anestrus, chronic dermatitis, abnormal behavior (coprophagy) | [17] |
| 79,XXX | Silky Terrier | Infertility, abnormal estrous cycles, hypoplastic ovaries, absence of normal follicular structures, shy and timid behavior | [18] |
| 79,XXX | Labrador Retriever | Infertility, abnormal estrous cycles, hypoplastic ovaries, absence of normal follicular structures | [18] |
| 79,XXX/78,XX | Boston Terrier | Estrus symptoms occurred once, ovary with corpora lutea | [14] |
| Karyotype | Breed | Characteristic Feature of Phenotype | Reference |
|---|---|---|---|
| 79,XXY | German Shorthair Pointer | Testicular hypoplasia, lack of spermatogenesis, ventricular septal defect, congenital heart abnormalities | [19] |
| 79,XXY | Great Dane | Female external and internal genitalia, structure reminiscent of a vestigial scrotal sac | [23] |
| 79,XXY | Norwich Terrier | Testicular dysgenesis, azoospermia | [24] |
| 79,XXY | West Highland White Terrier | High stature, rugae of the dermis and hypodermis, low level of testosterone, Sertoli cell tumor | [22] |
| 79,XXY/78,XY | Miniature Schnauzer | Alopecia, gynecomastia, bilateral cryptorchidism, Sertoli cell tumor | [20] |
| 79,XXY[18%]/78,XY[82%] | Poodle | Bilateral cryptorchidism, testes with vacuolation of the seminal cells and small nests of Leydig cells, total absence of sperm cells | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczerbal, I.; Switonski, M. Clinical Cytogenetics of the Dog: A Review. Animals 2021, 11, 947. https://doi.org/10.3390/ani11040947
Szczerbal I, Switonski M. Clinical Cytogenetics of the Dog: A Review. Animals. 2021; 11(4):947. https://doi.org/10.3390/ani11040947
Chicago/Turabian StyleSzczerbal, Izabela, and Marek Switonski. 2021. "Clinical Cytogenetics of the Dog: A Review" Animals 11, no. 4: 947. https://doi.org/10.3390/ani11040947






