Immunological Responses and the Antioxidant Status in African Catfish (Clarias gariepinus) Following Replacement of Dietary Fish Meal with Plant Protein
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Fish
2.3. Experimental Diet and Design
2.4. Sampling and Analytical Methods
2.4.1. Blood Sample Collection
2.4.2. Leukogram, Serum Total Protein, and Electrophoretic Fraction Analysis
2.4.3. Serum Oxidant/Antioxidant Status
2.4.4. Nonspecific Immune Analysis
2.4.5. Expression of Pituitary Adenylate Cyclase-Activating Polypeptide Gene
2.5. Aeromonas sobria Challenge Test
2.6. Statistical Analysis
3. Results
3.1. Leukogram, Serum Total Protein, and Electrophoretic Fraction
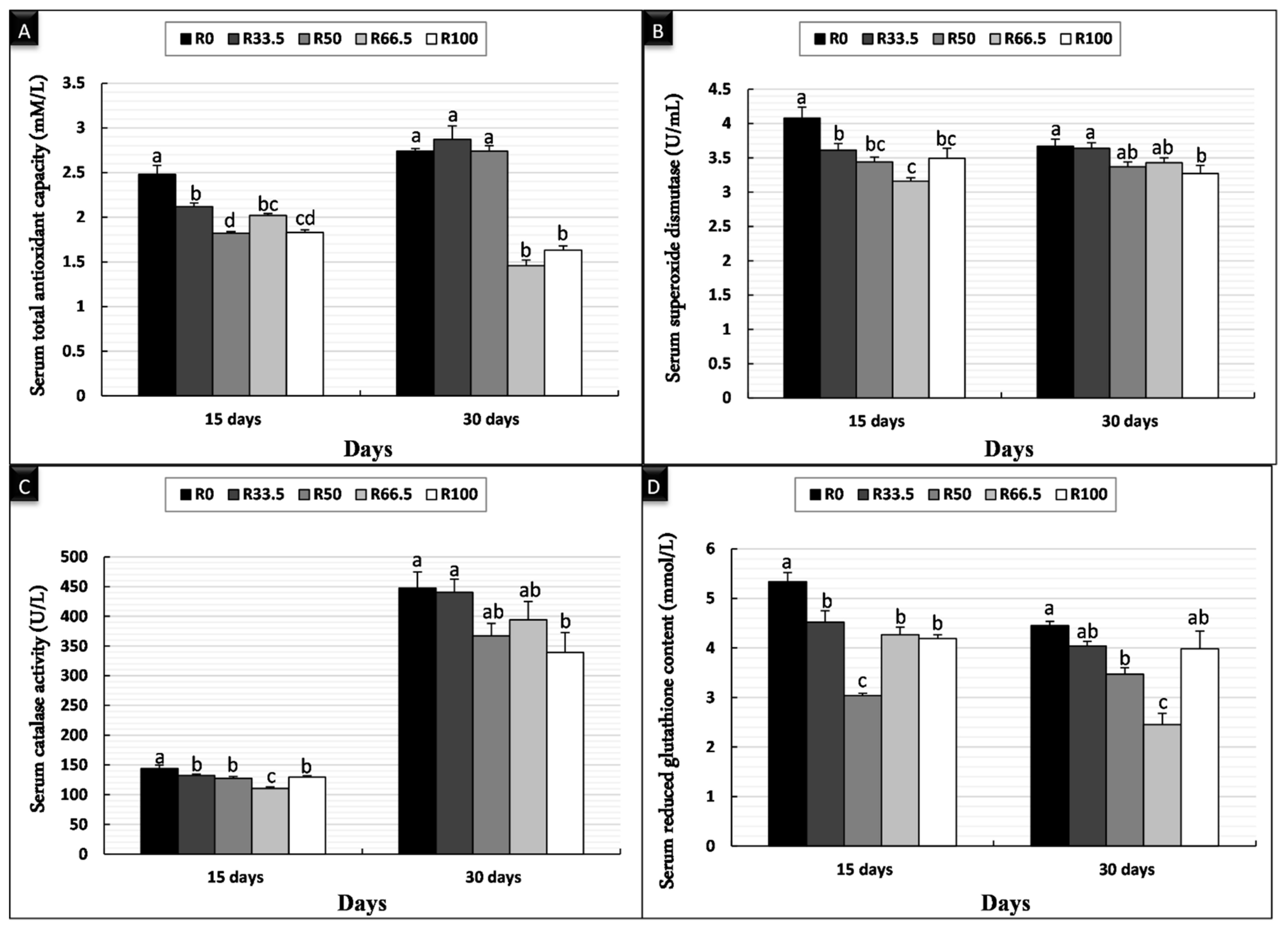
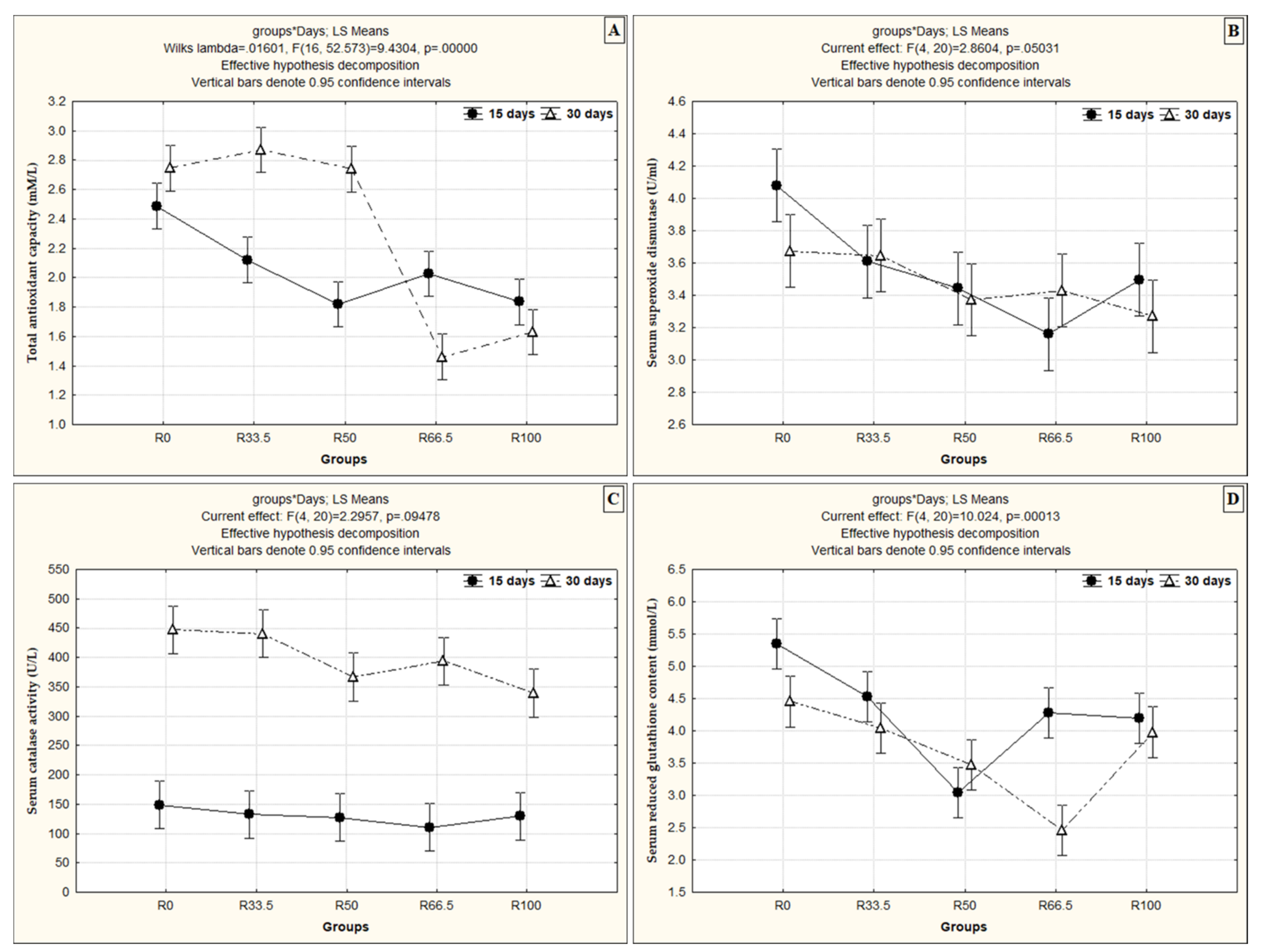
3.2. Serum Oxidant/Antioxidant Activity
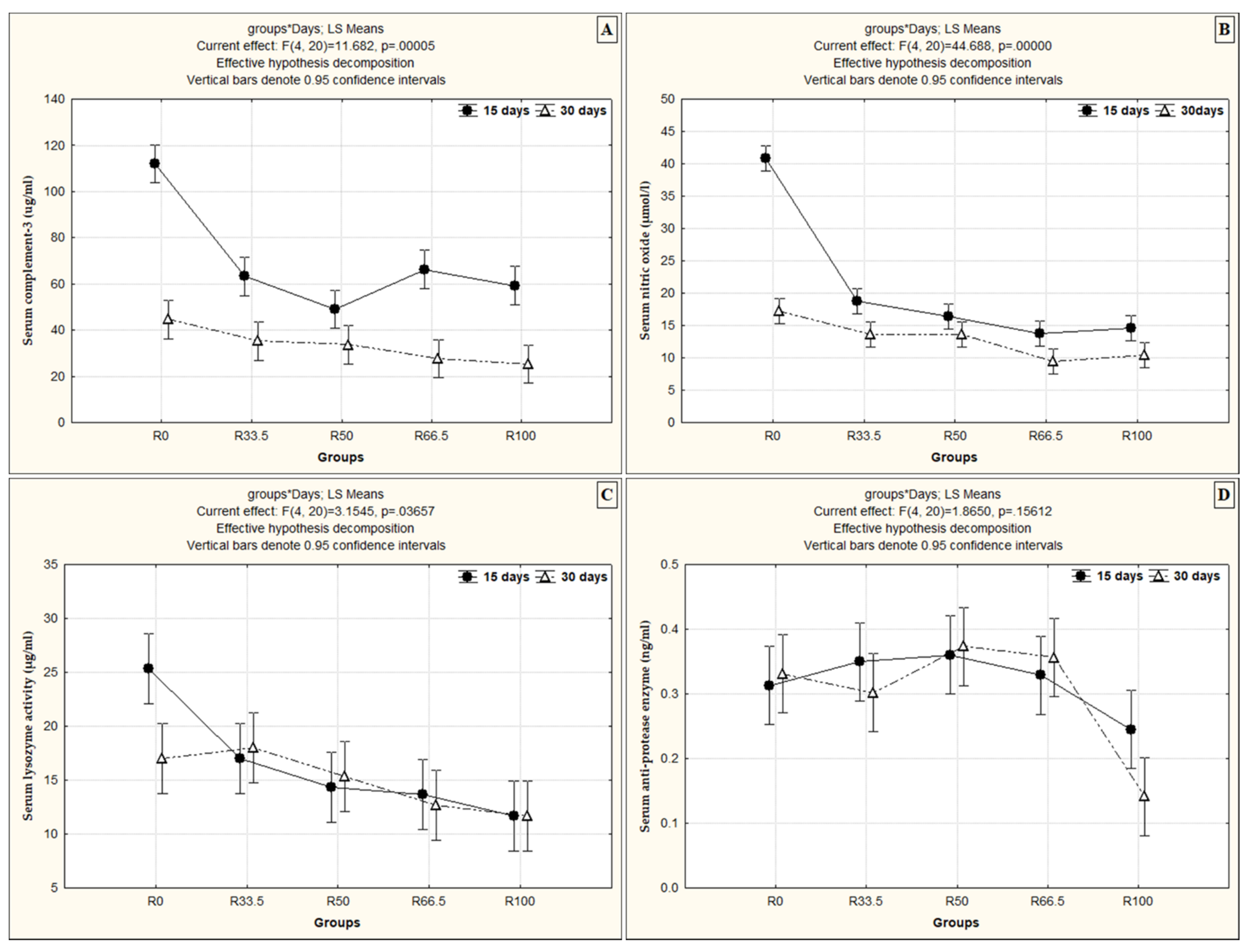
3.3. Nonspecific Immune Parameters
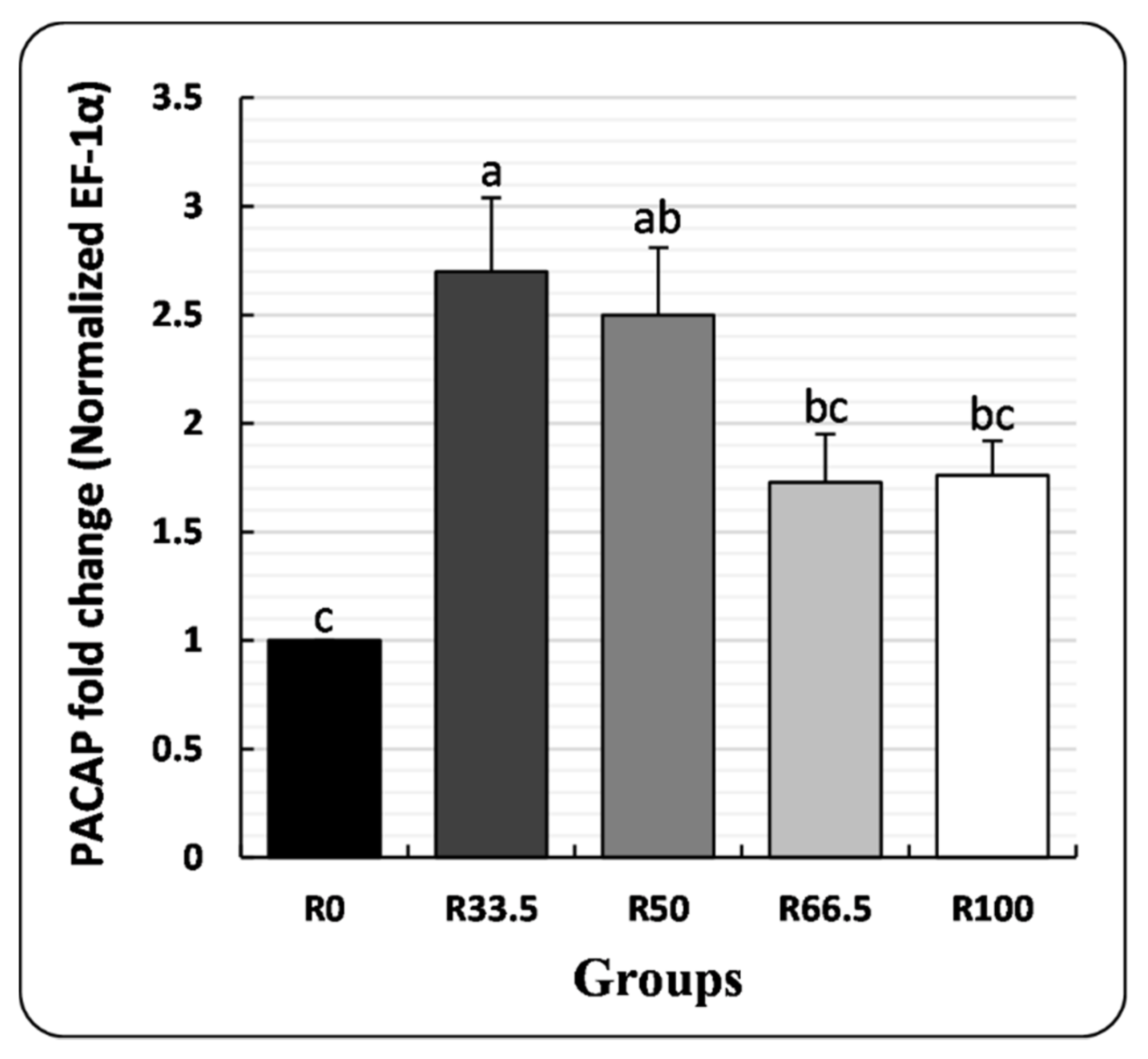
3.4. PACAP Gene Expression
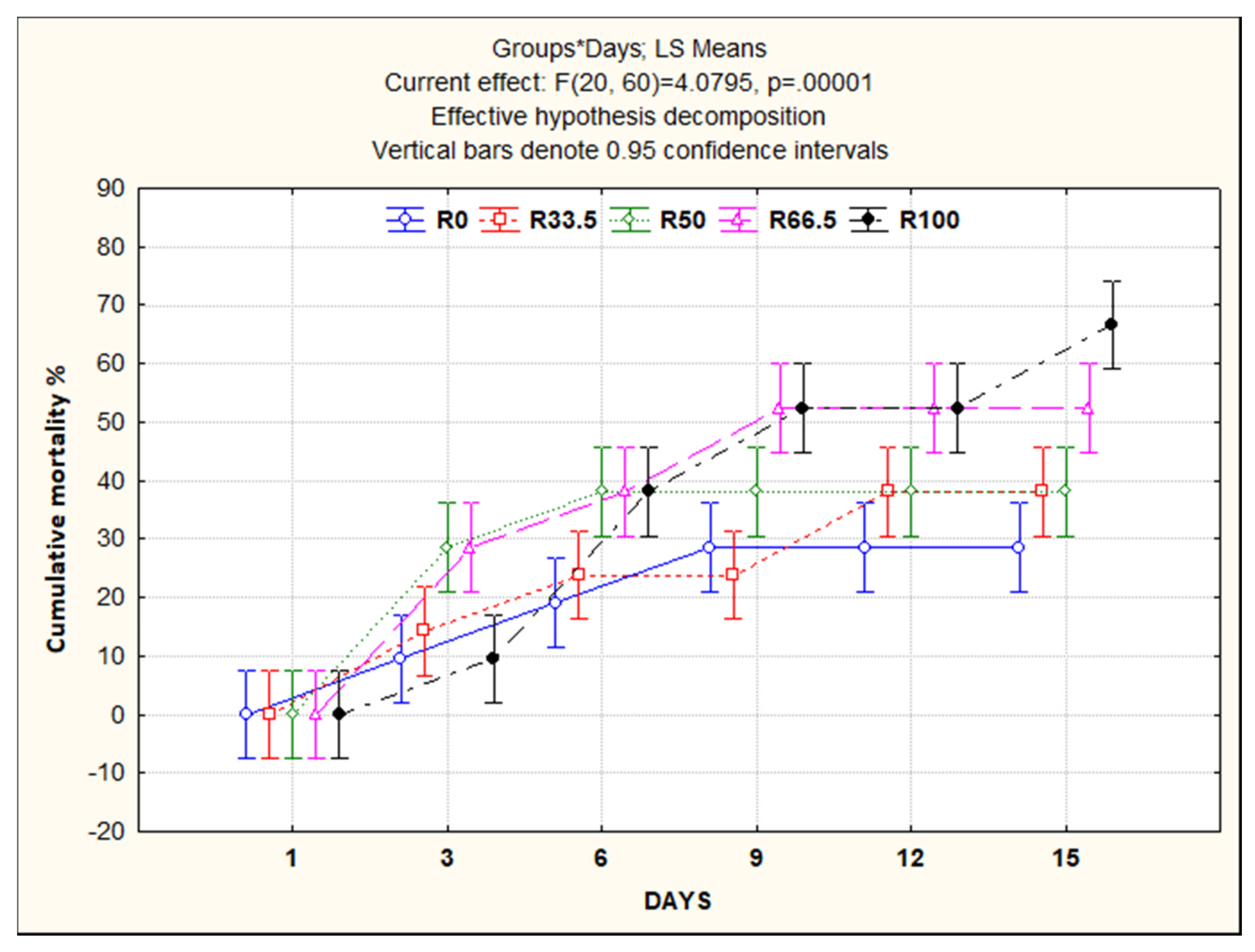
3.5. Resistance to Aeromonas sobria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tidwell, J.H.; Allan, G.L. Fish as food: Aquaculture’s contribution. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, R.L.; Hasan, M.R. A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Food Sci. Technol. 2012, 27, 120–128. [Google Scholar] [CrossRef]
- Artemenkov, D.V. Comparative characteristics of catfish growth of Silurus glanis and Clarias gariepinus species. Fish Beeding Fish. 2017, 2, 14–19. [Google Scholar]
- Belão, T.C.; Leite, C.A.C.; Florindo, L.H.; Kalinin, A.L.; Rantin, F.T. Cardiorespiratory responses to hypoxia in the African catfish, Clarias gariepinus (Burchell 1822), an air-breathing fish. J. Comp. Physiol. B. 2011, 181, 905–916. [Google Scholar] [CrossRef]
- Clay, D. Preliminary observations on salinity tolerance of Clarias lazera from Israel. Bamidgeh 1977, 29, 102–109. [Google Scholar]
- Baßmann, B.; Brenner, M.; Palm, H.W. Stress and Welfare of African Catfish (Clarias gariepinus Burchell, 1822) in a Coupled Aquaponic System. Water 2017, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Huisman, E.; Richter, C. Reproduction, growth, health control and aquacultural potential of the African catfish, Clarias gariepinus (Burchell 1822). Aquaculture 1987, 63, 1–14. [Google Scholar] [CrossRef]
