Susceptibility of Broiler Chickens to Deoxynivalenol Exposure via Artificial or Natural Dietary Contamination
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Test Feedstuffs and Cultured DON
2.3. Diets and Experimental Design
2.4. Gut Leakage Evaluation
2.5. Measurements
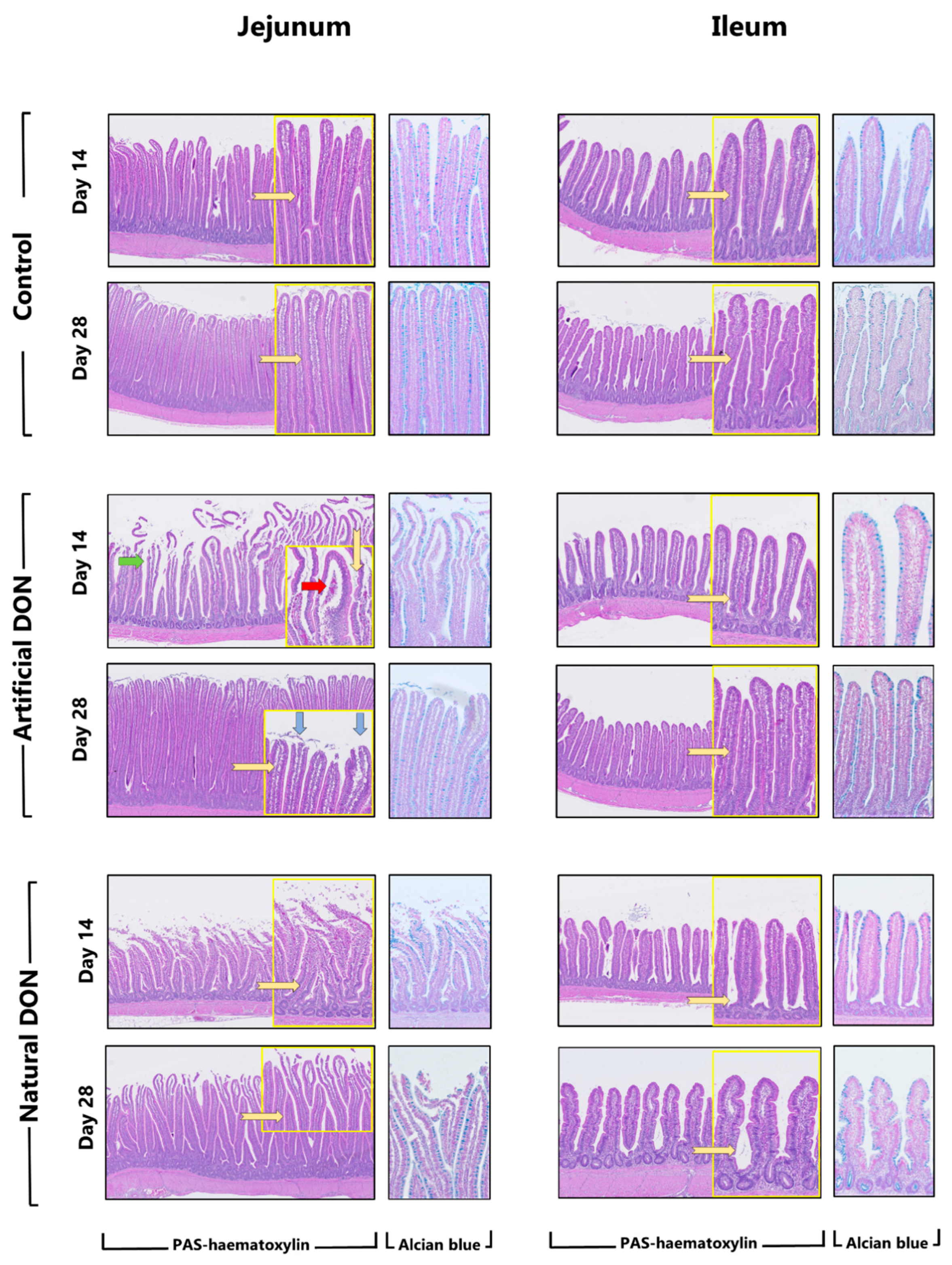
2.5.1. Histological Analysis of Jejunum and Ileum
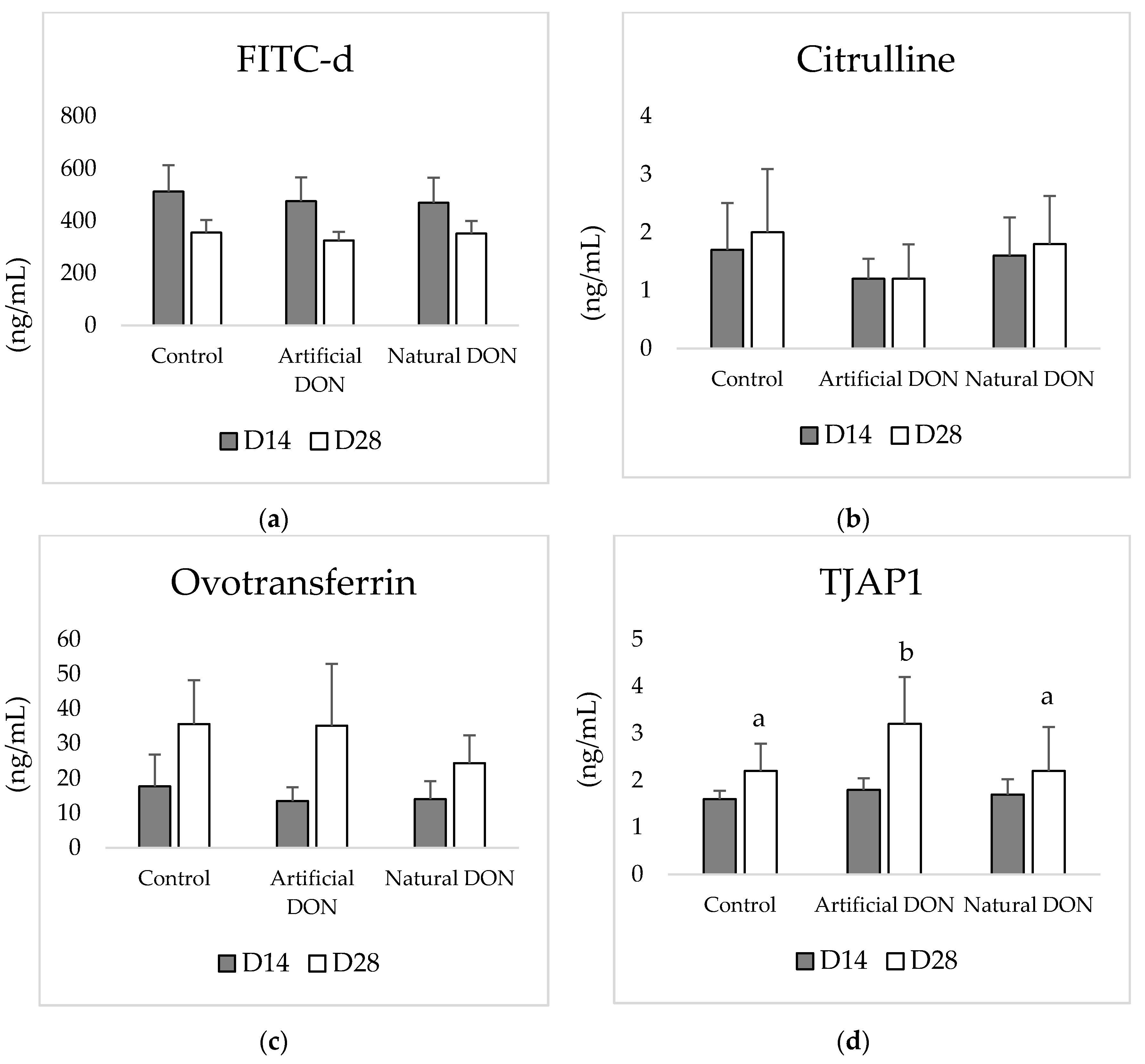
2.5.2. Serum Analysis
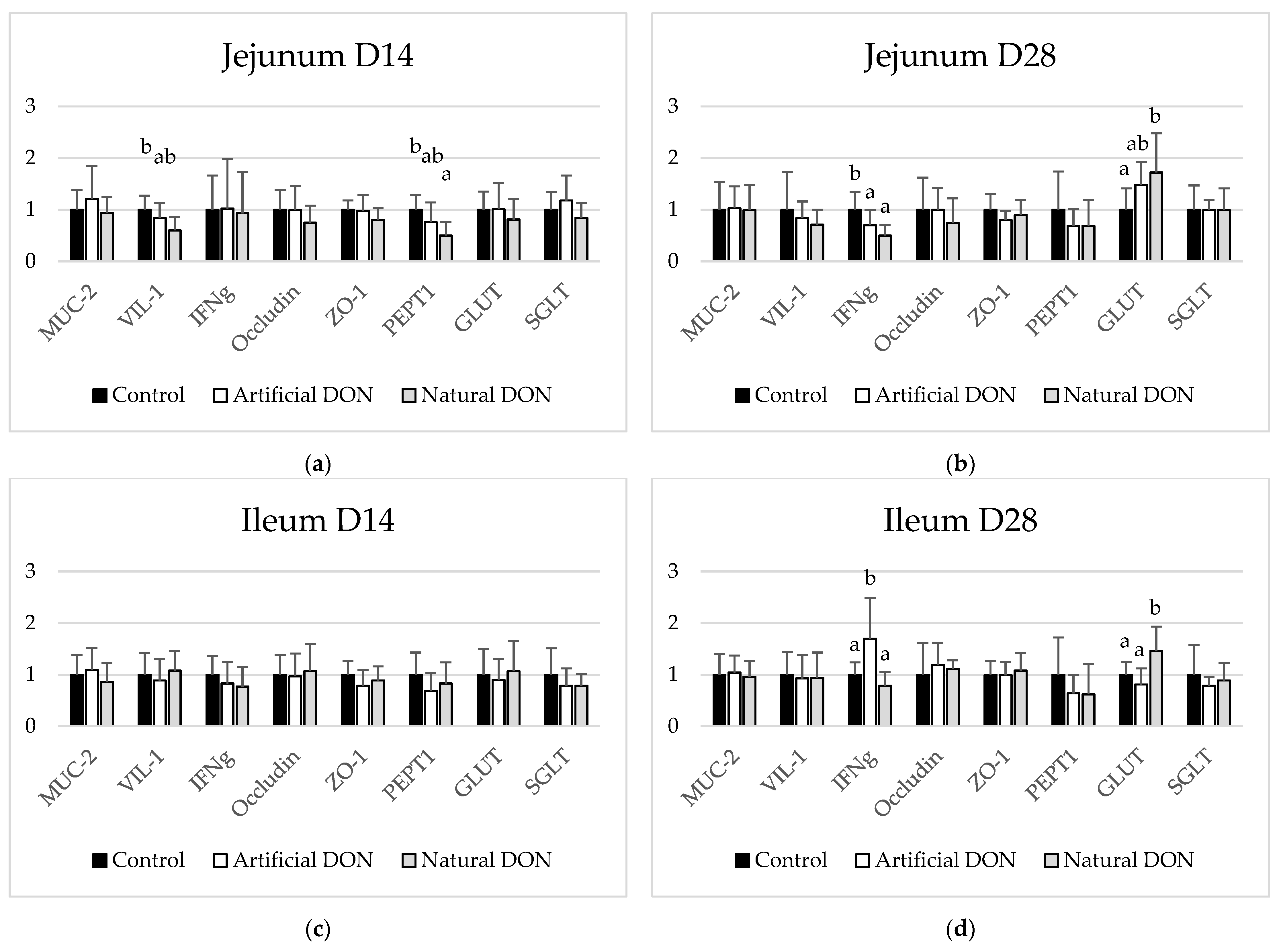
2.5.3. mRNA Expression in Jejunum and Ileum
2.6. Statistical Analysis
3. Results
3.1. Intestinal Morphometry and Morphological Scoring
3.2. Serum Analysis
3.3. mRNA Expression in the Jejunum and Ileum
3.4. Macroscopic Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Poel, A.F.B.; Abdollahi, M.R.; Cheng, H.; Colovic, R.; den Hartog, L.A.; Miladinovic, D.; Page, G.; Sijssens, K.; Smillie, J.F.; Thomas, M.; et al. Future Directions of Animal Feed Technology Research to Meet the Challenges of a Changing World. Anim. Feed Sci. Tech. 2020, 270, 114692. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of Mycotoxin-contaminated Feed on Farm Animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; Molist, F. Effect of Different Dietary Levels of Corn Naturally Contaminated with DON and Its Derivatives 3 + 15 Ac-DON and DON-3-glucoside on the Performance of Broilers. Heliyon 2020, 6, e05257. [Google Scholar] [CrossRef]
- Santos, R.R.; van Eerden, E. Impaired Performance of Broiler Chickens Fed Diets Naturally Contaminated with Moderate Levels of Deoxynivalenol. Toxins 2021, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Riahi, I.; Marquis, V.; Ramos, A.J.; Brufau, J.; Esteve-Garcia, E.; Pérez-Vendrell, A.M. Effects of Deoxynivalenol-contaminated Diets on Productive, Morphological, and Physiological Indicators in Broiler Chickens. Animals 2020, 10, 1795. [Google Scholar] [CrossRef]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.P.; Eeckhaut, V.; Eeckhout, M.; et al. The Mycotoxin Deoxynivalenol Predisposes for the Development of Clostridium perfringens-induced Necrotic Enteritis in Broiler Chickens. PLoS ONE 2014, 9, e108775. [Google Scholar] [CrossRef] [Green Version]
