A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Ethical Statement
2.2. Sea Urchin Collagen Extraction, Production of 2D Membranes, and 3D Scaffolds
2.3. Surgical Procedure and Animal Model
2.4. Clinical Follow-Up
2.5. Histopathological and Immunohistochemical Analysis
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
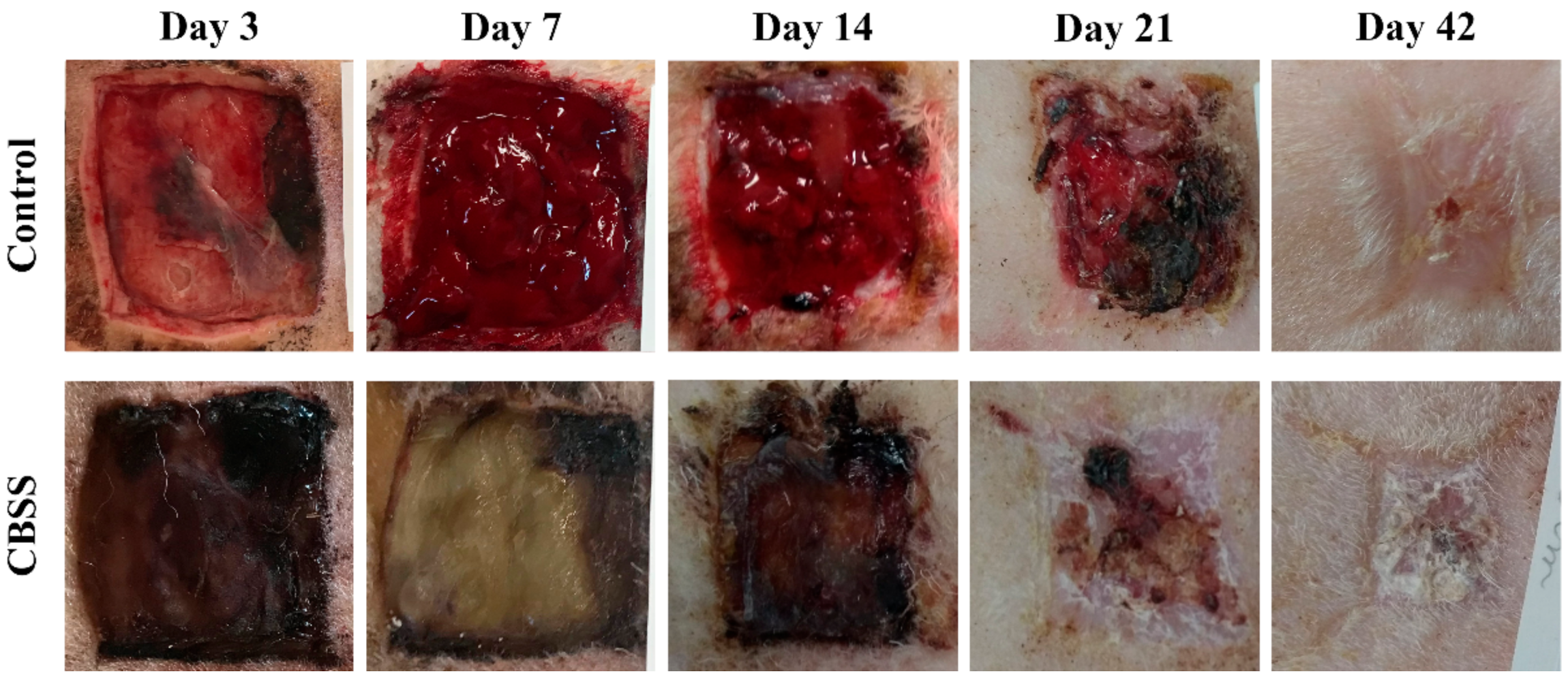
3.1. Clinical Follow-Up
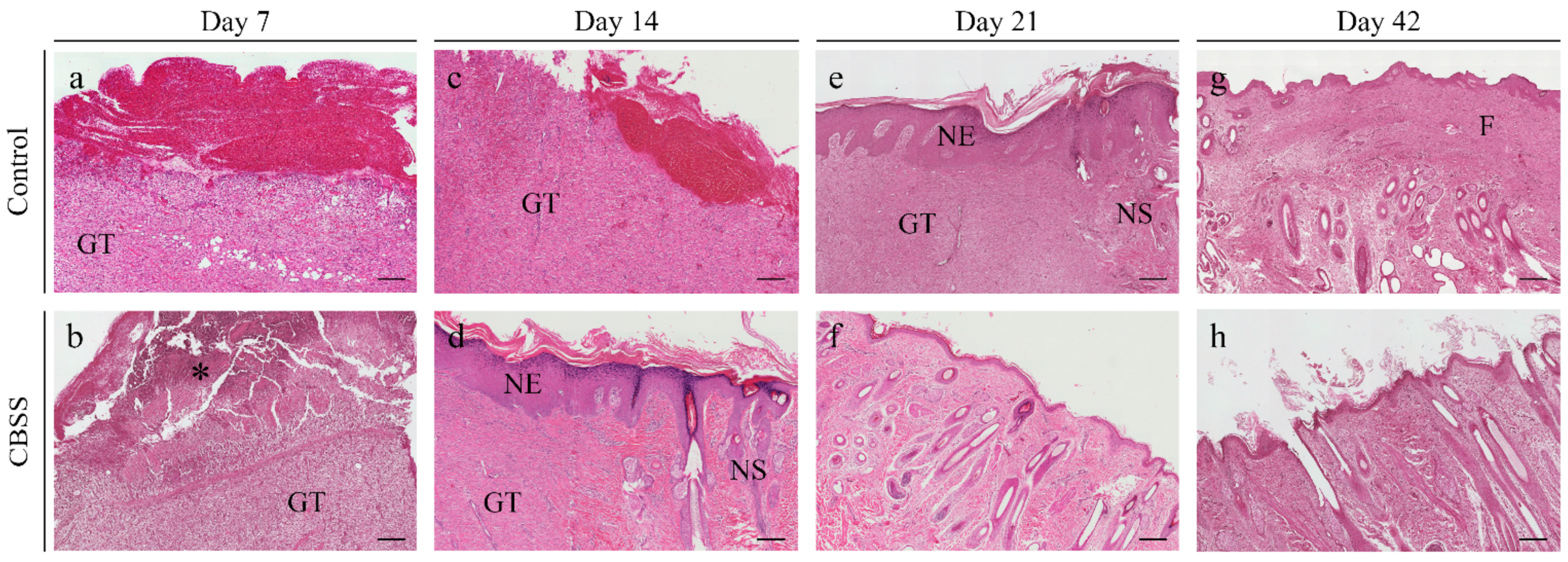
3.2. Histopathological Analysis
3.2.1. Superficial Inflammation
3.2.2. Deep Inflammation
3.2.3. Immature Granulation Tissue
3.2.4. Skin Adnexa
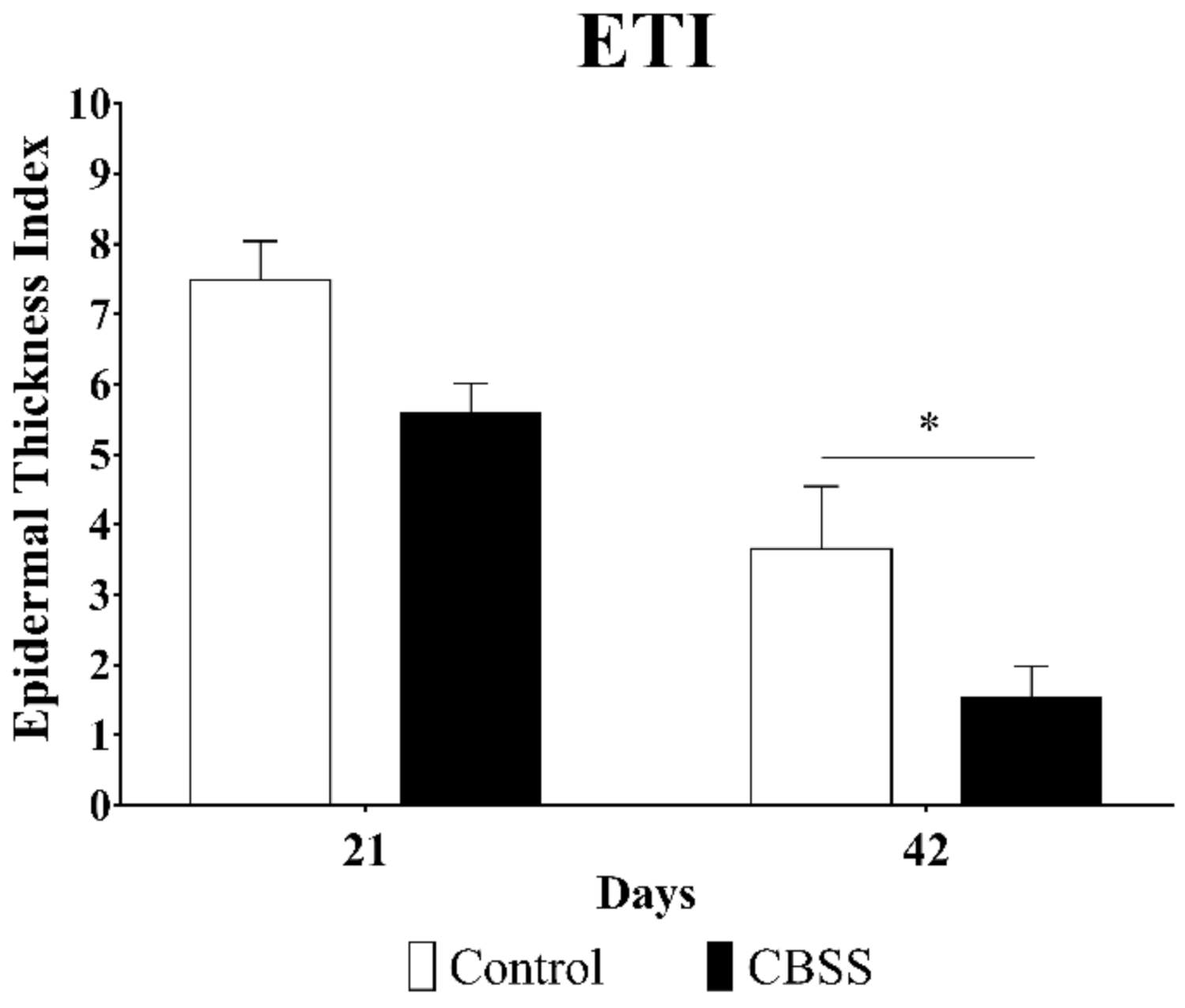
3.2.5. Epidermal Thickness Index (ETI)
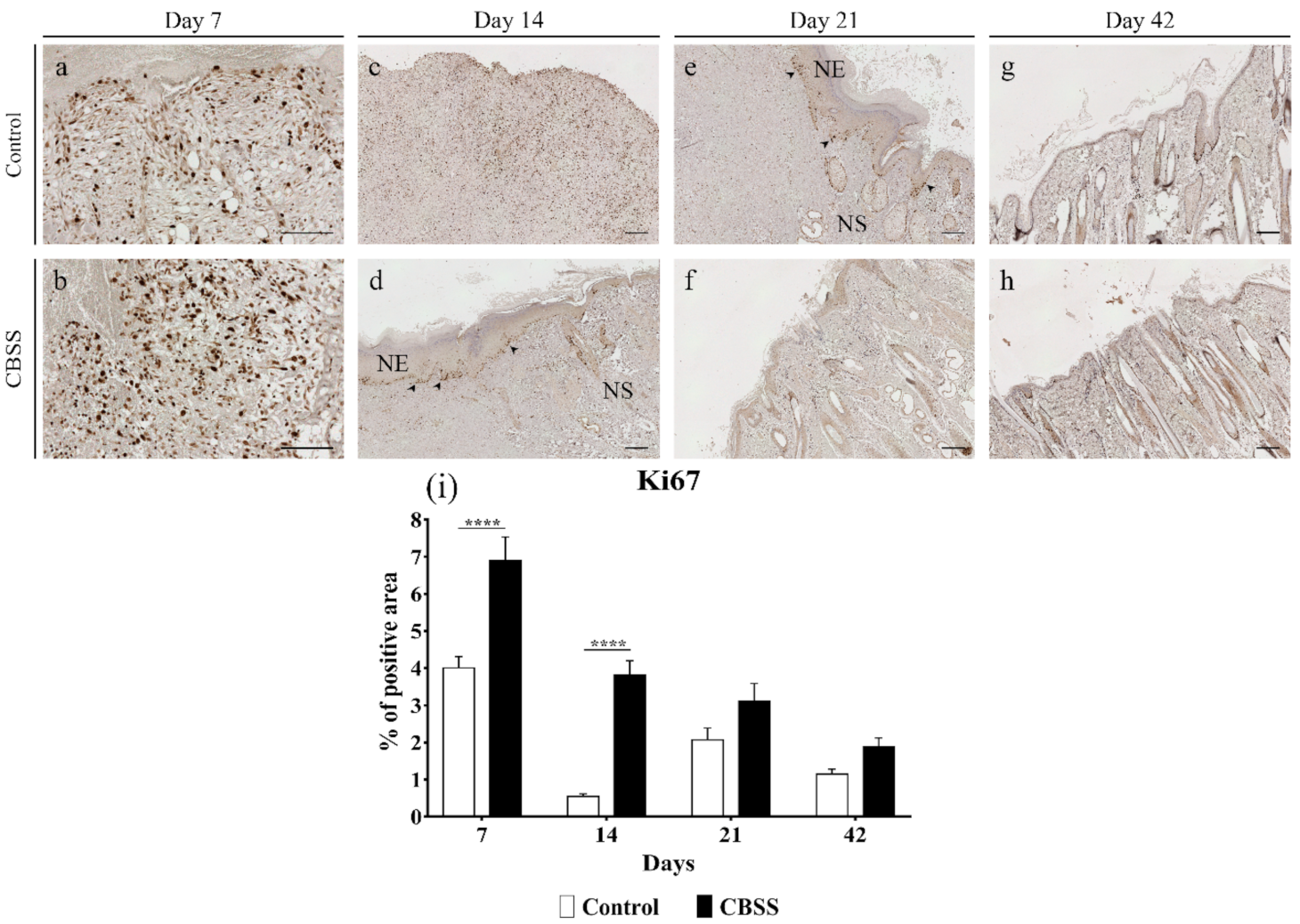
3.2.6. Ki67 Immunohistochemistry
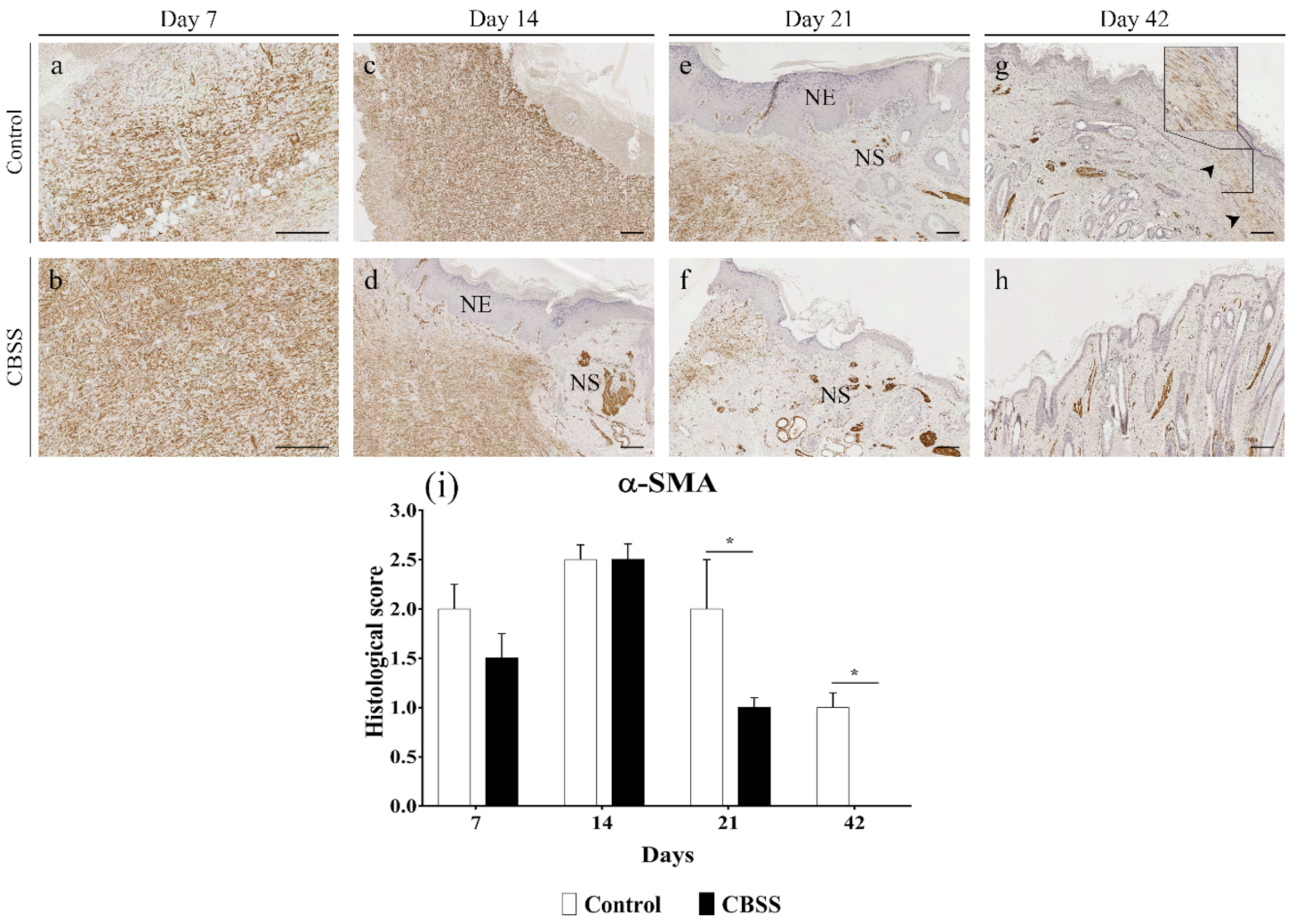
3.2.7. α-SMA Immunohistochemistry
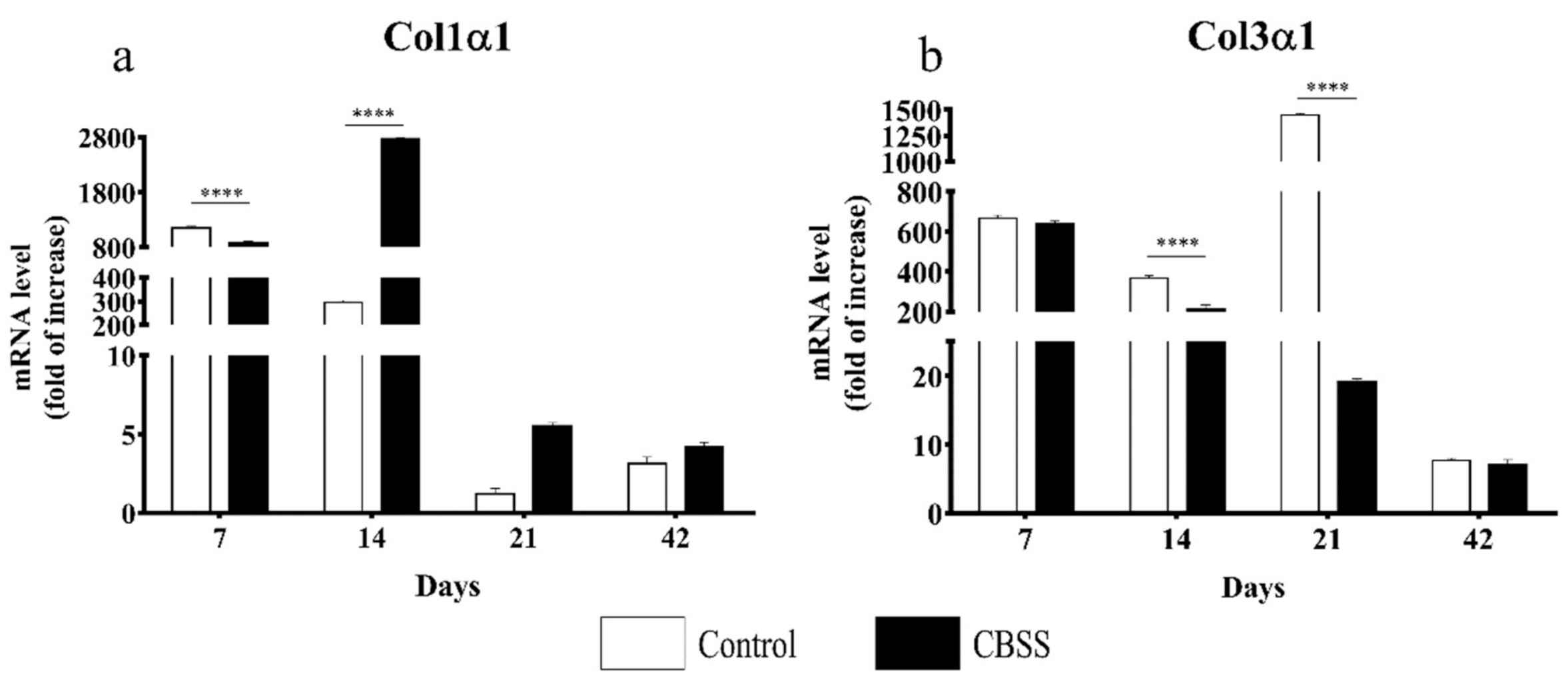
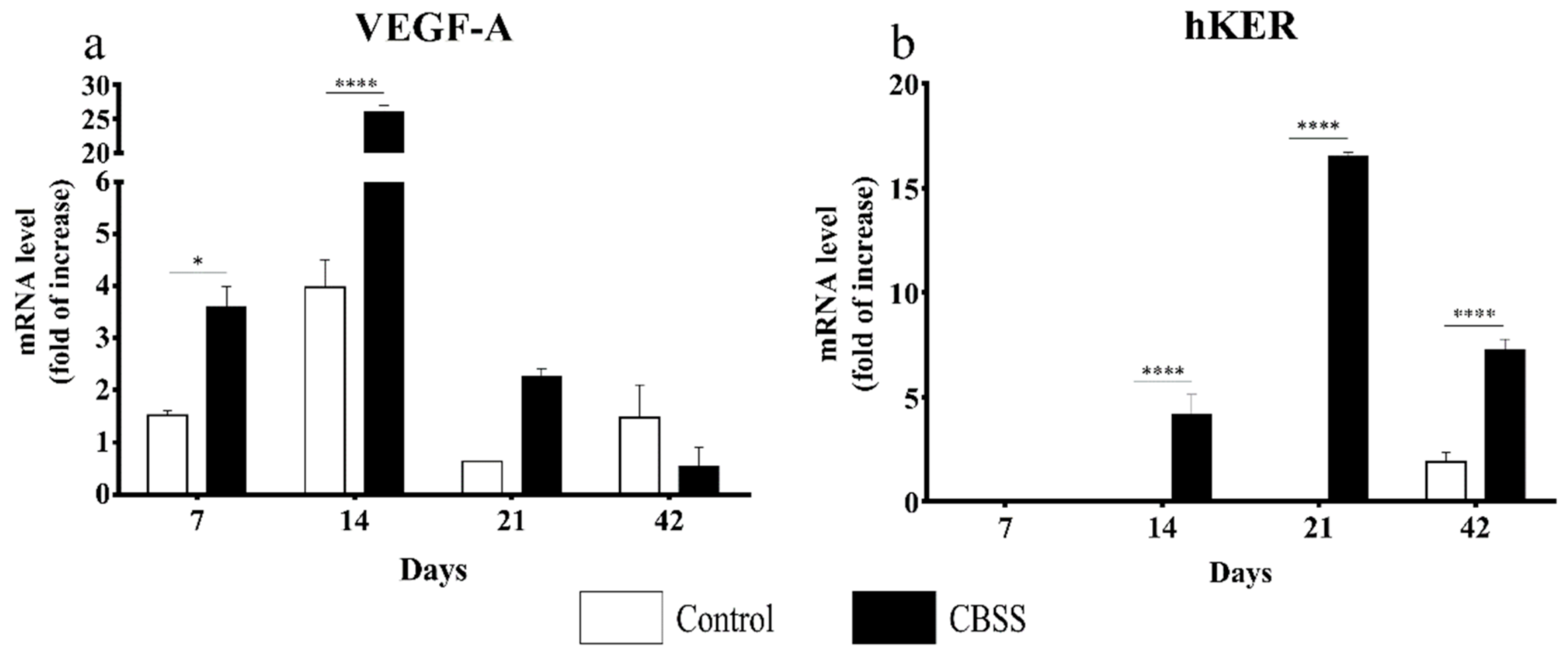
3.3. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theoret, C.L. The pathophysiology of wound repair. Vet. Clin. N. Am. Equine Pract. 2005, 21, 1–13. [Google Scholar] [CrossRef]
- Frykfors von Hekkel, A.K.; Pegram, C.; Halfacree, Z.J. Thoracic dog bite wounds in dogs: A retrospective study of 123 cases (2003–2016). Vet. Surg. 2020, 49, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Rothe, K.; Tsokos, M.; Handrick, W. Animal and Human Bite Wounds. Dtsch. Arztebl. Int. 2015, 112, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.T.; Pirraco, R.P.; Marques, A.P. Stem Cells in Skin Wound Healing: Are We There Yet? Adv. Wound Care 2016, 5, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sparks, H.D.; Sigaeva, T.; Tarraf, S.; Mandla, S.; Pope, H.; Hee, O.; Di Martino, E.S.; Biernaskie, J.; Radisic, M.; Scott, W.M. Biomechanics of Wound Healing in an Equine Limb Model: Effect of Location and Treatment with a Peptide-Modified Collagen-Chitosan Hydrogel. ACS Biomater. Sci. Eng. 2021, 7, 265–278. [Google Scholar] [CrossRef]
- Fowler, D. Distal Limb and Paw Injuries. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 819–845. [Google Scholar] [CrossRef]
- Dernell, W.S. Initial Wound Management. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 713–738. [Google Scholar] [CrossRef]