- Rumsey, G.L. Fish Meal and Alternate Sources of Protein in Fish Feeds Update 1993. Fisheries 1993, 18, 14–19. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Bendiksen, E. Åsgard; Johnsen, C.A.; Olsen, H.J.; Jobling, M. Sustainable aquafeeds: Progress towards reduced reliance upon marine ingredients in diets for farmed Atlantic salmon (Salmo salar L.). Aquaculture 2011, 314, 132–139. [Google Scholar] [CrossRef]
- Hertrampf, J.W.; Piedad-Pascual, F. Handbook on Ingredients for Aquaculture Feeds; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2000; pp. 364–369. [Google Scholar]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Ketola, H.G. Effect of phosphorus in trout diets on water pollution. Salmonid 1982, 6, 12–15. [Google Scholar]
- Bergheim, A.; Sveier, H. Replacement of fish meal in salmonid diets by soya meal reduces phosphorus excretion. Aquac. Int. 1995, 3, 265–268. [Google Scholar] [CrossRef]
- Jahan, P.; Watanabe, T.; Kiron, V.; Satoh, S. Improved carp diets based on plant protein sources reduce environmental phosphorus loading. Fish. Sci. 2003, 69, 219–225. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Nasr, M.; Reda, R.; Ismail, T.; Moustafa, A. Growth, Hemato-Biochemical Parameters, Body Composition, and Myostatin Gene Expression of Clarias gariepinus Fed by Replacing Fishmeal with Plant Protein. Animals 2021, 11, 889. [Google Scholar] [CrossRef]
- Ogello, E.O.; Kembenya, E.M.; Githukia, C.M.; Aera, C.N.; Munguti, J.M.; Nyamweya, C.S. Substitution of fish meal with sunflower seed meal in diets for Nile tilapia (Oreochromis niloticus L.) reared in earthen ponds. J. Appl. Aquac. 2017, 29, 81–99. [Google Scholar] [CrossRef]
- Barros, M.M.; Lim, C.; Klesius, P.H. Effect of soybean meal replacement by cottonseed meal and iron supplementation on growth, immune response and resistance of Channel Catfish (Ictalurus puctatus) to Edwardsiella ictaluri challenge. Aquaculture 2002, 207, 263–279. [Google Scholar] [CrossRef]