- Ruhnau, D.; Hess, C.; Grenier, B.; Doupovec, B.; Schatzmayr, D.; Hess, M.; Awad, W.A. The Mycotoxin Deoxynivalenol (DON) Promotes Campylobacter jejuni Multiplication in the Intestine of Broiler Chickens with Consequences on Bacterial Translocation and Gut Integrity. Front. Vet. Sci. 2020, 7, 573894. [Google Scholar] [CrossRef]
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol Impairs Hepatic and Intestinal Gene Expression of Selected Oxidative Stress, Tight Junction and Inflammation Proteins in Broiler Chickens, but Addition of an Adsorbing Agent Shifts the Effects to the Distal Parts of the Small Intestine. PLoS ONE 2013, 8, e69014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EC-European Comission. Commission Recommendation 2016/1319/EC of 29 July 2016 Amending Commission Recommendation 2006/576/EC on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Off. J. Eur. Union 2016, L208, 58–60. [Google Scholar]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New Insights into Mycotoxin Mixtures: The Toxicity of Low Doses of Type B Trichothecenes on Intestinal Epithelial Cells is Synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low Doses of Mycotoxin Mixtures Below EU Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study. Toxins 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.C.; King, W.D.; Verax, M.; Fox, U.; Kudupoje, M.B.; Mathis, G.; Lumpkins, B.; Yiannikouris, A. Impact of Chronic Levels of Naturally Multi-contaminated Feed with Fusarium Mycotoxins on Broiler Chickens and Evaluation of the Mitigation Properties of Different Titers of Yeast Cell Wall Extract. Toxins 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The Mycotoxin Deoxynivalenol Affects Nutrient Absorption in Human Intestinal Epithelial Cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar] [CrossRef] [Green Version]
- Fodor, J.; Nemeth, M.; Kametler, L.; Posa, R.; Kovacs, M.; Horn, P. Novel Methods of Fusarium Toxins’ Production for Toxicological Experiments. Acta Agrar. Kvar. 2006, 10, 277–285. [Google Scholar]
