Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Experimental Design
2.2. Rearing Conditions and Zootechnical Measurements
- Specific growth rate (SGR, %BW d−1) = [100 × (ln (FBW/IBW))] × d−1, where FBW and IBW are final and initial body weight, respectively, and d is the number of days between FBW and IBW;
- Feed conversation ratio (FCR) = feed intake × (FBW − IBW)−1;
- Net apparent retention (%) = (0.61 × FBW × FFA) − (0.61 × IBW × IFA) × (total feed × fatty acid concentration in feed)−1 FFA and IFA represent final and initial fatty acid composition and 0.61 corresponds to the edible portion of the fish.
2.3. Analytics
2.3.1. Muscle Full Fatty Acid Profile and Retention
2.3.2. Lipid Peroxidation
2.3.3. Contaminants in Feed and Fish Fillet Samples
2.3.4. Sensory Analysis
2.4. Data Analysis
2.4.1. Contrasting Theoretically Expected to Actually Observe Nutritional Content of Feeds
2.4.2. Zootechnical Performance
2.4.3. Fatty Acid Profile, Retention, and Lipid Oxidation
2.4.4. Heavy Metals, Dioxins, and DL-PCBs
2.4.5. Sensory Data
3. Results
3.1. Feed Analysis and Comparison between Theoretical and Observed Fatty Acid Composition
3.2. Survival and Growth Performance
3.3. Muscle Fatty Acid Profile, Retention, and Lipid Oxidation
3.4. Heavy Metals, Dioxins and DL-PCBs
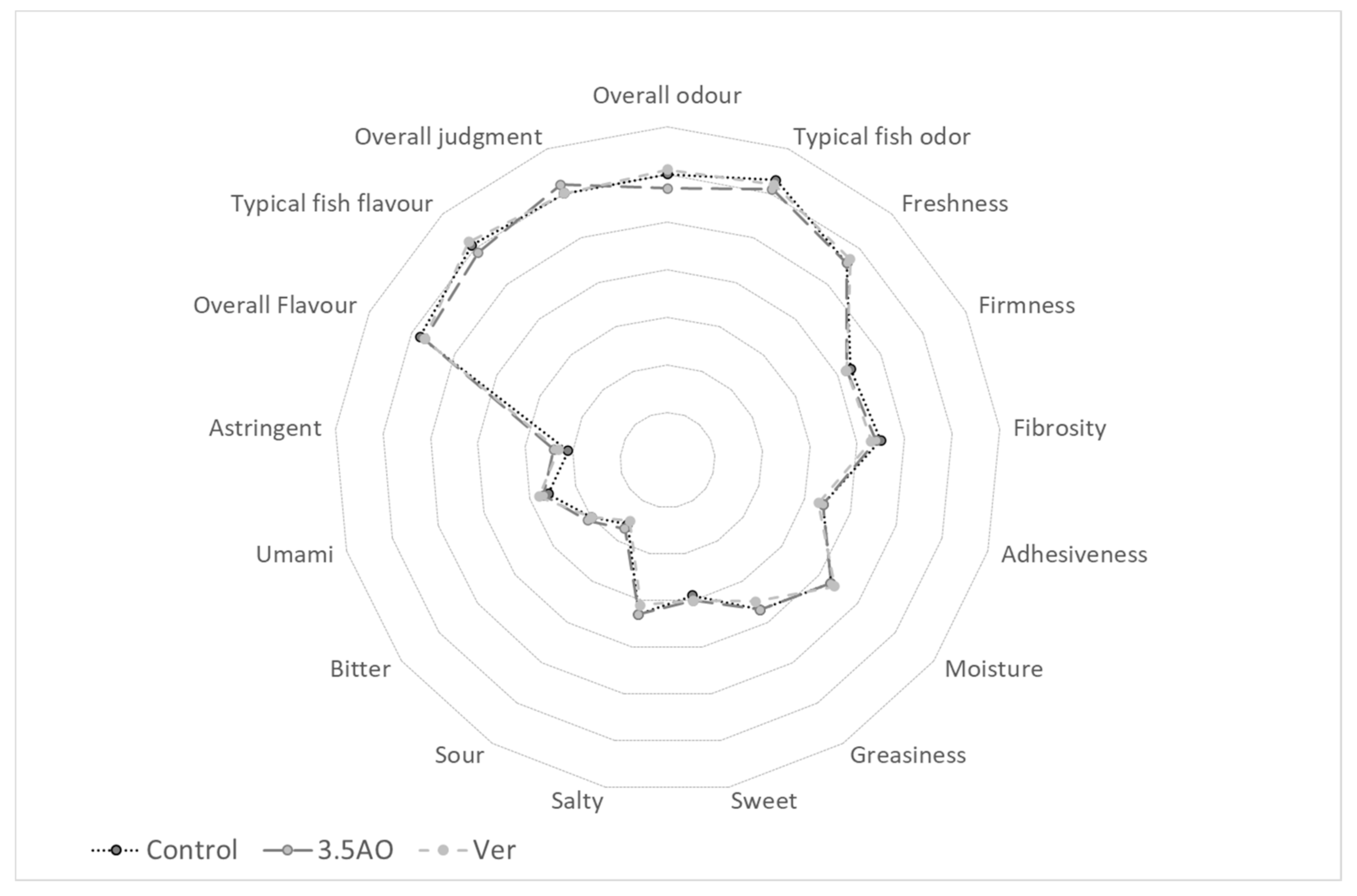
3.5. Sensory
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Saturated Fats | |
| C 12:0 (Lauric acid) | 0.2% |
| C 14:0 (Myristic acid) | 2.5% |
| C 15:0 (Pentadecanoic acid) | 0.7% |
| C 16:0 (Palmitic acid) | 29.6% |
| C 17:0 (Margaric acid) | 0.4% |
| C 18:0 (Stearic acid) | 2.2% |
| C 20:0 (Arachidic acid) | 0.6% |
| C 22:0 (Behenic acid) | 0.1% |
| C 23:0 (Tricosanoic acid) | 0.2% |
| C 24:0 (Lignoceric acid) | 0.1% |
| Total Saturated fats | 36.5% |
| Monosaturated fats | |
| C 22:1n9 (Erucic acid) | 0.2% |
| Polyunsaturated fats | |
| C 18:4n3 (Stearidonic acid) | 0.2% |
| C 20:3n6 (Homo-gamma-linolenic acid) | 0.1% |
| C 20:3n3 (Eicosatrienoic acid) | 0.1% |
| C 20:4n6 (Arachidonic acid) | 1.9% |
| C 20:4n3 (Eicosatetraenoic acid) | 0.8% |
| C 20:5n3 (Eicosapentaenoic acid) | 15.7% |
| C 22:5n6 (Docosapentaenoic acid) | 2.1% |
| C 22:5n3 (Docosapentaenoic acid) | 1.5% |
| C 22:6n3 (Docosahexaenoic acid) | 39.8% |
| Total Omega-3 | 58% |
| Total Omega-6 | 4.2% |
| Omega-3/Omega-6 ratio | 14 |
| NC (An) | PC (An) | 3.5AO (An) | 0.7AO (An) | NC (Th) | PC (Th) | 3.5AO (Th) | 0.7AO (Th) | |
|---|---|---|---|---|---|---|---|---|
| C14:0 | 0.22 ± 0.00b | 0.76 ± 0.02a | 0.21 ± 0.01b | 0.15 ± 0.01c | 0.20 | 0.94 | 0.17 | 0.08 |
| (C16:0) | 1.73 ± 0.00c | 3.18 ± 0.02a | 2.15 ± 0.01b | 1.63 ± 0.01d | 1.20 | 2.52 | 1.92 | 1.16 |
| (C16:1) | 0.30 ± 0.00b | 0.92 ± 0.02a | 0.20 ± 0.01c | 0.19 ± 0.01c | 0.25 | 1.01 | 0.23 | 0.11 |
| (C17:0) | 0.02 ± 0.00b | 0.05 ± 0.01a | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.02 | 0.08 | 0.02 | 0.01 |
| (C18:0) | 0.47 ± 0.01b | 0.54 ± 0.02a | 0.46 ± 0.01b | 0.48 ± 0.01b | 0.37 | 0.56 | 0.57 | 0.36 |
| (C18:1) | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.07 | 0.01 ± 0.01 | 0.01 | 0.06 | 0.01 | 0.00 |
