The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fish
2.3. Experimental Design
2.3.1. Experiment 1 (Acute Toxicity Study)
2.3.2. Experiment 2 (Subacute Toxicity Study)
2.4. Monitoring of Fish during the Experiment
2.5. Blood and Tissue Sampling
2.6. Evaluation of Hematological Indices
2.7. Immunological Assay and Protein Profile
2.8. Oxidative Stress Biomarkers in Serum and Acetyle Choline Esterase Enzyme (AchE)
2.9. Transcriptional Profile Changes of Immune-Related Genes—Tumor Necrosis Factor α (TNF-α), Interleukin-1β (IL-1β), Interleukin-10 (IL-10), and CC and CXC Chemokines
2.10. Challenge Test
2.11. Data Analysis
3. Results
3.1. Growth Performance
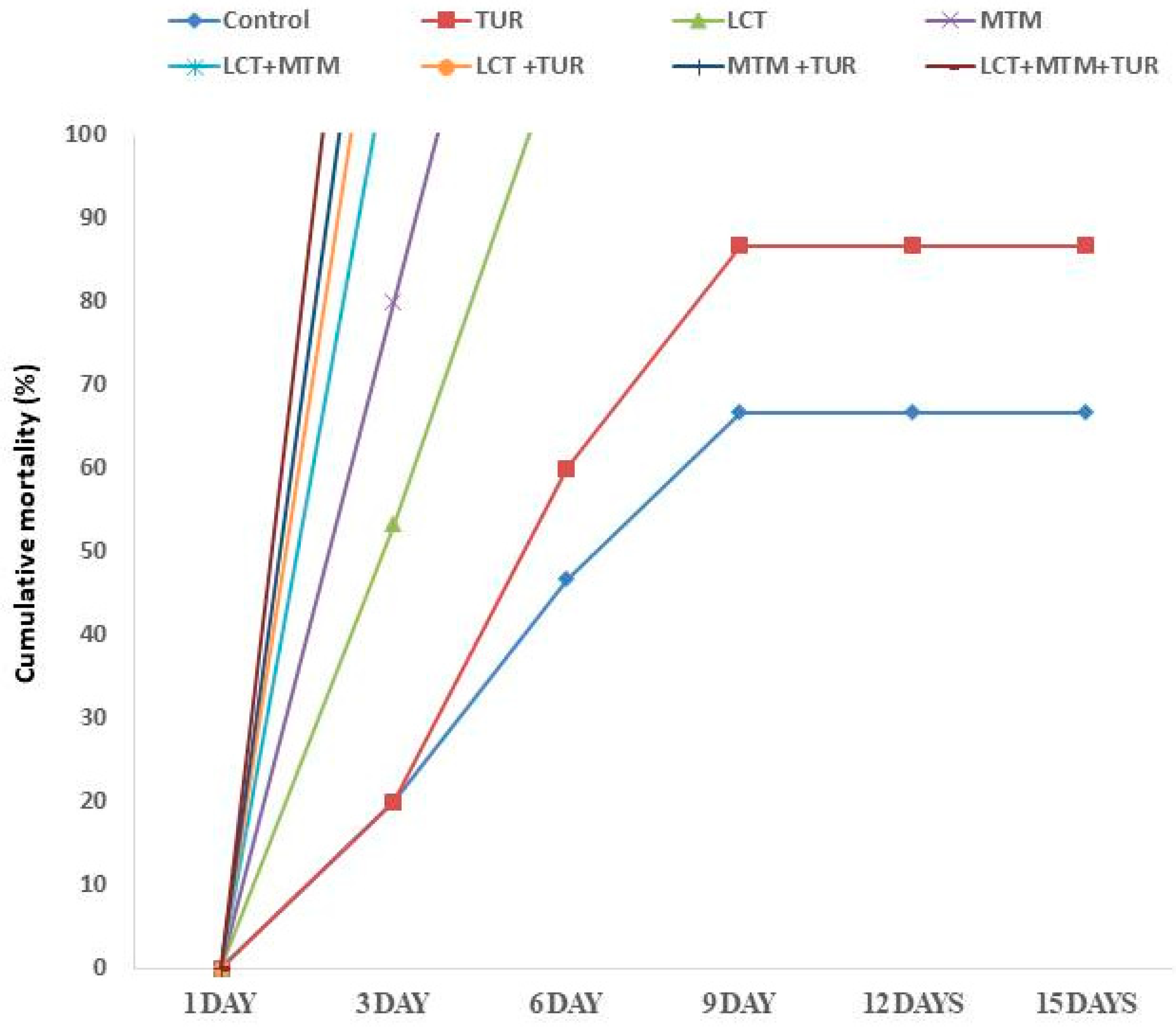
3.2. Mortality Rate and Clinical Observations
3.3. The Effects of Exposure to LCT and/or MTM and Their Combination on Hematological Variables
3.4. Effects on AchE and 8-OHdG
3.5. Immunological Response Indices and Protein Profile
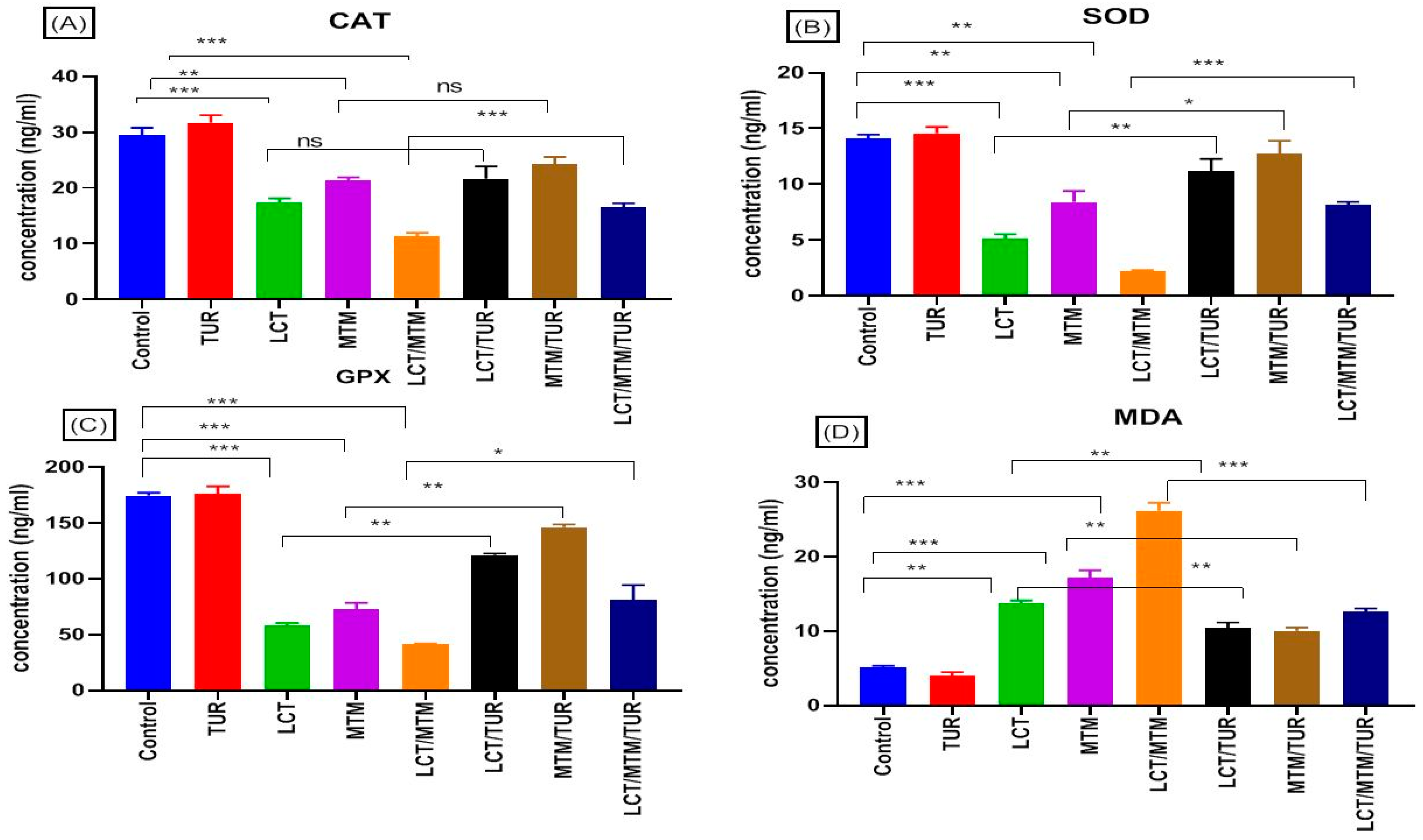
3.6. Properties of Oxidative Stress Indicators
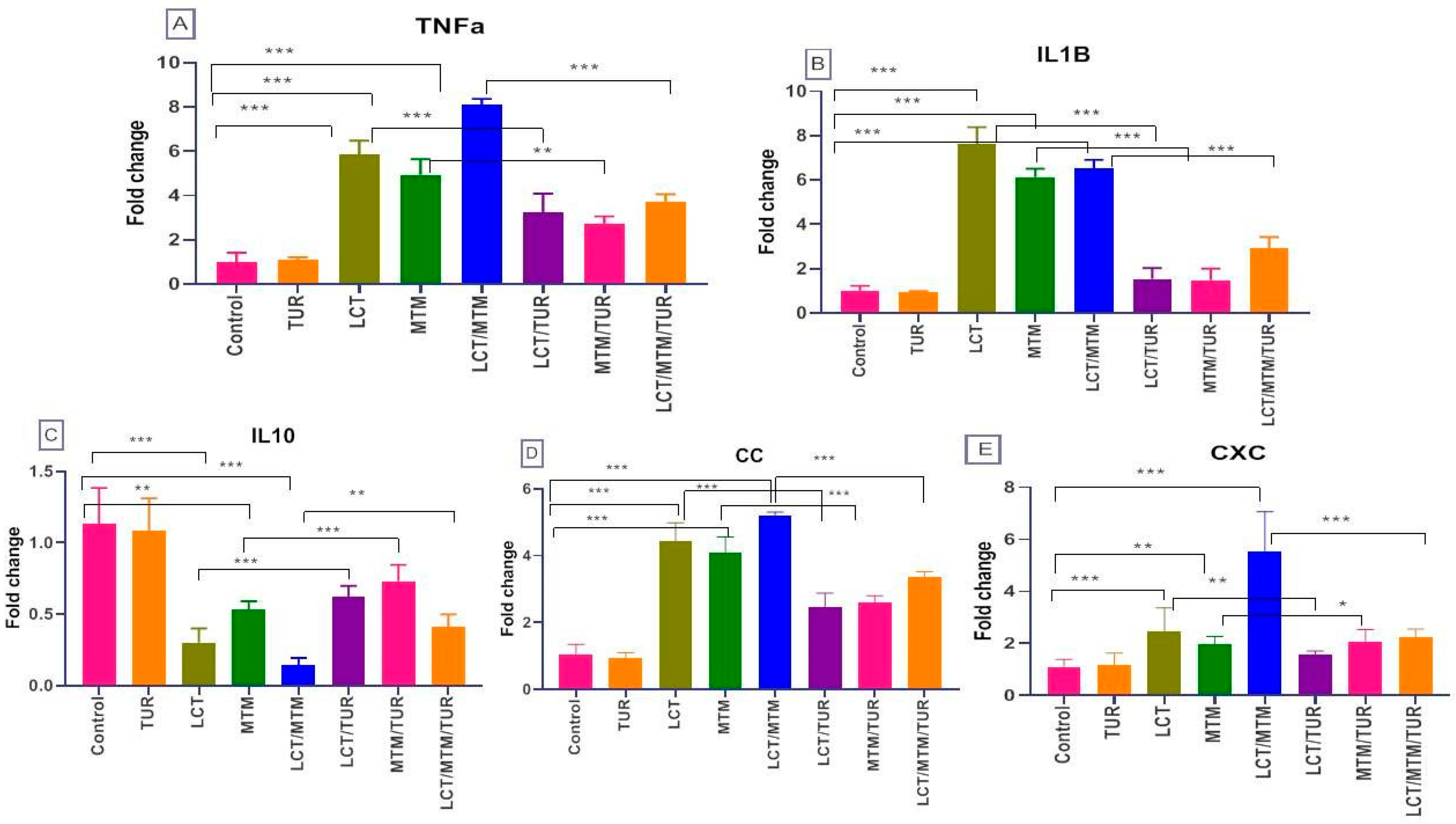
3.7. Immune-Related Genes’ Relative Expression Changes
3.8. Outcomes of Challenges with A. hydrophila
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alalibo, K.; Patricia, U.A.; Ransome, D.E. Effects of Lambda Cyhalothrin on the behaviour and histology of gills of Sarotherodon melanotheron in brackish water. Sci. Afr. 2019, 6, e00178. [Google Scholar] [CrossRef]
- Rahman, A.N.A.; Mohamed, A.A.-R.; Mohammed, H.H.; Elseddawy, N.M.; Salem, G.A.; El-Ghareeb, W.R. The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquaculture 2020, 517, 734777. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Rahman, A.N.A.; Mohammed, H.H.; Ebraheim, L.L.; Abo-Elmaaty, A.M.; Ali, S.A.; Elhady, W.M. Neurobehavioral, apoptotic, and DNA damaging effects of sub-chronic profenofos exposure on the brain tissue of Cyprinus carpio L.: Antagonistic role of Geranium essential oil. Aquat. Toxicol. 2020, 224, 105493. [Google Scholar] [CrossRef]
- Kaplan, A.; Savory, J. Evaluation of a Cellulose-Acetate Electrophoresis System for Serum Protein Fractionation. Clin. Chem. 1965, 11, 937–942. [Google Scholar] [CrossRef]