- Vicuna, E.A.; Kuttappan, V.A.; Tellez, G.; Hernandez-Velasco, X.; Seeber-Galarza, R.; Latorre, J.D.; Faulkner, O.B.; Wolfenden, A.D.; Hargis, B.M.; Bielke, L.R. Dose Titration of FITC-D for Optimal Measurement of Enteric Inflammation in Broiler Chicks. Poult. Sci. 2015, 94, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.; Tersteeg-Zijderveld, M.H.G.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric Analysis of Heat Stress Related Damage in the Small Intestines of Broiler Chickens. Avian Pathol. 2015, 44, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; van Kempen, T.A.T.G.; Tersteg-Zijderveld, M.H.G.; Koolmees, P.A.; Smits, C.; Fink-Gremmels, J. Effects of a Feed Additive Blend on Broilers Challenged with Heat Stress. Avian Pathol. 2019, 48, 582–601. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in Susceptibility to Heat Stress Along the Chicken Intestine and the Protective Effects of Galacto-oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [Green Version]
- Forder, R.E.; Nattrass, G.S.; Geier, M.S.; Hughes, R.J.; Hynd, P.I. Quantitative Analyses of Genes Asociated with Mucin Sunthesis of Broiler Chickens with Induced Necrotic Enteritis. Poult. Sci. 2012, 91, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Mott, C.R.; Siegel, P.B.; Webb, K.E., Jr.; Wong, E.A. Gene Expression of Nutrient Transporters in the Small Intestine of Chickens from Lines Divergently Selected for High or Low Juvenile Body Weight. Poult. Sci. 2008, 87, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D. Chicken Intestinal Development in Health and Disease: Transcriptomic and Modeling Approaches. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2012. [Google Scholar]
- Mahmoud, K.Z.; Edens, F.W. Breeder Age Affects Small Intestine Development of Broiler Chicks with Immediate or Delayed Access to Feed. Br. Poult. Sci. 2012, 53, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cheat, S.; Pinton, P.; Cossalter, A.-M.; Cognie, J.; Vilariño, M.; Callu, P.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. The Mycotoxins Deoxynivalenol and Nivalenol Show in vivo Synergism on Jejunum Enterocytes Apoptosis. Food Chem. Toxicol. 2016, 87, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Coudrier, E.; Arpin, M.; Dudouet, B.; Finidori, J.; Garcia, A.; Huet, C.; Pringault, E.; Robine, S.; Sahuquillo-Merino, C.; Louvard, D. Villin as a Structural Marker to Study the Assembly of the Brush Border. Protoplasma 1988, 145, 99–105. [Google Scholar] [CrossRef]