| (C18:1 n-9) | 6.46 ± 0.05a | 3.56 ± 0.05d | 5.21 ± 0.07c | 6.03 ± 0.04b | 6.44 | 2.43 | 7.12 | 5.95 |
| (C18:1 n-7) | 0.50 ± 0.01a | 0.46 ± 0.01b | 0.40 ± 0.014c | 0.45 ± 0.01b | 0.17 | 0.23 | 0.53 | 0.13 |
| (C18:2 n-6) (LA) | 3.61 ± 0.05 | 2.12 ± 0.09 | 3.36 ± 0.08 | 2.65 ± 1.89 | 2.70 | 0.67 | 4.42 | 2.94 |
| (C18:3 n-3) (ALA) | 2.73 ± 0.01c | 0.73 ± 0.02d | 2.99 ± 0.06b | 3.77 ± 0.09a | 1.95 | 0.26 | 4.44 | 2.72 |
| (C18:3 n-6) | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 | 0.05 | 0.02 | 0.01 |
| (C18:4 n-3) | 0.06 ± 0.04b | 0.42 ± 0.03a | 0.07 ± 0.00b | 0.05 ± 0.01b | 0.07 | 0.35 | 0.06 | 0.02 |
| (C20:0) | 0.07 ± 0.00a | 0.05 ± 0.01b | 0.07 ± 0.0a | 0.07 ± 0.01a | 0.09 | 0.06 | 0.08 | 0.10 |
| (C20:1 n-9) | 0.26 ± 0.00b | 0.47 ± 0.0a | 0.20 ± 0.01c | 0.22 ± 0.02c | 0.54 | 0.54 | 0.26 | 0.67 |
| (C20:2 n-6) | 0.02 ± 0.02 | 0.03 ± 0.04 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.07 | 0.06 | 0.03 | 0.10 |
| (C20:3 n-6) | 0.13 ± 0.12 | 0.37 ± 0.37 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.03 | 0.03 | 0.00 | 0.04 |
| (C20:3 n-3) | 0.01 ± 0.01ab | 0.02 ± 0.01a | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.00 | 0.01 | 0.01 | 0.00 |
| (C20:4 n-6) | 0.04 ± 0.00ab | 0.10 ± 0.00a | 0.08 ± 0.00b | 0.04 ± 0.01b | 0.03 | 0.12 | 0.04 | 0.03 |
| (C20:4 n-3) | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.02 | 0.10 | 0.15 | 0.02 |
| (C20:5 n-3) (EPA) | 0.34 ± 0.01c | 1.31 ± 0.05a | 0.59 ± 0.01b | 0.26 ± 0.00d | 0.34 | 1.81 | 0.31 | 0.20 |
| (C22:1 n-11) | 0.14 ± 0.11 | 0.40 ± 0.35 | 0.15 ± 0.02 | 0.14 ± 0.01 | 0.18 | 0.67 | 0.17 | 0.10 |
| (C22:1 n-9) | 0.02 ± 0.02 | 0.04 ± 0.03 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.08 | 0.11 | 0.02 | 0.10 |
| (C22:2 n-6) | 0.04 ± 0.01c | 0.11 ± 0.01a | 0.05 ± 0.00b | 0.03 ± 0.00c | 0.02 | 0.07 | 0.03 | 0.01 |
| (C22:5 n-3) (DPA) | 0.04 ± 0.01c | 0.13 ± 0.00a | 0.07 ± 0.01b | 0.03 ± 0.01c | 0.06 | 0.27 | 0.04 | 0.04 |
| (C22:6 n-3) (DHA) | 0.52 ± 0.03c | 1.80 ± 0.02a | 1.59 ± 0.01b | 0.54 ± 0.02c | 0.36 | 1.56 | 0.64 | 0.42 |
References
- FAO. The State of World Fisheries and Aquaculture 2016; SOPHIA: Rome, Italy, 2016. [Google Scholar]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M.J. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.; Fischer, A.R.; van Boekel, M.A.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Runge, C.A.; Gentry, R.R.; Gaines, S.D.; Halpern, B.S. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natl. Acad. Sci. USA 2018, 115, 5295–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santigosa, E.; Constant, D.; Prudence, D.; Wahli, T.; Verlhac-Trichet, V. A novel marine algal oil containing both EPA and DHA is an effective source of omega-3 fatty acids for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2020, 51, 649–665. [Google Scholar] [CrossRef]