- Prpich, C.Y.; Santamaria, A.C.; Simcock, J.O.; Wong, H.K.; Nimmo, J.S.; Kuntz, C.A. Second intention healing after wide local excision of soft tissue sarcomas in the distal aspects of the limbs in dogs: 31 cases (2005–2012). J. Am. Vet. Med. Assoc. 2014, 244, 187–194. [Google Scholar] [CrossRef]
- Theoret, C. Tissue engineering in wound repair: The three “r”s—Repair, replace, regenerate. Vet. Surg. 2009, 38, 905–913. [Google Scholar] [CrossRef]
- Riggs, J.; Jennings, J.L.F.; Friend, E.J.; Halfacree, Z.; Nelissen, P.; Holmes, M.A.; Demetriou, J.L. Outcome of full-thickness skin grafts used to close skin defects involving the distal aspects of the limbs in cats and dogs: 52 cases (2005–2012). J. Am. Vet. Med. Assoc. 2015, 247, 1042–1047. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Paggiaro, A.O.; Isaac, C.; Teixeira Neto, N.; Santos, G.B. dos Substitutos cutâneos: Conceitos atuais e proposta de classificação. Rev. Bras. Cir. Plástica 2011, 26, 696–702. [Google Scholar] [CrossRef]
- Cuono, C.B.; Langdon, R.; Birchall, N.; Barttelbort, S.; McGuire, J. Composite autologous-allogeneic skin replacement: Development and clinical application. Plast. Reconstr. Surg. 1987, 80, 626–635. [Google Scholar] [CrossRef]
- Balasubramani, M.; Kumar, T.R.; Babu, M. Skin substitutes: A review. Burns 2001, 27, 534–544. [Google Scholar] [CrossRef]
- Hansbrough, J.F.; Morgan, J.; Greenleaf, G.; Underwood, J. Development of a temporary living skin replacement composed of human neonatal fibroblasts cultured in Biobrane, a synthetic dressing material. Surgery 1994, 115, 633–644. [Google Scholar]
- Jones, I.; Currie, L.; Martin, R. A guide to biological skin substitutes. Br. J. Plast. Surg. 2002, 55, 185–193. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef]
- Araujo, T.A.T.; Almeida, M.C.; Avanzi, I.; Parisi, J.; Simon Sales, A.F.; Na, Y.; Renno, A. Collagen membranes for skin wound repair: A systematic review. J. Biomater. Appl. 2020. [Google Scholar] [CrossRef]
- Jansen, L.A.; De Caigny, P.; Guay, N.A.; Lineaweaver, W.C.; Shokrollahi, K. The evidence base for the acellular dermal matrix AlloDerm: A systematic review. Ann. Plast. Surg. 2013, 70, 587–594. [Google Scholar] [CrossRef]