- Guedegba, N.L.; Toko, I.I.; Agbohessi, P.T.; Zoumenou, B.; Douny, C.; Mandiki, S.N.; Schiffers, B.; Scippo, M.-L.; Kestemont, P. Comparative acute toxicity of two phytosanitary molecules, lambda-cyhalothrin and acetamiprid, on Nile Tilapia (Oreochromis Niloticus) juveniles. J. Environ. Sci. Health Part B 2019, 54, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Maund, S.J.; Hamer, M.J.; Warinton, J.S.; Kedwards, T.J. Aquatic ecotoxicology of the pyrethroid insecticide lambda-cyhalothrin: Considerations for higher-tier aquatic risk assessment. Pestic. Sci. 1998, 54, 408–417. [Google Scholar] [CrossRef]
- Richterová, Z.; Máchová, J.; Stara, A.; Tumová, J.; Velisek, J.; Sevcikova, M.; Svobodová, Z. Effects of Cyhalothrin-Based Pesticide on Early Life Stages of Common Carp (Cyprinus carpio L.). BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Inyang, I.R.; Izah, S.C.; Johnson, D.T.; Ejomarie, O.-O.A. Effects of Lambda cyhalothrin on some electrolytes and metabolites in organs of Parpohiocephalus obscurus. Biotechnol. Res. 2016, 3, 6–10. [Google Scholar]
- Giddings, J.M.; Barber, I.; Warren-Hicks, W. Comparative aquatic toxicity of the pyrethroid insecticide lambda-cyhalothrin and its resolved isomer gamma-cyhalothrin. Ecotoxicology 2009, 18, 239–249. [Google Scholar] [CrossRef]
- Heba, H.M.; Adel, A.S. Mycological and histopathological identification of potential fish pathogens in O-niloticus. Aquaculture 2020, 530, 735849. [Google Scholar]
- Anderson, T.A.; Salice, C.J.; Erickson, R.A.; McMurry, S.T.; Cox, S.B.; Smith, L.M. Effects of landuse and precipitation on pesticides and water quality in playa lakes of the southern high plains. Chemosphere 2013, 92, 84–90. [Google Scholar] [CrossRef]
- Tsaboula, A.; Papadakis, E.-N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Papadopoulou-Mourkidou, E. Environmental and human risk hierarchy of pesticides: A prioritization method, based on monitoring, hazard assessment and environmental fate. Environ. Int. 2016, 91, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.; Cunha, M.L.; Dores, E.F.; Calheiros, D.F. Pesticide residues in river sediments from the Pantanal Wetland, Brazil. J. Environ. Sci. Health Part B 2008, 43, 717–722. [Google Scholar] [CrossRef]