- Pelyhe, C.; Kövesi, B.; Szabó-Fodor, J.; Zándoki, E.; Erdélyi, M.; Kovács, B.; Mézes, M.; Balogh, K. Age-dependent Effects of Short-term Exposure of T-2 Toxin or Deoxynivalenol on Lipid Peroxidation and Glutathione Redox System in Broiler Chickens. World Mycotox. J. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Setyabudi, F.M.C.S.; Razzazi-Fazeli, E.; Böhm, J.; Zentek, J. In vitro Effects of Deoxynivalenol on Small Intestinal D-glucose Uptake and Absorption of Deoxynivalenol Across the Isolated Jejunal Epithelium of Laying Hens. Poult. Sci. 2007, 86, 15–20. [Google Scholar] [CrossRef]
- Schaefer, C.M.; Corsiglia, C.M.; Mireles Jr, A.; Koutos, E.A. Turkey Breeder Hen Age Affects Growth and Systemic and Intestinal Inflammatory Responses in Female Poults Examined at Different Ages Posthatch. Poult. Sci. 2006, 85, 1755–1763. [Google Scholar] [CrossRef]
- Geyra, A.; Uni, Z.; Sklan, D. Enterocyte Dynamics and Mucosal Development in the Posthatch Chick. Poult. Sci. 2001, 80, 776–782. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Daniel, H. Peptide Transporters and Their Roles in Physiological Processes and Drug Disposition. Xenobiotica 2008, 38, 1022–1042. [Google Scholar] [CrossRef]
- Madsen, S.L.; Wong, E.A. Expression of the Chicken Peptide Transporter 1 and the Peroxisome Proliferator-activated Receptor α Following Feed Restriction and Subsequent Refeeding. Poult. Sci. 2011, 90, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Duan, Y.; Wang, F.; Guo, F.; Yan, F.; Yang, X.; Yang, X. Intestinal Toxicity of Deoxynivalenol is Limited by Supplementation with Lactobacillus plantarum JM113 and Consequentially Altered Gut Microbiota in Broiler Chickens. J. Anim. Sci. Biotechnol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.; Sweazea, K.L. Glucose Regulation in Birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Sakata, M.; Okamoto, Y.; Yasui, Y.; Tadokoro, C.; Yoshimoto, Y.; Yamaguchi, M.; Kurachi, H.; Maeda, T.; Murata, Y. 8-bromo-cyclic AMP Stimulates Glucose Transporter-1 Expression in a Human Choriocarcinoma Cell Line. J. Endocrinol. 