- Wester, J.L.; Pittman, A.L.; Lindau, R.H.; Wax, M.K. AlloDerm with split-thickness skin graft for coverage of the forearm free flap donor site. Otolaryngol. Head Neck Surg. USA 2014, 150, 47–52. [Google Scholar] [CrossRef]
- Fourman, M.S.; Phillips, B.T.; Fritz, J.R.; Conkling, N.; McClain, S.A.; Simon, M.; Dagum, A.B. Laser-Assisted Indocyanine Green Dye Angiography Accurately Predicts the Split-Thickness Graft Timing of Integra Artificial Dermis. Ann. Plast. Surg. 2014, 73, 150–155. [Google Scholar] [CrossRef]
- Notodihardjo, S.C.; Morimoto, N.; Munisso, M.C.; Le, T.M.; Mitsui, T.; Kakudo, N.; Kusumoto, K. A comparison of the wound healing process after the application of three dermal substitutes with or without basic fibroblast growth factor impregnation in diabetic mice. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1547–1555. [Google Scholar] [CrossRef]
- Cervelli, V.; Brinci, L.; Spallone, D.; Tati, E.; Palla, L.; Lucarini, L.; De Angelis, B. The use of MatriDerm ® and skin grafting in post-traumatic wounds. Int. Wound J. 2011, 8, 400–405. [Google Scholar] [CrossRef]
- Min, J.H.; Yun, I.S.; Lew, D.H.; Roh, T.S.; Lee, W.J. The use of Matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch. Plast. Surg. 2014, 41, 330–336. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Azizur Rahman, M. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Ellis, D.L.; Yannas, I.V. Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers. Biomaterials 1996, 17, 291–299. [Google Scholar] [CrossRef]
- Chung, K.-H.; Bhadriraju, K.; Spurlin, T.A.; Cook, R.F.; Plant, A.L. Nanomechanical Properties of Thin Films of Type I Collagen Fibrils. Langmuir 2010, 26, 3629–3636. [Google Scholar] [CrossRef]
- Bozec, L.; Van Der Heijden, G.; Horton, M. Collagen fibrils: Nanoscale ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.C.; et al. Production, characterization and biocompatibility of marine collagen matrices from an alternative and sustainable source: The sea urchin Paracentrotus lividus. Mar. Drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Coccè, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, S.; Barbaglio, A.; Burlini, N.; del Giacco, L.; Ghilardi, A.; Sugni, M.; Di Benedetto, C.; Bonasoro, F.; Wilkie, I.C.; Carnevali, M.D.C. New insights into the mutable collagenous tissue of Paracentrotus lividus: Preliminary results. Zoosymposia 2012, 7, 279–285. [Google Scholar] [CrossRef]