- Daiyong, W.; Yuantu, Y.; Baotong, Z. Effects of cotton seed meal and rapeseed meal on growth performance, non-specific immune indexes and body compositions of Litopenaeus vannamei. China Feed 2009, 23, 12. [Google Scholar]
- Guo, Y.-X.; Dong, X.-H.; Tan, B.-P.; Chi, S.-Y.; Yang, Q.-H.; Chen, G.; Zhang, L. Partial replacement of soybean meal by sesame meal in diets of juvenile Nile tilapia, Oreochromis niloticus L. Aquac. Res. 2011, 42, 1298–1307. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Kasper, C.S.; Kohler, C.C. Challenges and Opportunities in Finfish Nutrition. N. Am. J. Aquac. 2006, 68, 122–140. [Google Scholar] [CrossRef]
- Zhou, Q.-C.; Mai, K.-S.; Tan, B.-P.; Liu, Y.-J. Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentron canadum). Aquac. Nutr. 2005, 11, 175–182. [Google Scholar] [CrossRef]
- Watanabe, T. Strategies for further development of aquatic feeds. Fish. Sci. 2002, 68, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Peres, H.; Lim, C.; Klesius, P.H. Nutritional value of heat-treated soybean meal for channel catfish (Ictalurus punctatus). Aquaculture 2003, 225, 67–82. [Google Scholar] [CrossRef]
- Choi, D.G.; He, M.; Fang, H.; Wang, X.L.; Li, X.Q.; Leng, X.J. Replacement of fish meal with two fermented soybean meals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2020, 26, 37–46. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Poolsawat, L.; Guo, Z.; Yao, W.; Zhang, C.; Leng, X. Effects of fish meal replaced by fermented soybean meal on growth performance, intestinal histology and microbiota of largemouth bass (Micropterus salmoides). Aquac. Nutr. 2020, 26, 1058–1071. [Google Scholar] [CrossRef]
- Alam, S.; Watanabe, W.O.; Myers, A.R.; Rezek, T.C.; Carroll, P.M.; Longfellow, S. Effects of Replacement of Menhaden Fish Meal Protein by Solvent-Extracted Soybean Meal Protein Supplemented with or without l-Methionine and l-Lysine in the Diet of Juvenile Southern Flounder. N. Am. J. Aquac. 2011, 73, 350–359. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.; Davis, D.A. Evaluation of differently processed soybean meal products as ingredients in practical diets for Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2020, 26, 287–295. [Google Scholar] [CrossRef]