1999, 164, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.; Liaubert, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal Toxicity of the Masked Mycotoxin Deoxynivalenol-3-β-D-glucoside. Arch. Toxicol. 2015, 90, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef]
- Benthem de Grave, X.; Saltzmann, J.; Laurain, J.; Rodriguez, M.A.; Molist, F.; Dänicke, S.; Santos, R.R. Transmission of Zearalenone, Deoxynivalenol, and Their Derivatives from Sows to Piglets during Lactation. Toxins 2021, 6, 37. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Hoerr, F.J.; McKee, S.R.; Conner, D.E. Influence of Salmonella Enterica Serovar Typhimurium Infection on Intestinal Goblet Cells and Villous Morphology in Broiler Chicks. Avian Dis. 2010, 54, 841–847. [Google Scholar] [CrossRef]
- Reisinger, N.; Ganner, A.; Masching, S.; Schatzmayr, G.; Applegate, T.J. Efficacy of a Yeast Derivative on Broiler Performance, Intestinal Morphology and blood profile. Livest. Sci. 2012, 143, 195–200. [Google Scholar] [CrossRef]
- Pascual, A.; Pauletto, M.; Giantin, M.; Radaelli, G.; Ballarin, C.; Birolo, M.; Zomeño, C.; Dacasto, M.; Bortoletti, M.; Vascellari, M.; et al. Effect of Dietary Supplementation with Yeast Cell Wall Extracts on Performance and Gut Response in Broiler Chickens. J. Anim. Sci. Biotechnol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Baxter, M.F.A.; Latorre, J.D.; Dridi, S.; Merino-Guzman, R.; Hernandez-Velasco, X.ç.; Hargis, B.M.ç.; Tellez-Isaias, G. Identification of Serum Biomarkers for Intestinal Integrity in a Broiler Chicken Malabsorption Model. Front. Vet. Sci. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, N.C.; Anthony, N.B.; Kannan, L.; Huff, W.E.; Chapman, H.D.; Erf, G.F.; Wakenell, P. Serum Ovotransferrin as a Biomarker of Inflammatory Diseases in Chickens. Poult. Sci. 2009, 88, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Soodoi, C.; Sasgary, S.; Strasser, A.; Böhm, J. Effects of Feed Contaminant Deoxynivalenol on Plasma Cytokines and mRNA Expression of Immune Genes in the Intestine of Broiler Chickens. PLoS ONE 2013, 8, e71492. [Google Scholar] [CrossRef] [Green Version]