- Hunt, L.; Bonetto, C.; Resh, V.H.; Buss, D.F.; Fanelli, S.; Marrochi, N.; Lydy, M.J. Insecticide concentrations in stream sediments of soy production regions of South America. Sci. Total Environ. 2016, 547, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piner, P.; Üner, N. Oxidative and apoptotic effects of lambda-cyhalothrin modulated by piperonyl butoxide in the liver of Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2012, 33, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, B.; Pandey, R.S. Alterations in nitrogen metabolism in freshwater fishes, Channa punctatus and Clarias batrachus, exposed to a commercial-grade λ-cyhalothrin, REEVA-5. Int. J. Exp. Pathol. 2012, 93, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, B.; Pandey, R.S. λ-Cyhalothrin and cypermethrin induce stress in the freshwater muddy fish, Clarias batrachus. Toxicol. Environ. Chem. 2014, 96, 136–149. [Google Scholar] [CrossRef]
- Vieira, C.E.D.; Martinez, C.B.D.R. The pyrethroid λ-cyhalothrin induces biochemical, genotoxic, and physiological alterations in the teleost Prochilodus lineatus. Chemosphere 2018, 210, 958–967. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, B.C.; Pandey, R.S. Assessment of acute toxicity of λ-cyhalothrin to a freshwater catfish, Clarias batrachus. Environ. Chem. Lett. 2009, 9, 43–46. [Google Scholar] [CrossRef]
- Meng, S.L.; Chen, J.Z.; Hu, G.D.; Song, C.; Fan, L.M.; Qiu, L.P.; Xu, P. Effects of chronic exposure of methomyl on the antioxidant system in liver of O-niloticus (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2014, 101, 1–6. [Google Scholar] [CrossRef]
- Tomlin, C.D. The Pesticide Manual: A World Compendium; British Crop Production Council: Hampshire, UK, 2009. [Google Scholar]
- Kongphonprom, K.; Burakham, R. Determination of Carbamate Insecticides in Water, Fruit, and Vegetables by Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction and High-Performance Liquid Chromatography. Anal. Lett. 2016, 49, 753–767. [Google Scholar] [CrossRef]
- Driskell, W.J.; Groce, D.F.; Hill, R.H., Jr.; Birky, M.M. Methomyl in the blood of a pilot who crashed during aerial spraying. J. Anal. Toxicol. 1991, 15, 339–340. [Google Scholar] [CrossRef]
- Toumi, H.; Burga-Pérez, K.F.; Férard, J.-F. Acute and chronic ecotoxicity of carbaryl with a battery of aquatic bioassays. J. Environ. Sci. Health Part B 2015, 51, 57–62. [Google Scholar] [CrossRef]