- Gomez-Requeni, P.; Mingarro, M.; Calduch-Giner, J.A.; Medale, F.; Martin, S.A.M.; Houlihan, D.F.; Kaushik., S.; Perez-Sanchez, J. Protein growth performance, amino acid utilisation and somatotropic axis responsive-ness to fish meal replacement by plant protein sourcesin gilthead sea bream (Sparus aurata). Aquaculture 2004, 232, 493–510. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Feed Matters: Satisfying the Feed Demand of Aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Naylor, R.; Burke, M. Aquaculture and Ocean Resources: Raising Tigers of the Sea. Annu. Rev. Environ. Resour. 2005, 30, 185–218. [Google Scholar] [CrossRef] [Green Version]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Crawford, M.; Galli, C.; Visioli, F.; Renaud, S.; Simopoulos, A.P.; Spector, A.A. Role of Plant-Derived Omega–3 Fatty Acids in Human Nutrition. Ann. Nutr. Metab. 2000, 44, 263–265. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- AbuMweis, S.; Jew, S.; Tayyem, R.; Agraib, L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: A meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2017, 31, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Bosschieter, E.B.; Coulander, C.D.L. The Inverse Relation between Fish Consumption and 20-Year Mortality from Coronary Heart Disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef]
- Hawthorne, A.B.; Daneshmend, T.K.; Hawkey, C.J.; Belluzzi, A.; Everitt, S.J.; Holmes, G.K.; Malkinson, C.; Shaheen, M.Z.; Willars, J.E. Treatment of ulcerative colitis with fish oil supplementation: A prospective 12 month randomised controlled trial. Gut 1992, 33, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.; Weber, P.C.; Szimnau, P.; Heldwein, W.; Strasser, T.; Loeschke, K. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease-a randomized, placebo-controlled, double-blind cross-over trial. J. Intern. Med. 1989, 225, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bittiner, S.B.; Tucker, W.F.; Cartwright, I.; Bleehen, S.S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet 1988, 1, 378–380. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [Green Version]
- Fountoulaki, E.; Vasilaki, A.; Hurtado, R.; Grigorakis, K.; Karacostas, I.; Nengas, I.; Rigos, G.; Kotzamanis, Y.; Venou, B.; Alexis, M. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile. Aquaculture 2009, 289, 317–326. [Google Scholar] [CrossRef]
- Sprague, M.; Fawcett, S.; Betancor, M.; Struthers, W.; Tocher, D. Variation in the nutritional composition of farmed Atlantic salmon (Salmo salar L.) fillets with emphasis on EPA and DHA contents. J. Food Compos. Anal. 2020, 94, 103618. [Google Scholar] [CrossRef]
- Cheng, K.; Bou, M.; Ruyter, B.; Pickova, J.; Ehtesham, E.; Du, L.; Venegas, C.; Moazzami, A.A.; Buyter, B. Impact of Reduced Dietary Levels of Eicosapentaenoic Acid and Docosahexaenoic Acid on the Composition of Skin Membrane Lipids in Atlantic Salmon (Salmo salar L.). J. Agric. Food Chem. 2018, 66, 8876–8884. [Google Scholar] [CrossRef] [PubMed]
- Sissener, N.; Waagbø, R.; Rosenlund, G.; Tvenning, L.; Susort, S.; Lea, T.; Oaland, Ø.; Chen, L.; Breck, O. Reduced n-3 long chain fatty acid levels in feed for Atlantic salmon (Salmo salar L.) do not reduce growth, robustness or product quality through an entire full scale commercial production cycle in seawater. Aquaculture 2016, 464, 236–245. [Google Scholar] [CrossRef]