- Girgis, G.N.; Sharif, S.; Barta, J.R.; Boermans, H.J.; Smith, T.K. Immunomodulatory Effects of Feed-borne Fusarium Mycotoxins in Chickens Infected with Coccidia. Exp. Biol. Med. 2008, 233, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Fluctuation of Zonulin Levels in Blood vs. Stability of Antibodies. World J. Gastroenterol. 2017, 23, 5669–5679. [Google Scholar] [CrossRef]
- Pelyhe, C.; Kövesi, B.; Zándoki, E.; Kovács, B.; Erdélyi, M.; Kulcsár, S.; Mézes, M.; Balogh, K. Multi-trichothecene Mycotoxin Exposure Activates Glutathione-redox System in Broiler Chicken. Toxicon 2018, 153, 53–57. [Google Scholar] [CrossRef]
- Quezada, T.; Cuellar, H.; Jaramillo-Juarez, F.; Valdivia, A.G.; Reyes, J.L. Effects of Aflatoxin B(1) on the Liver and Kidney of Broiler Chickens during Development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 265–272. [Google Scholar] [CrossRef]
- Pozzo, L.; Cavallarin, L.; Antoniazzi, S.; Guerre, P.; Biasbetti, E.; Capucchio, M.T.; Schiavone, A. Feeding a Diet Contaminated with Ochratoxin A for Broiler Chickens at the Maximum Level Recommended by the EU for Poultry Feeds (0.1 mg/kg). 2. Effects on Meat Quality, Oxidative Stress, Residues and Histological Traits. J. Anim. Physiol. Anim. Nutr. 2013, 97, 23–31. [Google Scholar] [CrossRef]
- Mavilia, M.G.; Pakala, T.; Molina, M.; Wu, G.Y. Differentiating Cystic Liver Lesions: A Review of Imaging Modalities, Diagnosis and Management. J. Clin. Transl. Hepatol. 2018, 6, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, D.; Kaunitz, J.D. The “Leaky Gut”: Tight Junctions but Loose Associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Mycotoxins | Control | Artificial DON | Natural DON |
|---|---|---|---|
| DON | 233 | 4040 | 3810 |
| 3 + 15 Ac-DON | - | 180 | 88.1 |
| DON-3-Glucoside | 78.3 | 191 | 1640 |
| Zearalenone | 39.3 | 105 | 577 |
| Fumonisins B1 + B2 | 61.3 | 103 | 32.7 |