- González-Pérez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Shchekoldina, T.; Aider, M. Production of low chlorogenic and caffeic acid containing sunflower meal protein isolate and its use in functional wheat bread making. J. Food Sci. Technol. 2012, 51, 2331–2343. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.B.; Molnár, T.; Ardó, L.; Jeney, G.; Hancz, C. Oral administration of Basella alba leaf methanol extract and genistein enhances the growth and non-specific immune responses of Oreochromis niloticus. Turk. J. Fish. Aquat. Sci. 2015, 15, 167–173. [Google Scholar] [CrossRef]
- Espe, M.; Lemme, A.; Petri, A.; El-Mowafi, A. Assessment of lysine requirement for maximal protein accretion in Atlantic salmon using plant protein diets. Aquac. 2007, 263, 168–178. [Google Scholar] [CrossRef]
- Burr, G.; Barrows, F.; Gaylord, G.; Wolters, W. Apparent digestibility of macro-nutrients and phosphorus in plant-derived ingredients for Atlantic salmon, Salmo salar and Arctic charr, Salvelinus alpinus. Aquac. Nutr. 2011, 17, 570–577. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Ray, A.K. Effects of amino acid supplementation on the nutritive quality of fermented linseed meal protein in the diets for rohu, Labeo rohita, fingerlings. J. Appl. Ichthyol. 2001, 17, 220–226. [Google Scholar] [CrossRef]
- Takagi, S.; Shimeno, S.; Hosokawa, H.; Ukawa, M. Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream Pagrus major. Fish. Sci. 2001, 67, 1088–1096. [Google Scholar] [CrossRef]
- Langar, H.; Guillaume, J.; Métailler, R.; Fauconneau, B. Augmentation of Protein Synthesis and Degradation by Poor Dietary Amino Acid Balance in European Sea Bass (Dicentrarchus labrax). J. Nutr. 1993, 123, 1754–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Geng, Y.; Wang, K.; Chen, D.; Huang, X.L.; Ou, Y.; Lai, W.; Zhong, Z.J.; He, C.L. Aeromonas veronii Infection in Cultured Channel Catfish, Ictalurus punctatus, in Southwest China. Isr. J. Aquac. Bamidgeh 2016, 68, 20839. [Google Scholar] [CrossRef]