- Magalhães, R.; Guerreiro, I.; Santos, R.A.; Coutinho, F.; Couto, A.; Serra, C.R.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Oxidative status and intestinal health of gilthead sea bream (Sparus aurata) juveniles fed diets with different ARA/EPA/DHA ratios. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Magalhães, R.; Guerreiro, I.; Coutinho, F.; Moutinho, S.; Sousa, S.; Delerue-Matos, C.; Domingues, V.; Olsen, R.; Peres, H.; Oliva-Teles, A. Effect of dietary ARA/EPA/DHA ratios on growth performance and intermediary metabolism of gilthead sea bream (Sparus aurata) juveniles. Aquaculture 2020, 516, 734644. [Google Scholar] [CrossRef]
- Bou, M.; Berge, G.M.; Baeverfjord, G.; Sigholt, T.; Østbye, T.-K.K.; Ruyter, B. Low levels of very-long-chain n-3 PUFA in Atlantic salmon (Salmo salar) diet reduce fish robustness under challenging conditions in sea cages. J. Nutr. Sci. 2017, 6, e32. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, M. Essential fatty acid requirements in Mediterranean fish species. Mediterr. Fish Nutr. 2005, 63, 91–102. [Google Scholar]
- Rosenlund, G.; Torstensen, B.E.; Stubhaug, I.; Usman, N.; Sissener, N.H. Atlantic salmon require long-chain n-3 fatty acids for optimal growth throughout the seawater period. J. Nutr. Sci. 2016, 5, e19. [Google Scholar] [CrossRef] [Green Version]
- Ruyter, B.; Røsjø, C.; Einen, O.; Thomassen, M.S. Essential fatty acids in Atlantic salmon: Effects of increasing dietary doses of n-6 and n-3 fatty acids on growth, survival and fatty acid composition of liver, blood and carcass. Aquac. Nutr. 2000, 6, 119–127. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. (n-3) Fatty Acids and Cardiovascular Health: Are Effects of EPA and DHA Shared or Complementary? J. Nutr. 2012, 142, 614S–625S. [Google Scholar] [CrossRef] [Green Version]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bou, M.; Berge, G.M.; Baeverfjord, G.; Sigholt, T.; Østbye, T.-K.; Romarheim, O.H.; Hatlen, B.; Leeuwis, R.; Venegas, C.; Ruyter, B. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L): Effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br. J. Nutr. 2017, 117, 30–47. [Google Scholar] [CrossRef] [Green Version]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding aquaculture in an era of finite resources. Proc. Natl Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [Green Version]
- Wallberg, P.; Jonsson, P.R.; Andersson, A. Trophic transfer and passive uptake of a polychlorinated biphenyl in experimental marine microbial communities. Environ. Toxicol. Chem. 2001, 20, 2158–2164. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Wang, W.-X. Bioaccumulation and trophic transfer of dioxins in marine copepods and fish. Environ. Pollut. 2011, 159, 3390–3397. [Google Scholar] [CrossRef]
- Kiron, V.; Phromkunthong, W.; Huntley, M.E.; Archibald, I.; De Scheemaker, G. Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012, 18, 521–531. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Replacement of fish oil with thraustochytrid Schizochytrium sp. L oil in Atlantic salmon parr (Salmo salar L) diets. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 382–392. [Google Scholar] [CrossRef]