| Enniatin B | - | 6.5 | 7.8 |
| Beauvericin | 8.2 | 7.7 | 6.4 |
| Mycotoxins | Control | Artificial DON | Natural DON |
|---|---|---|---|
| DON | 234 | 3860 | 3950 |
| 3 + 15 Ac-DON | - | 320 | 59.8 |
| DON-3-Glucoside | 75.7 | 118 | 895 |
| Zearalenone | 31.6 | 56 | 727 |
| Aflatoxin B1 | 1.3 | - | - |
| Aflatoxin B1 + B2 + G1 + G2 | 1.3 | - | - |
| Fumonisins B1 + B2 | 56.1 | 263 | - |
| Enniatin B | - | 7.8 | - |
| Beauvericin | 5.4 | 20.9 | - |
| Genes | Primer Sequence | Annealing T° | Reference |
|---|---|---|---|
| HKG | - | - | - |
| HPRT | F: GTTGCTGTCTCTACTTAAGCAG R: ATATCCCACACTTCGAGGAG | 65 | [19] |
| ACTB | F: ATGTGGATCAGCAAGCAGGAGTA R: TTTATGCGCATTTATGGGTTTTGT | 65 | [20] |
| GOI | - | - | - |
| MUC-2 | F: ATGCGATGTTAACACAGGACTC R: GTGGAGCACAGCAGACTTTG | 61 | [21] |
| VIL-1 | F: GGCACCAACGAGTACAACACCA R: TGCAGCCCTTCCCATACCAGA | 65 | [20] |
| IFNg | F: CAAGCTCCCGATGAACGAC R: CAATTGCATCTCCTCTGAGAC | 64 | [20] |
| Occludin | F: TTCATGATGCCTGCTCTTGTG R: CCTGAGCCTTGGTACATTCTTGT | 61 | [20] |
| ZO-1 | F: CTTCAGGTGTTTCTCTTCCTCCTC R: CTGTGGTTTCATGGCTGGATC | 59 | [19] |
| PEPT-1 | F: CCCCTGAGGAGGATCACTTGTT R: CAAAAGAGCAGCAGCAACGA | 59 | [19] |
| GLUT1 | F: TTGCTGGCTTTGGGTTGTG R: GGAGGTTGAGGGCCAAAGTC | 57 | [20] |
| SGLT | F: TGTCTCTCTGGCAAGAACATGTC R: GGGCAAGAGCTTCAGGTATCC | 59 | [22] |
| Intestinal Section | Control | Artificial DON | Natural DON | p-Value | LSD |
|---|---|---|---|---|---|
| Jejunum | - | - | - | - | - |
| Villus height (μm) | 873.3 b | 713.2 a | 656.4 a | 0.02 | 152.84 |
| Crypt depth (μm) | 168.5 | 134.4 | 144.6 | 0.09 | 31.06 |
| VH/CD | 5.34 | 5.66 | 4.98 | 0.51 | 1.18 |
| Villus area (μm2) | 93.2 | 81.9 | 60.8 | 0.18 | 34.88 |
| Goblet cells (number) | 142.0 b | 125.2 ab | 93.1 a | 0.02 | 32.45 |
| Goblet cells density 1 | 1.5 | 1.7 | 1.6 | 0.50 | 0.29 |
| Integrity score 2 | 0.84 a | 3.24 b | 3.48 b | < 0.001 | 0.98 |
| Ileum | - | - | - | - | - |
| Villus height (μm) | 493.2 | 450.1 | 489.4 | 0.52 | 84.46 |
| Crypt depth (μm) | 134.8 | 115.4 | 134.0 | 0.22 | 25.14 |
| VH/CD | 3.80 | 4.02 | 3.88 | 0.89 | 0.93 |
| Villus area (μm2) | 45.7 | 39.5 | 44.8 | 0.24 | 7.99 |