- Trachantong, W.; Saenphet, S.; Saenphet, K.; Chaiyapo, M. Lethal and sublethal effects of a methomyl-based insecticide in Hoplobatrachus rugulosus. J. Toxicol. Pathol. 2017, 30, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queirós, L.; Martins, A.; Krum, B.; Ke, T.; Aschner, M.; Pereira, J.; Gonçalves, F.; Milne, G.; Pereira, P. Assessing the neurotoxicity of the carbamate methomyl in Caenorhabditis elegans with a multi-level approach. Toxicology 2021, 451, 152684. [Google Scholar] [CrossRef]
- Fazio, F.; Faggio, C.; Torre, A.; Sanfilippo, M.; Piccione, G. Effect of water quality on hematological and biochemical parameters of Gobius niger caught in Faro lake (Sicily). Iran. J. Fish. Sci. 2013, 12, 219–231. [Google Scholar]
- El-Sayed, A.-F.M. Tilapia Culture; Elsevier: Amsterdam, The Netherlands, 2020; p. 274. [Google Scholar]
- Rahman, A.N.A.; ElHady, M.; Shalaby, S.I. Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of O-niloticus (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 2019, 505, 92–97. [Google Scholar] [CrossRef]
- Khalil, S.R.; Elhakim, Y.A.; El-Fattah, A.H.A.; Farag, M.R.; El-Hameed, N.E.A.; El-Murr, A.E. Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): Stress and immune encoding gene expression. Fish Shellfish. Immunol. 2020, 100, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Islamy, R.A.; Yanuhar, U.; Hertika, A.M.S. Assessing the Genotoxic Potentials of Methomyl-based Pesticide in Tilapia (Oreochromis niloticus) Using Micronucleus Assay. J. Exp. Life Sci. 2017, 7, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Michelato, M.; Furuya, W.M.; Gatlin, D.M., III. Metabolic responses of O-niloticus Oreochromis niloticus to methionine and taurine supplementation. Aquaculture 2018, 485, 66–72. [Google Scholar] [CrossRef]
- Takahashi, K.; Harada, H.; Schaffer, S.W.; Azuma, J. Effect of taurine on intracellular calcium dynamics of cultured myocardial cells during the calcium paradox. In Taurine; Springer: Berlin/Heidelberg, Germany, 1992; pp. 153–161. [Google Scholar]
- Stapleton, P.; Bloomfield, F. Effect of zwitterions on the respiratory burst. J. Biomed. Sci. 1993, 3, 79–84. [Google Scholar]
- El-Sayed, A.F.M. Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev. Aquac. 2014, 6, 241–255. [Google Scholar] [CrossRef]