- Castro, C.; Corraze, G.; Firmino-Diógenes, A.; Larroquet, L.; Panserat, S.; Oliva-Teles, A. Regulation of glucose and lipid metabolism by dietary carbohydrate levels and lipid sources in gilthead sea bream juveniles. Br. J. Nutr. 2016, 116, 19–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, S.J.; Karalazos, V.; Tinsley, J.; Betancor, M.B.; Martin, S.A.; Tocher, D.R.; Monroig, O. The compositional and metabolic responses of gilthead seabream (Sparus aurata) to a gradient of dietary fish oil and associated n-3 long-chain PUFA content. Br. J. Nutr. 2017, 118, 1010–1022. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Pkrez, J.A.; Diaz, M.; Ferngndez-Palacios, H.; Lorenzo, A. Influence of the EPA/DHA ratio in rotifers on gilthead seabream (Sparus aurata) larval development. Aquaculture 1997, 150, 77–89. [Google Scholar] [CrossRef]
- Jin, M.; Monroig, Ó.; Lu, Y.; Yuan, Y.; Li, Y.; Ding, L.; Tocher, D.R.; Zhou, Q. Dietary DHA/EPA ratio affected tissue fatty acid profiles, antioxidant capacity, hematological characteristics and expression of lipid-related genes but not growth in juvenile black seabream (Acanthopagrus schlegelii). PLoS ONE 2017, 12, e0176216. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org (accessed on 26 August 2020).
- Izquierdo, M.; Forster, I.; Divakaran, S.; Conquest, L.; DeCamp, O.; Tacon, A. Effect of green and clear water and lipid source on survival, growth and biochemical composition of Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2006, 12, 192–202. [Google Scholar] [CrossRef]
- Katerina, K.; Berge, G.M.; Turid, M.; Aleksei, K.; Grete, B.; Trine, Y.; Mats, C.; John, S.; Bente, R. Microalgal Schizochytrium limacinum Biomass Improves Growth and Filet Quality When Used Long-Term as a Replacement for Fish Oil, in Modern Salmon Diets. Front. Mar. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Izquierdo, M.; Montero, D.; Robaina, L.; Caballero, M.; Rosenlund, G.; Ginés, R. Alterations in fillet fatty acid profile and flesh quality in gilthead seabream (Sparus aurata) fed vegetable oils for a long term period. Recovery of fatty acid profiles by fish oil feeding. Aquaculture 2005, 250, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.; Grasso, V.; Izquierdo, M.; Ganga, R.; Real, F.; Tort, L.; Caballero, M.; Acosta, F. Total substitution of fish oil by vegetable oils in gilthead sea bream (Sparus aurata) diets: Effects on hepatic Mx expression and some immune parameters. Fish. Shellfish. Immunol. 2008, 24, 147–155. [Google Scholar] [CrossRef]
- Wassef, E.A.; Saleh, N.E.; El-Hady, H.A.E.-A. Vegetable oil blend as alternative lipid resources in diets for gilthead seabream, Sparus aurata. Aquac. Int. 2008, 17, 421–435. [Google Scholar] [CrossRef]
- Montero, D.; Kalinowski, T.; Obach, A.; Robaina, L.; Tort, L.; Caballero, M.; Izquierdo, M. Vegetable lipid sources for gilthead seabream (Sparus aurata): Effects on fish health. Aquaculture 2003, 225, 353–370. [Google Scholar] [CrossRef]