- Dong, H.T.; Techatanakitarnan, C.; Jindakittikul, P.; Thaiprayoon, A.; Taengphu, S.; Charoensapsri, W.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Aeromonas jandaei and Aeromonas veronii caused disease and mortality in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2017, 40, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, S.-H. Identification and Pathogenicity of Aeromonas sobria on Tail-rot Disease in Juvenile Tilapia Oreochromis niloticus. Curr. Microbiol. 2011, 62, 623–627. [Google Scholar] [CrossRef]
- Ashida, T.; Okimasu, E. Immunostimulatory effects of fermented vegetable product on the non-specific immunity of Japanese flounder Paralichthys olivaceus. Fish. Sci. 2005, 71, 257–262. [Google Scholar] [CrossRef]
- Azeredo, R.; Machado, M.; Kreuz, E.; Wuertz, S.; Oliva-Teles, A.; Enes, P.; Costas, B. The European seabass (Dicentrarchus labrax) innate immunity and gut health are modulated by dietary plant-protein inclusion and prebiotic supplementation. Fish Shellfish. Immunol. 2017, 60, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lilleeng, E.; Penn, M.H.; Haugland, Ø.; Xu, C.; Bakke, A.M.; Krogdahl, Å.; Landsverk, T.; Frøystad-Saugen, M.K. Decreased expression of TGF-β, GILT and T-cell markers in the early stages of soybean enteropathy in Atlantic salmon (Salmo salar L.). Fish Shellfish. Immunol. 2009, 27, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bransden, M.P.; Carter, C.G.; Nowak, B.F. Effects of dietary protein source on growth, immune function, blood chemistry and disease resistance of Atlantic salmon (Salmo salar L.) parr. Anim. Sci. 2001, 73, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Li, P.-Y.; Wang, J.-Y.; Song, Z.-D.; Zhang, L.-M.; Zhang, H.; Li, X.-X.; Pan, Q. Evaluation of soy protein concentrate as a substitute for fishmeal in diets for juvenile starry flounder (Platichthys stellatus). Aquaculture 2015, 448, 578–585. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Hepher, B.; Liao, I.; Cheng, S.; Hsieh, C. Food utilization by red tilapia—Effects of diet composition, feeding level and temperature on utilization efficiencies for maintenance and growth. Aquaculture 1983, 32, 255–275. [Google Scholar] [CrossRef]
- AOAC. 15th edn. Association of Official Analysis of Chemist, Washington. 2000. Available online: http://webpages.icav.up.pt/PTDC/CVT-NUT/4294/2012/AOAC%202000.pdf (accessed on 1 January 2021).