| Goblet cells (number) | 99.5 | 81.4 | 99.3 | 0.13 | 19.95 |
| Goblet cells density 1 | 2.2 | 2.2 | 2.4 | 0.63 | 0.05 |
| Integrity score 2 | 0.12 | 0.14 | 0.24 | 0.79 | 0.37 |
| Intestinal Section | Control | Artificial DON | Natural DON | p-Value | LSD |
|---|---|---|---|---|---|
| Jejunum | - | - | - | - | - |
| Villus height (μm) | 1277 | 1224 | 1144 | 0.35 | 185.49 |
| Crypt depth (μm) | 287.6 | 300.4 | 259.1 | 0.45 | 67.40 |
| VH/CD | 4.61 | 4.46 | 4.59 | 0.95 | 0.96 |
| Villus area (μm2) | 209.2 | 213.4 | 155.0 | 0.47 | 107.23 |
| Goblet cells (number) | 165.1 | 156.6 | 169.5 | 0.78 | 37.99 |
| Goblet cells density 1 | 0.80 a | 0.91 a | 1.20 b | 0.02 | 0.23 |
| Integrity score 2 | 0.38 a | 0.62 ab | 1.32 b | 0.04 | 0.734 |
| Ileum | - | - | - | - | - |
| Villus height (μm) | 682.5 | 600.0 | 637.5 | 0.27 | 102.23 |
| Crypt depth (μm) | 188.7 | 179.2 | 169.2 | 0.63 | 40.67 |
| VH/CD | 3.82 | 3.55 | 3.96 | 0.51 | 0.724 |
| Villus area (μm2) | 119.15 | 90.62 | 80.85 | 0.16 | 40.54 |
| Goblet cells (number) | 144.2 | 121.3 | 129.6 | 0.20 | 25.71 |
| Goblet cells density 1 | 1.40 | 1.60 | 1.80 | 0.22 | 0.43 |
| Integrity score 2 | 0.01 a | 0.06 b | 0.04 b | 0.03 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.R.; Oosterveer-van der Doelen, M.A.M.; Tersteeg-Zijderveld, M.H.G.; Molist, F.; Mézes, M.; Gehring, R. Susceptibility of Broiler Chickens to Deoxynivalenol Exposure via Artificial or Natural Dietary Contamination. Animals 2021, 11, 989. https://doi.org/10.3390/ani11040989
Santos RR, Oosterveer-van der Doelen MAM, Tersteeg-Zijderveld MHG, Molist F, Mézes M, Gehring R. Susceptibility of Broiler Chickens to Deoxynivalenol Exposure via Artificial or Natural Dietary Contamination. Animals. 2021; 11(4):989. https://doi.org/10.3390/ani11040989
Chicago/Turabian StyleSantos, Regiane R., Marjolein A. M. Oosterveer-van der Doelen, Monique H. G. Tersteeg-Zijderveld, Francesc Molist, Miklós Mézes, and Ronette Gehring. 2021. "Susceptibility of Broiler Chickens to Deoxynivalenol Exposure via Artificial or Natural Dietary Contamination" Animals 11, no. 4: 989. https://doi.org/10.3390/ani11040989