- Russel, W.; Burch, R. The Principles of Human Experimental Technique; Methiun & Co: London, UK, 1959; ISBN 0900767782 9780900767784. [Google Scholar]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Melotti, L.; Martinello, T.; Perazzi, A.; Martines, E.; Zuin, M.; Modenese, D.; Cordaro, L.; Ferro, S.; Maccatrozzo, L.; Iacopetti, I.; et al. Could cold plasma act synergistically with allogeneic mesenchymal stem cells to improve wound skin regeneration in a large size animal model? Res. Vet. Sci. 2021, 136, 97–110. [Google Scholar] [CrossRef]
- Rahmani-Neishaboor, E.; Yau, F.M.; Jalili, R.; Kilani, R.T.; Ghahary, A. Improvement of hypertrophic scarring by using topical anti-fibrogenic/anti-inflammatory factors in a rabbit ear model. Wound Repair Regen. 2010, 18, 401–408. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Fibrosis: Recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol. Rep. 2010, 2, 78. [Google Scholar] [CrossRef]
- Balsa, I.M.; Culp, W.T.N. Wound Care. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 1049–1065. [Google Scholar] [CrossRef]
- Eggleston, R.B. Wound Management: Wounds with Special Challenges. Vet. Clin. N. Am. Equine Pract. 2018, 34, 511–538. [Google Scholar] [CrossRef]
- Theoret, C.L.; Bolwell, C.F.; Riley, C.B. A cross-sectional survey on wounds in horses in New Zealand. N. Z. Vet. J. 2016, 64, 90–94. [Google Scholar] [CrossRef]
- Chocarro-Wrona, C.; López-Ruiz, E.; Perán, M.; Gálvez-Martín, P.; Marchal, J.A. Therapeutic strategies for skin regeneration based on biomedical substitutes. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 484–496. [Google Scholar] [CrossRef]
- Ramió-Lluch, L.; Cerrato, S.; Brazis, P.; Rabanal, R.M.; Fondevila, D.; Puigdemont, A. Proof of concept of a new autologous skin substitute for the treatment of deep wounds in dogs. Vet. J. 2017, 230, 36–40. [Google Scholar] [CrossRef]