- Llames, C.R.; Fontaine, J. Determination of Amino Acids in Feeds: Collaborative Study. J. AOAC Int. 1994, 77, 1362–1402. [Google Scholar] [CrossRef]
- Neiffer, D.L.; Stamper, M.A. Fish Sedation, Anesthesia, Analgesia, and Euthanasia: Considerations, Methods, and Types of Drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Thomas, L. Clinical Laboratory Diagnostics: Use and Assessment of Clinical Laboratory Results, 1st ed.; TH-Books Verlagsgesellschaft: Frankfurt/Main, Germany, 1998; ISBN 3-9805215-4-0. [Google Scholar]
- Rajaraman, V.; Nonnecke, B.; Franklin, S.; Hammell, D.; Horst, R. Effect of Vitamins A and E on Nitric Oxide Production by Blood Mononuclear Leukocytes from Neonatal Calves Fed Milk Replacer. J. Dairy Sci. 1998, 81, 3278–3285. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Fletcher, T.C., Anderson, D.P., Roberson, B.S., Van Muiswinkel, W.B., Eds.; SOS Publications: Fair Haven, CT, USA, 1990; pp. 101–103. [Google Scholar]
- Bowden, T.; Butler, R.; Bricknell, I.; Ellis, A. Serum trypsin-inhibitory activity in five species of farmed fish. Fish Shellfish. Immunol. 1997, 7, 377–385. [Google Scholar] [CrossRef]
- Gröner, F.; Ziková, A.; Kloas, W. Effects of the pharmaceuticals diclofenac and metoprolol on gene expression levels of enzymes of biotransformation, excretion pathways and estrogenicity in primary hepatocytes of Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2015, 167, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.M.; Carpio, Y.; Oliva, A.; Morales, A.; Estrada, M.P. Pituitary adenylate cyclase-activating polypeptide (PACAP): A regulator of the innate and acquired immune functions in juvenile fish. Fish Shellfish. Immunol. 2010, 29, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M.; Honda, Y.; Mamauag, R.E.; Laining, A. Growth, nutrient utilization, oxidative condition, and element composition of juvenile red sea bream Pagrus major fed with fermented soybean meal and scallop by-product blend as fishmeal replacement. Fish. Sci. 2010, 77, 119–128. [Google Scholar] [CrossRef]
- Pickering, A.D. Stress responses of farmed fish. In Biology of Farmed Fish; Black, K.D., Pickering, A.D., Eds.; Sheffield Academic Press: Sheffield, UK, 1998; pp. 222–255. [Google Scholar]
- Adeparusi, E.; Ajayi, A. Haematological characteristics of Nile Tilapia (Oreochromis niloticus) fed differently processed lima bean (Phaseolus lunatus L.) diets. J. Res. Sci. Manage. 2004, 1, 73–80. [Google Scholar]
- Akinwande, A.A.; Dada, A.A.; Sogbesan, O.A.; Umar, I.O. Haematological response of Heterobranchus longifilis fed varying dietary protein levels. Afr. J. Gen. Agric. 2016, 2, 17–21. [Google Scholar]
- Bello-Olusoji, O.A.; Omoare, V.Y.; Nwana, L.C. Comparative studies on the haematological characteristics of pond cultured and wild tilapia (Oreochromis niloticus) Linnaeus, 1857. Niger. J. For. 2006, 36, 134–141. [Google Scholar]
- Jalili, R.; Tukmechi, A.; Agh, N.; Noori, F.; Ghasemi, A. Replacement of dietary fish meal with plant sources in rainbow trout (Oncorhynchus mykiss); effect on growth performance, immune responses, blood indices and disease resistance. Iran. J. Fish. Sci. 2013, 12, 577–591. [Google Scholar]
- Jimoh, W.; Fagbenro, O.; Adeparusi, E. Haematological Profile of Blood of African Catfish (Clarias gariepinus, Burchell, 1822) Fed Sunflower and Sesame Meal Based Diets. J. Fish. Aquat. Sci. 2013, 8, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.-R.; Zhou, Q.-C. Effect of replacing soybean meal with cottonseed meal on growth, feed utilization, and hematological indexes for juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Aquaculture 2008, 284, 185–189. [Google Scholar] [CrossRef]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Johnson, P. Antioxidant enzyme expression in health and disease: Effects of exercise and hypertension. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 493–505. [Google Scholar] [CrossRef]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Kentouri, M.; Alexis, M.; Rigos, G. Effects of graded dietary levels of soy protein concentrate supplemented with methionine and phosphate on the immune and antioxidant responses of gilthead sea bream (Sparus aurata L.). Fish Shellfish. Immunol. 2017, 64, 111–121. [Google Scholar] [CrossRef]
- Xu, Q.-Y.; Li, C.; Yang, P.; Xu, H.; Wang, C.-A. Effects of partial replacement of fishmeal with soy protein isolated and meat bone meal on growth and non-specific immunity in rainbow trout. J. Dalian Fish. Univ. 2008, 23, 8–12. [Google Scholar]
- Bu, X.; Chen, A.; Lian, X.; Chen, F.; Zhang, Y.; Muhammad, I.; Ge, X.; Yang, Y. An evaluation of replacing fish meal with cottonseed meal in the diet of juvenile Ussuri catfish Pseudobagrus ussuriensis: Growth, antioxidant capacity, nonspecific immunity and resistance to Aeromonas hydrophila. Aquaculture 2017, 479, 829–837. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; He, R.; Xu, W.; Mai, K.; He, G. Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 2016, 464, 87–94. [Google Scholar] [CrossRef]
- Xie, S.-W.; Liu, Y.-J.; Zeng, S.; Niu, J.; Tian, L.-X. Partial replacement of fish-meal by soy protein concentrate and soybean meal based protein blend for juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2016, 464, 296–302. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Mui, J.-J. Comparison of dietary inclusion of commercial and fermented soybean meal on oxidative status and non-specific immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2017, 63, 208–212. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Rigos, G.; Henry, M.; Alexis, M.; Kentouri, M. Effects of Fish Meal Replacement by a Soybean Protein on Growth, Histology, Selected Immune and Oxidative Status Markers of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2015, 46, 115–128. [Google Scholar] [CrossRef]