- Al-Feky, S.; El-Sayed, A.F.; Ezzat, A. Dietary taurine enhances growth and feed utilization in larval N ile tilapia (O reochromis niloticus) fed soybean meal-based diets. Aquac. Nutr. 2016, 22, 457–464. [Google Scholar] [CrossRef]
- Richard, N.; Colen, R.; Aragão, C. Supplementing taurine to plant-based diets improves lipid digestive capacity and amino acid retention of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 2017, 468, 94–101. [Google Scholar] [CrossRef]
- Poppi, D.A.; Moore, S.S.; Glencross, B.D. The effect of taurine supplementation to a plant-based diet for barramundi (Lates calcarifer) with varying methionine content. Aquac. Nutr. 2018, 24, 1340–1350. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient requirements of fish; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Galal, A.A.; Reda, R.M.; Mohamed, A.A.-R. Influences of Chlorella vulgaris dietary supplementation on growth performance, hematology, immune response and disease resistance in Oreochromis niloticus exposed to sub-lethal concentrations of penoxsulam herbicide. Fish Shellfish. Immunol. 2018, 77, 445–456. [Google Scholar] [CrossRef]
- Weiss, D.J.; Wardrop, K.J. Schalm’s Veterinary Hematology; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Misra, H.P.; Fridovich, I. Superoxide dismutase: A photochemical augmentation assay. Arch. Biochem. Biophys. 1977, 181, 308–312. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, E.N.; Mahboub, H.H.H. Studies on the effect of Lactococcus garvieae of dairy origin on both cheese and O-niloticus (O. niloticus). Int. J. Vet. Sci. Med. 2018, 6, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richterova, Z.; Machova, J.; Stara, A.; Tumova, J.; Velisek, J.; Sevcikova, M.; Svobodová, Z. Effects of a cypermethrin-based pesticide on early life stages of common carp (Cyprinus carpio L.). Veterinární Med. 2016, 60, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Sasaki, T.; Awa, M.; Inomata, M.; Honryo, T.; Agawa, Y.; Ando, M.; Sawada, Y. Effect of dietary taurine enhancement on growth and development in red sea bream Pagrus major larvae. Aquac. Res. 2016, 47, 1168–1179. [Google Scholar] [CrossRef]