- Kaasi, A.; Lima-Neto, J.F.; Matiello-Filho, J.A.; Calejo, M.H.S.; Jardini, A.L.; Kharmandayan, P. Regenerative collagen biomembrane: Interim results of a phase I veterinary clinical trial for skin repair. F1000 Res 2018, 7. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Fernández-Cervantes, I.; Rodríguez-Fuentes, N.; León-Deniz, L.V.; Alcántara Quintana, L.E.; Cervantes-Uc, J.M.; Herrera Kao, W.A.; Cerón-Espinosa, J.D.; Cauich-Rodríguez, J.V.; Castaño-Meneses, V.M. Cell-free scaffold from jellyfish Cassiopea andromeda (Cnidaria; Scyphozoa) for skin tissue engineering. Mater. Sci. Eng. C 2020, 111, 110748. [Google Scholar] [CrossRef]
- Cruz, M.A.; Araujo, T.A.; Avanzi, I.R.; Parisi, J.R.; de Andrade, A.L.M.; Rennó, A.C.M. Collagen from Marine Sources and Skin Wound Healing in Animal Experimental Studies: A Systematic Review. Mar. Biotechnol. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing inflammation in skin wound healing: A review. Int. J. Inflam. 2015, 2015. [Google Scholar] [CrossRef]
- Farndale, R.W. Collagen-induced platelet activation. Blood Cells Mol. Dis. 2006, 36, 162–165. [Google Scholar] [CrossRef]
- Kehrel, B. Platelet receptors for collagens. Platelets 1995, 6, 11–16. [Google Scholar] [CrossRef]
- Harrison, S.; Vavken, P.; Kevy, S.; Jacobson, M.; Zurakowski, D.; Murray, M.M. Platelet Activation by Collagen Provides Sustained Release of Anabolic Cytokines. Am. J. Sports Med. 2011, 39, 729–734. [Google Scholar] [CrossRef]
- Ollivier, V.; Syvannarath, V.; Gros, A.; Butt, A.; Loyau, S. Collagen Can Selectively Trigger a Platelet Secretory Phenotype via Glycoprotein VI. PLoS ONE 2014, 9, e104712. [Google Scholar] [CrossRef]
- Sonmez, O.; Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. [Google Scholar] [CrossRef]
- Das, A.; Abas, M.; Biswas, N.; Banerjee, P.; Ghosh, N.; Rawat, A.; Khanna, S.; Roy, S.; Sen, C.K. A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Häkkinen, L.; Larjava, H.; Koivisto, L. Granulation tissue formation and remodeling. Endod. Top. 2011, 24, 94–129. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ikeda, K.; Yamaya, Y.; Yamashita, K.; Saito, T.; Hoshino, Y.; Koga, T.; Enari, H.; Suto, S.; Yotsuyanagi, T. The usefulness of the collagen and elastin sponge derived from salmon as an Artificial dermis and scaffold for tissue engineering. Biomed. Res. 2011, 32, 29–36. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Kim, K.O.; Lee, Y.; Hwang, J.W.; Kim, H.; Kim, S.M.; Chang, S.W.; Lee, H.S.; Choi, Y.S. Wound healing properties of a 3-D scaffold comprising soluble silkworm gland hydrolysate and human collagen. Coll. Surf. B Biointerfaces 2014, 116, 318–326. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Eweida, A.M.; Marei, M.K. Naturally Occurring Extracellular Matrix Scaffolds for Dermal Regeneration: Do They Really Need Cells? Biomed. Res. Int. 2015, 2015, 839694. [Google Scholar] [CrossRef] [PubMed]
- Bluff, J.E.; Ferguson, M.W.J.; O’Kane, S.; Ireland, G. Bone marrow-derived endothelial progenitor cells do not contribute significantly to new vessels during incisional wound healing. Exp. Hematol. 2007, 35, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Chen, B.; Yan, X.; Lin, Y.; Xiao, Z.; Hou, X.; Dai, J. Promotion of diabetic wound healing by collagen scaffold with collagen-binding vascular endothelial growth factor in a diabetic rat model. J. Tissue Eng. Regen. Med. 2014, 8, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Elbialy, Z.I.; Atiba, A.; Abdelnaby, A.; Al-Hawary, I.I.; Elsheshtawy, A.; El-Serehy, H.A.; Abdel-Daim, M.M.; Fadl, S.E.; Assar, D.H. Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and α-SMA genes expression. BMC Vet. Res. 2020, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Ben Amar, M.; Wu, M. Re-epithelialization: Advancing epithelium frontier during wound healing. J. R. Soc. Interface 2014, 11, 20131038. [Google Scholar] [CrossRef]
- Dang, Q.F.; Liu, H.; Yan, J.Q.; Liu, C.S.; Liu, Y.; Li, J.; Li, J.J. Characterization of collagen from haddock skin and wound healing properties of its hydrolysates. Biomed. Mater. 2015, 10, 015022. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Rudenko, T.G.; Istranov, L.P.; Guller, A.E.; Borodulin, R.R.; Vanin, A.F. Dinitrosyl iron complexes with glutathione incorporated into a collagen matrix as a base for the design of drugs accelerating skin wound healing. Eur. J. Pharm. Sci. 2015, 78, 8–18. [Google Scholar] [CrossRef]