- López-Bote, C.; Diez, A.; Corraze, G.; Arzel, J.; Alvarez, M.; Dias, J.; Kaushik, S.; Bautista, J. Dietary protein source affects the susceptibility to lipid peroxidation of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax) muscle. Anim. Sci. 2001, 73, 443–449. [Google Scholar] [CrossRef]
- Betancor, M.B.; Sprague, M.; Montero, D.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Izquierdo, M.; Tocher, D.R. Replacement of Marine Fish Oil with de novo Omega-3 Oils from Transgenic Camelina sativa in Feeds for Gilthead Sea Bream (Sparus aurata L.). Lipids 2016, 51, 1171–1191. [Google Scholar] [CrossRef]
- Betancor, M.B.; Sprague, M.; Sayanova, O.; Usher, S.; Metochis, C.; Campbell, P.J.; Napier, J.A.; Tocher, U.R. Nutritional Evaluation of an EPA-DHA Oil from Transgenic Camelina sativa in Feeds for Post-Smolt Atlantic Salmon (Salmo salar L.). PLoS ONE 2016, 11, e0159934. [Google Scholar] [CrossRef]
- Eryalçın, K.M.; Roo, J.; Saleh, R.; Atalah, E.; Benítez, T.; Betancor, M.; Hernandez-Cruz, M.D.C.; Izquierdo, M. Fish oil replacement by different microalgal products in microdiets for early weaning of gilthead sea bream (Sparus aurata, L.). Aquac. Res. 2013, 44, 819–828. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Turchini, G.; Mailer, R. Rapeseed (Canola) Oil and Other Monounsaturated Fatty Acid-Rich Vegetable Oils. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; Apple Academic Press: Boca Raton, FL, USA, 2010; pp. 161–208. [Google Scholar]
- World Health Organisation. Food Safety Issues Associated with Products from Aquaculture; Report of a Joint FAO/NACA (WHO Study Group), WHO Technical Report Series; World Health Organisation: Geneva, Switzerland, 1999; p. 883. [Google Scholar]
- Jacobs, M.N.; Covaci, A.; Schepens, P. Investigation of Selected Persistent Organic Pollutants in Farmed Atlantic Salmon (Salmo salar), Salmon Aquaculture Feed, and Fish Oil Components of the Feed. Environ. Sci. Technol. 2002, 36, 2797–2805. [Google Scholar] [CrossRef]
- Ratledge, C. Single Cell Oils for the 21st Century. In Single Cell Oils: Microbial and Algal Oils: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 3–26. [Google Scholar]
- Karl, H.; Kuhlmann, H.; Ruoff, U. Transfer of PCDDs and PCDFs into the edible parts of farmed rainbow trout, Oncorhynchus mykiss (Walbaum), via feed. Aquac. Res. 2003, 34, 1009–1014. [Google Scholar] [CrossRef]
- Isosaari, P.; Kiviranta, H.; Lie, O.; Lundebye, A.-K.; Ritchie, G.; Vartiainen, T. Accumulation and Distribution of Polychlorinated Dibenzo-P-Dioxin, Dibenzofuran, and Polychlorinated Biphenyl Congeners in Atlantic Salmon (Salmo Salar). Environ. Toxicol. Chem. 2004, 23, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Lundebye, A.-K.; Berntssen, M.; Lie, O.; Ritchie, G.; Isosaari, P.; Kiviranta, H.; Vartiainen, T. Dietary uptake of dioxins (PCDD/PCDFs) and dioxin-like PCBs in Atlantic salmon (Salmo salar). Aquac. Nutr. 2004, 10, 199–207. [Google Scholar] [CrossRef]