- Yue, Y.R.; Liu, Y.J.; Tian, L.X.; Gan, L.; Yang, H.J.; Liang, G.Y.; He, J.Y. The effect of dietary taurine supplementation on growth performance, feed utilization and taurine contents in tissues of juvenile white shrimp (L itopenaeus vannamei, Boone, 1931) fed with low-fishmeal diets. Aquac. Res. 2013, 44, 1317–1325. [Google Scholar] [CrossRef]
- Shen, G.; Wang, S.; Dong, J.; Feng, J.; Xu, J.; Xia, F.; Wang, X.; Ye, J. Metabolic Effect of Dietary Taurine Supplementation on Grouper (Epinephelus coioides): A 1H-NMR-Based Metabolomics Study. Molecules 2019, 24, 2253. [Google Scholar] [CrossRef] [Green Version]
- Adeshina, I.; Abdel-Tawwab, M. Dietary taurine incorporation to high plant protein-based diets improved growth, biochemical, immunity, and antioxidants biomarkers of African catfish, Clarias gariepinus (B.). Fish Physiol. Biochem. 2020, 46, 1323–1335. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N. Stimulatory effect of dietary taurine on growth performance, digestive enzymes activity, antioxidant capacity, and tolerance of common carp, Cyprinus carpio L., fry to salinity stress. Fish Physiol. Biochem. 2018, 44, 639–649. [Google Scholar] [CrossRef]
- Bibi, N.; Zuberi, A.; Naeem, M.; Ullah, I.; Sarwar, H.; Atika, B. Evaluation of acute toxicity of karate and its sub-lethal effects on protein and acetylcholinestrase activity in Cyprinus carpio. Int. J. Agric. Biol. 2014, 16, 731–737. [Google Scholar]
- Pamanji, R.; Yashwanth, B.; Rao, J.V. Profenofos induced biochemical alterations and in silico modelling of hatching enzyme, ZHE1 in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 2016, 45, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, B.; Pandey, R.S. Preliminary Evaluation of The Acute Toxicity of Cypermethrin and λ-Cyhalothrin to Channa Punctatus. Bull. Environ. Contam. Toxicol. 2007, 79, 613–616. [Google Scholar] [CrossRef]
- Han, S.-Y.; Kim, J.-H.; Gwon, G.-Y.; Yeom, D.-H. Effects on Biomarkers and Endocrine in Muddy Loach (Misgurnus anguillicaudatus) under 21 day Exposure to Methomyl. Korean J. Pestic. Sci. 2012, 16, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Ceccotti, C.; Al-Sulaivany, B.S.; Al-Habbib, O.A.; Saroglia, M.; Rimoldi, S.; Terova, G. Protective Effect of Dietary Taurine from ROS Production in European Seabass under Conditions of Forced Swimming. Animals 2019, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Parveen, N.; Firdaus, F.; Shadab, G.G.H.A. Assessment of Micronuclei and Chromosomal Aberrations in Channa punctatus Exposed to Lead Nitrate. Adv. Sci. Eng. Med. 2013, 5, 683–687. [Google Scholar] [CrossRef]