- Ouyang, Q.Q.; Hu, Z.; Lin, Z.P.; Quan, W.Y.; Deng, Y.F.; Li, S.D.; Li, P.W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Lesperance, M.M.; Francis, T.L.; Norton, B. Postsurgical Soft Tissue Healing. In Postsurgical Orthopedic Sports Rehabilitation; Elsevier Inc.: Amsterdam, The Netherlands, 2006; pp. 3–18. ISBN 9780323027021. [Google Scholar]
- Li, S.; Van Den Diepstraten, C.; D’Souza, S.J.; Chan, B.M.C.; Pickering, J.G. Vascular smooth muscle cells orchestrate the assembly of type I collagen via α2β1 integrin, RhoA, and fibronectin polymerization. Am. J. Pathol. 2003, 163, 1045–1056. [Google Scholar] [CrossRef]
- Schultz, G.S.; Davidson, J.M.; Kirsner, R.S.; Bornstein, P.; Herman, I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011, 19, 134–148. [Google Scholar] [CrossRef]
- Toriseva, M.; Laato, M.; Carpén, O.; Ruohonen, S.T.; Savontaus, E.; Inada, M.; Krane, S.M.; Kähäri, V.-M. MMP-13 Regulates Growth of Wound Granulation Tissue and Modulates Gene Expression Signatures Involved in Inflammation, Proteolysis, and Cell Viability. PLoS ONE 2012, 7, e42596. [Google Scholar] [CrossRef]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A basic physiologic process in wound healing. Int. J. Low. Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Aravinthan, A.; Park, J.K.; Hossain, M.A.; Sharmila, J.; Kim, H.J.; Kang, C.W.; Kim, N.S.; Kim, J.H. Collagen-based sponge hastens wound healing via decrease of inflammatory cytokines. 3 Biotech 2018, 8, 487. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Belien, J.M.; Scheper, R.J.; Niessen, F.B.; Gibbs, S. Hypertrophic and keloid scars fail to progress from the CD34−/α-smooth muscle actin (α-SMA) + immature scar phenotype and show gradient differences in α-SMA and p16 expression. Br. J. Dermatol. 2020, 182, 974–986. [Google Scholar] [CrossRef]
- Slemp, A.E.; Kirschner, R.E. Keloids and scars: A review of keloids and scars, their pathogenesis, risk factors, and management. Curr. Opin. Pediatr. 2006, 18, 396–402. [Google Scholar] [CrossRef]
- Ng, C.P.; Hinz, B.; Swartz, M.A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci. 2005, 118, 4731–4739. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Wells, A.; Nuschke, A.; Yates, C.C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 2016, 49, 25–36. [Google Scholar] [CrossRef]