- Sprague, M.; Walton, J.; Campbell, P.; Strachan, F.; Dick, J.R.; Bell, J.G. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 2015, 185, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Rosenlund, G.; Baardsen, G.K.; Stubhaug, I.; Holme, M.H. Sensory quality of Atlantic salmon (S. salar) fed no fish meal–no fish oil diets. In Proceedings of the 18th International Symposium on Fish Nutrition & Feeding (ISFNF), Las Palmas, Spain, 3–7 June 2018; pp. 1–14. [Google Scholar]
- Li, M.H.; Robinson, E.H.; Tucker, C.S.; Manning, B.B.; Khoo, L. Effects of dried algae Schizochytrium sp., a rich source of docosahexaenoic acid, on growth, fatty acid composition, and sensory quality of channel catfish Ictalurus punctatus. Aquaculture 2009, 292, 232–236. [Google Scholar] [CrossRef]

| Ingredients (%) | NC | PC | 3.5AO | 0.7AO | ||
|---|---|---|---|---|---|---|
| Fish oil | 2.10 | 13.79 | 0 | 0 | ||
| Microalgal oil 1 | 0 | 0 | 3.5 | 0.71 | ||
| Rapeseed oil | 8.47 | 0 | 6.09 | 7.49 | ||
| Camelina oil | 3.22 | 0 | 4.20 | 5.60 | ||
| Fish meal | 19.96 | |||||
| Soybean meal | 8.54 | |||||
| Guar meal | 12.81 | |||||
| Wheat | 10.58 | |||||
| Corn gluten | 22.20 | |||||
| Wheat gluten | 3.77 | |||||
| Pea | 4.27 | |||||
| DL-methionine 2 | 0.60 | |||||
| Lysine HCl 3 | 1.02 | |||||
| Taurine 4 | 0.44 | |||||
| Monoammonium phosphate 5 | 0.68 | |||||
| Vitamin C 6 | 0.07 | |||||
| Vitamin and mineral premix 7 | 1.25 | |||||
| Protein 8 | 42.96 ± 0.12 | 43.05 ± 0.15 | 42.95 ± 0.20 | 43.03 ± 0.15 | ||
| Lipid 8 | 18.0 ± 0.09 | 18.1 ± 0.07 | 18.0 ± 0.07 | 17.9 ± 0.05 | ||
| Ash 8 | 4.92 ± 0.07 | 4.9 ± 0.09 | 4.93 ± 0.08 | 4.90 ± 0.09 | ||
| Fiber 8 | 2.86 ± 0.08 | 2.84 ± 0.07 | 2.82 ± 0.05 | 2.83 ± 0.09 | ||
| Dietary EPA | 0.34c | 1.31a | 0.59b | 0.26d | ||
| Dietary DHA | 0.52c | 1.80a | 1.59b | 0.54c | ||
| Dietary EPA + DHA | 0.86c | 3.11a | 2.18b | 0.80c | ||
| Dietary DHA:EPA | 1.54c | 1.37d | 2.67a | 2.09b | ||
| Dietary omega-3 | 3.83d | 4.54c | 5.43a | 4.79b | ||
| Dietary omega-6 | 3.84a | 2.79b | 3.51a | 3.85a | ||
| Dietary omega-3:omega-6 | 1.00c | 1.65a | 1.55ab | 1.25bc | ||
| FFDRoil 9 | 0.60 | 3.95 | 0 | 0 | ||
| Diets | NC | PC | 3.5AO | 0.7AO | Significance |
|---|---|---|---|---|---|
| Survival (%) | 99.6 ± 0.7 | 99.6 ± 0.7 | 99.6 ± 0.7 | 99.6 ± 0.7 | ns |
| IBW (g) | 64.94 ± 1.31 | 64.19 ± 1.27 | 64.83 ± 0.91 | 64.14 ± 1.00 | ns |
| FBW (g) | 146.66 ± 2.52 | 143.95 ± 1.30 | 145.69 ± 1.26 | 144.44 ± 3.00 | ns |
| SGR | 0.74 ± 0.02 | 0.73 ± 0.02 | 0.74 ± 0.01 | 0.74 ±0.01 | ns |
| FCR | 1.44 ± 0.04 | 1.43 ± 0.05 | 1.43 ± 0.04 | 1.44 ± 0.04 | ns |
| Fatty Acid | T0 | NC | PC | 3.5AO | 0.7AO | |
|---|---|---|---|---|---|---|
| Myristic acid | C14:0 | 2.48bc | 1.44ac | 2.50b | 1.41a | 1.35a |
| Pentadecanoic acid | C15:0 | 0.26bc | 0.19ac | 0.32b | 0.19ac | 0.18a |
| Palmitic acid | C16:0 | 13.02a | 11.70a | 15.81b | 12.63a | 11.65a |
| Palmitoleic acid | C16:1 | 3.92b | 2.41a | 4.36b | 2.31a | 2.36a |
| Margaric acid | C17:0 | 0.21ab | 0.17a | 0.24b | 0.15a | 0.16a |
| Heptadecenoic acid | C17:1 | 0.10a | 0.10a | 0.18b | 0.07a | 0.08a |
| Stearic acid | C18:0 | 3.17abc | 3.00ab | 3.23c | 2.86ab | 3.03ac |
| Elaidic acid | C18:1 | 0.10a | 0.15a | 0.51b | 0.09a | 0.15a |
| Oleic acid | C18:1n-9 | 26.12cd | 32.25a | 22.79bd | 28.43ac | 31.80a |