- Joshp, P.; Bose, M.; Harish, D. Changes in certain haematological parameters in a siluroid cat fish Clarias batrachus (Linn) exposed to cadmium chloride. Pollut. Res. 2002, 21, 129–131. [Google Scholar]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Kilawati, Y.; Brawijaya, U.; Islamy, R.A. The Antigenotoxic Activity of Brown Seaweed (Sargassum sp.) Extract Against Total Erythrocyte and Micronuclei of Tilapia Oreochromis niloticus Exposed by Methomyl-Base Pesticide. J. Exp. Life Sci. 2019, 9, 205–210. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, H.; Goto, T.; Hayashi, M.; Hatate, H.; Endo, M.; Yamashita, H.; Ukawa, M. Hemolytic suppression roles of taurine in yellowtail Seriola quinqueradiata fed non-fishmeal diet based on soybean protein. Fish. Sci. 2006, 72, 546–555. [Google Scholar] [CrossRef]
- López, L.M.; Flores-Ibarra, M.; Bañuelos-Vargas, I.; Galaviz, M.A.; True, C.D. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on growth performance, hematological and biochemical status, and liver histology of totoaba juveniles (Totoaba macdonaldi). Fish Physiol. Biochem. 2015, 41, 921–936. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish. Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Verma, V.K.; Rani, K.V.; Sehgal, N.; Prakash, O. Immunostimulatory response induced by supplementation of Ficus benghalensis root powder, in the artificial feed the Indian freshwater murrel, Channa punctatus. Fish Shellfish. Immunol. 2012, 33, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Jiang, J.; Tang, L.; Tang, W.; Zhang, Y.; Yang, J.; et al. Dietary taurine supplementation to a plant protein source-based diet improved the growth and intestinal immune function of young grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2019, 25, 873–896. [Google Scholar] [CrossRef]
- Saleh, N.E.; Wassef, E.A.; Ashry, A.M. Is a taurine supplement necessary in fishmeal-based feeds for juvenile European sea bass (Dicentrarchus labrax)? Aquac. Int. 2019, 28, 321–333. [Google Scholar] [CrossRef]