- Kader, A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M.; Nguyen, B.T.; Gao, J.; Laining, A. Can fermented soybean meal and squid by-product blend be used as fishmeal replacements for Japanese flounder (Paralichthys olivaceus)? Aquac. Res. 2011, 43, 1427–1438. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Ye, J.; Du, Z.; Kong, Y. An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: Growth, nonspecific immunity, and resistance to Aeromonas hydrophila. Fish Shellfish. Immunol. 2015, 44, 295–301. [Google Scholar] [CrossRef]
- Mokrani, A.; Ren, M.; Liang, H.; Yang, Q.; Ji, K.; Kasiya, H.C.; Ge, X. Effect of the total replacement of fishmeal with plant proteins and supplemental essential amino acids in the extruded diet on antioxidants genes, enzyme activities, and immune response in juvenile blunt snout bream. Aquac. Int. 2020, 28, 555–568. [Google Scholar] [CrossRef]
- Guo, Q.; Rimbach, G.; Moini, H.; Weber, S.; Packer, L. ESR and cell culture studies on free radical-scavenging and antioxidant activities of isoflavonoids. Toxicol. 2002, 179, 171–180. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Anderson, D.P. Immunostimulants, adjuvants, and vaccine carriers in fish: Applications to aquaculture. Annu. Rev. Fish Dis. 1992, 2, 281–307. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J. Recent advances on the complement system of teleost fish. Fish Shellfish. Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, C.; Figueras, A.; Novoa, B. Role of nitric oxide on the replication of viral haemorrhagic septicemia virus (VHSV), a fish rhabdovirus. Vet. Immunol. Immunopathol. 1999, 72, 249–256. [Google Scholar] [CrossRef]
- Bogdan, C.; Rollinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Jollès, P.; Jollès, J. What’s new in lysozyme research? Mol. Cell. Biochem. 1984, 63, 165–189. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.D.; Urbinati, E.C. Fish Immunology. The modification and manipulation of the innate immune system: Brazilian studies. An. Acad. Bras. Ciências 2014, 86, 1484–1506. [Google Scholar] [CrossRef] [Green Version]
- Hüner, G.F. Çocuklarda Beslenme ve Infeksiyon Iliskisi. Ankem Dergisi 2004, 18, 26–31. (In Turkish) [Google Scholar]
- Karacabey, K.; Ozdemir, N. The Effect of Nutritional Elements on the Immune System. J. Obes. Weight. Loss Ther. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Jobling, M. Fish nutrition research: Past, present and future. Aquac. Int. 2016, 24, 767–786. [Google Scholar] [CrossRef]
- Martin, S.A.; Król, E. Nutrigenomics and immune function in fish: New insights from omics technologies. Dev. Comp. Immunol. 2017, 75, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Corman, L.C. The Relationship Between Nutrition, Infection, and Immunity. Med Clin. N. Am. 1985, 69, 519–531. [Google Scholar] [CrossRef]
- Abasubong, K.P.; Liu, W.-B.; Zhang, D.-D.; Yuan, X.-Y.; Xia, S.-L.; Xu, C.; Li, X.-F. Fishmeal replacement by rice protein concentrate with xylooligosaccharides supplement benefits the growth performance, antioxidant capability and immune responses against Aeromonas hydrophila in blunt snout bream (Megalobrama amblycephala). Fish Shellfish. Immunol. 2018, 78, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Burrells, C.; Williams, P.; Southgate, P.; Crampton, V. Immunological, physiological and pathological responses of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Vet. Immunol. Immunopathol. 1999, 72, 277–288. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.; Roem, A. Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture 1998, 161, 365–379. [Google Scholar] [CrossRef]
- Arimura, A. Perspectives on Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) in the Neuroendocrine, Endocrine, and Nervous Systems. Jpn. J. Physiol. 1998, 48, 301–331. [Google Scholar] [CrossRef] [Green Version]
- Carpio, Y.; Lugo, J.M.; Leon, K.; Morales, R.; Estrada, M.P. Novel function of recombinant pituitary adenylate cyclase-activating polypeptide as stimulator of innate immunity in African catfish (Clarias gariepinus) fry. Fish Shellfish. Immunol. 2008, 25, 439–445. [Google Scholar] [CrossRef]
- Selim, K.M.; El-Sayed, H.M.; El-Hady, M.A.; Reda, R.M. In vitro evaluation of the probiotic candidates isolated from the gut of Clarias gariepinus with special reference to the in vivo assessment of live and heat-inactivated Leuconostoc mesenteroides and Edwardsiella sp. Aquac. Int. 2019, 27, 33–51. [Google Scholar] [CrossRef]
- Marjara, I.S.; Chikwati, E.M.; Valen, E.C.; Krogdahl, Å.; Bakke, A.M. Transcriptional regulation of IL-17A and other inflammatory markers during the development of soybean meal-induced enteropathy in the distal intestine of Atlantic salmon (Salmo salar L.). Cytokine 2012, 60, 186–196. [Google Scholar] [CrossRef]
- Sahlmann, C.; Sutherland, B.J.; Kortner, T.M.; Koop, B.F.; Krogdahl, Å.; Bakke, A.M. Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis. Fish Shellfish. Immunol. 2013, 34, 599–609. [Google Scholar] [CrossRef]