| Vaccenic acid | C18:1n-7 | 2.81a | 3.08a | 2.99a | 2.49b | 2.92a |
| Linoleic acid (LA) | C18:2n-6 | 19.98a | 19.01a | 12.96b | 17.74a | 19.23a |
| α-Linolenic acid (ALA) | C18:3n-3 | 6.42b | 9.78a | 4.06b | 10.64a | 11.36a |
| Gamma linoleic acid | C18:3n-6 | 0.44b | 0.88a | 0.37bc | 0.55bc | 0.63ab |
| Octadecatetraenoic acid | C18:4n-3 | 0.69a | 0.80a | 1.19b | 0.59a | 0.58a |
| Arachidic acid | C20:0 | 0.23a | 0.21ab | 0.19b | 0.22a | 0.23a |
| Eicosenoic acid | C20:1n-9 | 1.94d | 1.22ac | 1.66abd | 1.17ac | 1.32ab |
| Eicosadienoic acid | C20:2n-6 | 0.61a | 0.58a | 0.45b | 0.49a | 0.67a |
| Di-homo-linoleic acid | C20:3n-6 | 0.34a | 0.36a | 0.25b | 0.29ab | 0.32ab |
| Eicosatrienoic acid | C20:3n-3 | 0.38a | 0.40a | 0.23b | 0.44a | 0.50a |
| Arachidonic acid | C20:4n-6 | 0.45a | 0.35a | 0.71b | 0.53a | 0.38a |
| Eicosapentaenoic acid (EPA) | C20:5n-3 | 3.12b | 1.89d | 4.79a | 2.40c | 1.63d |
| Heneicosanoic acid | C21:0 | 0.04a | 0.04a | 0.04a | 0.02b | 0.04a |
| Behenic acid | C22:0 | 0.08b | 0.11a | 0.10ab | 0.11ab | 0.13a |
| Cetoleic acid | C22:1 n-11 | 1.45b | 0.84a | 1.79b | 0.73a | 0.72a |
| Erucic acid | C22:1n-9 | 0.64b | 0.43a | 0.45a | 0.37a | 0.44a |
| Docosadienoic acid | C22:2n-6 | 0.78b | 0.67ac | 0.86bc | 0.64ac | 0.65ac |
| Docosapentaenoic acid (DPA) | C22:5n-3 | 1.95b | 1.13a | 1.96b | 1.33a | 1.05a |
| Docosahexaenoic acid (DHA) | C22:6n-3 | 6.95c | 5.58cd | 13.61a | 9.97b | 5.20d |
| Tetracosanoic acid | C24:1n-9 | 0.57abc | 0.48ac | 0.65b | 0.42a | 0.47ac |
| Pentacosaenoic acid | C25:0 | 0.04b | 0.07a | 0.07a | 0.07a | 0.07a |
| EPA + DHA | 10.07c | 7.48d | 18.40a | 12.36b | 6.83d | |
| DHA: EPA | 2.23d | 2.95bc | 2.84c | 4.15a | 3.19b | |
| Total omega-3 | 20.22b | 20.28b | 26.54a | 26.03a | 21.01b | |
| Total omega-6 | 22.59a | 21.85b | 15.59d | 20.24c | 21.88b | |
| Omega-3:omega-6 | 0.90c | 0.93c | 1.71a | 1.29b | 0.96c | |
| SFA | 19.48b | 16.89d | 22.46a | 17.65c | 16.80d | |
| MUFA | 37.67b | 40.95a | 35.37c | 36.06bc | 40.27a | |
| PUFA | 42.81b | 42.12b | 42.13b | 46.26a | 42.89b |
| PC | 3.5AO | 0.7AO | ||||
|---|---|---|---|---|---|---|
| Feed | Fillets | Feed | Fillets | Feed | Fillets | |
| DL-PCB and dioxins (pg/g wet weight) | 0.464 | 0.455 ± 0.230 | 0.298 | 0.145 ± 0.073 | 0.307 | 0.131 ± 0.066 |
| Arsenic (mg/Kg) | 0.77 | 0.81 ± 0.24 | 0.6 | 0.75 ± 0.23 | 0.67 | 0.68 ± 0.20 |
| Mercury (mg/Kg) | 0.032 | 0.039 ± 0.012 | 0.03 | 0.060 ± 0.018 | 0.032 | 0.051 ± 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals 2021, 11, 971. https://doi.org/10.3390/ani11040971
Santigosa E, Brambilla F, Milanese L. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals. 2021; 11(4):971. https://doi.org/10.3390/ani11040971
Chicago/Turabian StyleSantigosa, Ester, Fabio Brambilla, and Luca Milanese. 2021. "Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture" Animals 11, no. 4: 971. https://doi.org/10.3390/ani11040971
APA StyleSantigosa, E., Brambilla, F., & Milanese, L. (2021). Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals, 11(4), 971. https://doi.org/10.3390/ani11040971