- Sharpe, J.R.; Martin, Y. Strategies Demonstrating Efficacy in Reducing Wound Contraction In Vivo. Adv. Wound Care 2013, 2, 167–175. [Google Scholar] [CrossRef]
- Greaves, N.S.; Iqbal, S.A.; Hodgkinson, T.; Morris, J.; Benatar, B.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen. 2015, 23, 483–494. [Google Scholar] [CrossRef]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- De Angelis, B.; Orlandi, F.; Fernandes Lopes Morais D’Autilio, M.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int. Wound J. 2018, 15, 695–706. [Google Scholar] [CrossRef]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar]
- Iacopetti, I.; Patruno, M.; Melotti, L.; Martinello, T.; Bedin, S.; Badon, T.; Righetto, E.M.; Perazzi, A. Autologous Platelet-Rich Plasma Enhances the Healing of Large Cutaneous Wounds in Dogs. Front. Vet. Sci. 2020, 7, 575449. [Google Scholar] [CrossRef]



| Antibody | Clonality | Host | Clone | Antigen Retrieval | Dilution | Catalog# |
|---|---|---|---|---|---|---|
| Ki67 | Monoclonal | Mouse | MIB-1 | HIER, sodium citrate 10 mM pH 6.0 | 1:50 | M7240 |
| α-SMA | Monoclonal | Mouse | 1A4 | - | 1:1000 | M0851 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melotti, L.; Martinello, T.; Perazzi, A.; Iacopetti, I.; Ferrario, C.; Sugni, M.; Sacchetto, R.; Patruno, M. A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep. Animals 2021, 11, 1219. https://doi.org/10.3390/ani11051219
Melotti L, Martinello T, Perazzi A, Iacopetti I, Ferrario C, Sugni M, Sacchetto R, Patruno M. A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep. Animals. 2021; 11(5):1219. https://doi.org/10.3390/ani11051219
Chicago/Turabian StyleMelotti, Luca, Tiziana Martinello, Anna Perazzi, Ilaria Iacopetti, Cinzia Ferrario, Michela Sugni, Roberta Sacchetto, and Marco Patruno. 2021. "A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep" Animals 11, no. 5: 1219. https://doi.org/10.3390/ani11051219
APA StyleMelotti, L., Martinello, T., Perazzi, A., Iacopetti, I., Ferrario, C., Sugni, M., Sacchetto, R., & Patruno, M. (2021). A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep. Animals, 11(5), 1219. https://doi.org/10.3390/ani11051219