- Hedrera, M.I.; Galdames, J.A.; Jimenez-Reyes, M.F.; Reyes, A.E.; Avendaño-Herrera, R.; Romero, J.; Feijóo, C.G. Soybean Meal Induces Intestinal Inflammation in Zebrafish Larvae. PLoS ONE 2013, 8, e69983. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.; Yildirim-Aksoy, M.; Li, M.H.; Welker, T.L.; Klesius, P.H. Influence of dietary levels of lipid and vitamin E on growth and resistance of Nile tilapia to Streptococcus iniae challenge. Aquaculture 2009, 298, 76–82. [Google Scholar] [CrossRef]
- Abou-El-Atta, M.E.; Abdel-Tawwab, M.; Abdel-Razek, N.; Abdelhakim, T.M.N. Effects of dietary probiotic Lactobacillus plantarum and whey protein concentrate on the productive parameters, immunity response and susceptibility of Nile tilapia, Oreochromis niloticus (L.), to Aeromonas sobria infection. Aquac. Nutr. 2019, 25, 1367–1377. [Google Scholar] [CrossRef]
- Reda, R.M.; Mahmoud, R.; Selim, K.M.; El-Araby, I.E. Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2016, 50, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, G.A.; Ross, A.J. Nutritional Factors in the Biochemical Pathology of Corynebacterial Kidney Disease in the Coho Salmon (Oncorhynchus kisutch). J. Fish. Res. Board Can. 1973, 30, 296–298. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Ingebrigtsen, K.; Bogwald, J. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish Dis. 1997, 20, 241–273. [Google Scholar] [CrossRef]


| Ingredient | Experimental Diets (g/kg) | ||||
|---|---|---|---|---|---|
| R0 | R33.5 | R50 | R66.5 | R100 | |
| Fishmeal 60% | 150 | 100 | 75 | 50 | 0 |
| Soybean meal 48% | 411 | 453 | 475 | 509 | 500 |
| Sunflower meal 36% | 0 | 50 | 71 | 81 | 100 |
| Wheat bran 14.5% | 128 | 68 | 38 | 0 | 0 |
| Ground yellow corn | 286 | 303 | 311 | 323 | 303 |
| Corn gluten 60% | 0 | 0 | 0 | 0 | 50 |
| Fish oil | 20.0 | 20.7 | 22.4 | 24.5 | 25.0 |
| Dicalcium phosphate | 0 | 0 | 2 | 06.6 | 15.0 |
| Vitamin mineral premix * | 3 | 3 | 3 | 3 | 3 |
| Dl-methionine | 2.0 | 2.3 | 2.3 | 2.5 | 2.7 |
| L-Lysine | 0 | 0 | 0.3 | 0.4 | 1.3 |
| Calculated composition (%Dry matter) ** | |||||
| Crude protein | 32.20 | 31.90 | 32.20 | 32.10 | 32.20 |
| Crude fiber | 3.75 | 3.95 | 3.98 | 4.05 | 4.12 |
| Starch | 21.10 | 21.00 | 20.80 | 21.00 | 20.90 |
| Ether extract | 5.35 | 5.11 | 5.13 | 5.14 | 5.12 |
| Lysine | 1.89 | 1.88 | 1.86 | 1.89 | 1.87 |
| Methionine | 0.80 | 0.80 | 0.79 | 0.78 | 0.79 |
| Cysteine | 0.42 | 0.41 | 0.43 | 0.43 | 0.42 |
| Threonine | 1.23 | 1.24 | 1.24 | 1.25 | 1.26 |
| Arginine | 2.26 | 2.29 | 2.30 | 2.30 | 2.28 |
| Histidine | 0.74 | 0.78 | 0.80 | 0.82 | 0.85 |
| Isoleucine | 1.33 | 1.40 | 1.43 | 1.45 | 1.49 |
| Leucine | 2.43 | 2.52 | 2.56 | 2.61 | 2.96 |
| Phenylalanine | 1.44 | 1.55 | 1.59 | 1.64 | 1.77 |
| Tyrosine | 1.09 | 1.10 | 1.11 | 1.13 | 1.21 |
| Tryptophan | 0.39 | 0.42 | 0.43 | 0.44 | 0.45 |
| Valine | 1.60 | 1.69 | 1.73 | 1.76 | 1.82 |
| Calcium | 0.99 | 0.77 | 0.69 | 0.68 | 0.63 |
| Available P | 0.53 | 0.39 | 0.35 | 0.35 | 0.35 |
| DE (kcal/kg diet) *** | 2660 | 2656 | 2658 | 2659 | 2662 |
| Parameters | R0 | R33.5 | R50 | R66.5 | R100 | p-Value |
|---|---|---|---|---|---|---|
| WBCs (103/µL) | 0.81 ± 0.034 | 0.78 ± 0.022 | 0.77 ± 0.056 | 0.75 ± 0.049 | 0.83 ± 0.060 | 0.749 |
| Neutrophil (103/µL) | 0.003 ± 0.002 b | 0.013 ± 0.005 a,b | 0.030 ± 0.007 a,b | 0.030 ± 0.010 a,b | 0.040 ± 0.014 a,b | 0.063 |
| Lymphocyte (103/µL) | 0.71 ± 0.07 | 0.71 ± 0.02 | 0.63 ± 0.05 | 0.66 ± 0.04 | 0.75 ± 0.04 | 0.466 |
| Monocyte (103/µL) | 0.063 ± 0.021 a | 0.016 ± 0.005 c,d | 0.053 ± 0.010 a,b | 0.033 ± 0.01 a,b,c | 0.010 ± 0.006 d | 0.021 |
| Eosinophil (103/µL) | 0.03 ± 0.015 | 0.02 ± 0.006 | 0.04 ± 0.010 | 0.02 ± 0.001 | 0.03 ± 0.015 | 0.718 |
| Total protein (g/dL) | 6.57 ± 0.09 a | 5.73 ± 0.06 b | 6.74 ± 0.10 a | 5.62 ± 0.06 b | 5.55 ± 0.03 b | <0.001 |
| Albumin (g/dL) | 3.80 ± 0.05 c | 3.42 ± 0.09 d | 4.49 ± 0.03 b | 4.41 ± 0.05 b | 4.85 ± 0.05 a | <0.001 |
| Total globulin (g/dL) | 2.77 ± 0.15 a | 2.31 ± 0.16 b | 2.25 ± 0.09 b | 1.21 ± 0.06 c | 0.70 ± 0.15 d | <0.001 |
| α1-globulin (g/dL) | 0.49 ± 0.020 a | 0.48 ± 0.060 a | 0.50 ± 0.015 a | 0.25 ± 0.015 b | 0.16 ± 0.020 b | <0.001 |
| α2-globulin (g/dL) | 0.70 ± 0.02 a | 0.70 ± 0.06 a | 0.73 ± 0.06 a | 0.33 ± 0.01 b | 0.15 ± 0.02 c | <0.001 |
| ß-globulin (g/dL) | 0.74 ± 0.04 a | 0.65 ± 0.02 a | 0.52 ± 0.06 b | 0.31 ± 0.08 c | 0.20 ± 0.03 c | <0.001 |
| γ-globulin (g/dL) | 0.82 ± 0.09 a | 0.46 ± 0.04 b | 0.49 ± 0.03 b | 0.3 1 ± 0.06 b,c | 0.18 ± 0.04 c | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reda, R.M.; Nasr, M.A.F.; Ismail, T.A.; Moustafa, A. Immunological Responses and the Antioxidant Status in African Catfish (Clarias gariepinus) Following Replacement of Dietary Fish Meal with Plant Protein. Animals 2021, 11, 1223. https://doi.org/10.3390/ani11051223
Reda RM, Nasr MAF, Ismail TA, Moustafa A. Immunological Responses and the Antioxidant Status in African Catfish (Clarias gariepinus) Following Replacement of Dietary Fish Meal with Plant Protein. Animals. 2021; 11(5):1223. https://doi.org/10.3390/ani11051223
Chicago/Turabian StyleReda, Rasha M., Mohammed A. F. Nasr, Tamer A. Ismail, and Amira Moustafa. 2021. "Immunological Responses and the Antioxidant Status in African Catfish (Clarias gariepinus) Following Replacement of Dietary Fish Meal with Plant Protein" Animals 11, no. 5: 1223. https://doi.org/10.3390/ani11051223
APA StyleReda, R. M., Nasr, M. A. F., Ismail, T. A., & Moustafa, A. (2021). Immunological Responses and the Antioxidant Status in African Catfish (Clarias gariepinus) Following Replacement of Dietary Fish Meal with Plant Protein. Animals, 11(5), 1223. https://doi.org/10.3390/ani11051223