- Fırat, Ö.; Cogun, H.Y.; Yüzereroğlu, T.A.; Gök, G.; Fırat, Ö.; Kargin, F.; Kötemen, Y. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2011, 37, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Ogueji, E.; Auta, J. Investigations of biochemical effects of acute concentrations of Lambda-cyhalothrin on African catfish, Clarias gariepinus–Teugels. J. Fish. INT 2007, 2, 86–90. [Google Scholar]
- Asadi, M.; Mirvaghefei, A.; Nematollahi, M.; Banaee, M.; Ahmadi, K. Effects of Watercress (Nasturtium nasturtium) extract on selected immunological parameters of rainbow trout (Oncorhynchus mykiss). Open Veter-J. 2012, 2, 32–39. [Google Scholar]
- Tong, S.; Wang, L.; Kalhoro, H.; Volatiana, J.A.; Shao, Q. Effects of supplementing taurine in all-plant protein diets on growth performance, serum parameters, and cholesterol 7α-hydroxylase gene expression in black sea bream, Acanthopagrus schlegelii. J. World Aquac. Soc. 2019, 51, 990–1001. [Google Scholar] [CrossRef]
- De Almeida, E.A.; Marques, S.D.A.; Klitzke, C.F.; Bainy, A.C.D.; De Medeiros, M.H.G.; Di Mascio, P.; Loureiro, A.P.D.M. DNA damage in digestive gland and mantle tissue of the mussel Perna perna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 295–303. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuro Endocrinol. Lett. 2009, 30, 2. [Google Scholar] [PubMed]
- Mohamed, A.A.-R.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.; Ahmed, A.I.; El-Hakim, Y.M.A. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Shen, W.; Lou, B.; Xu, C.; Yang, G.; Yu, R.; Wang, X.; Li, X.; Wang, Q.; Wang, Y. Lethal toxicity and gene expression changes in embryonic zebrafish upon exposure to individual and mixture of malathion, chlorpyrifos and lambda-cyhalothrin. Chemosphere 2020, 239, 124802. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.L.; Qu, J.H.; Fan, L.M.; Qiu, L.P.; Chen, J.Z.; Xu, P. Responses of glutathione-related antioxidant defense system in serum of O-niloticus (Oreochromis niloticus) exposed to sublethal concentration of methomyl and recovery pattern. Environ. Toxicol. 2015, 30, 483–489. [Google Scholar] [CrossRef]
- Li, M.; Lai, H.; Li, Q.; Gong, S.; Wang, R. Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 2016, 450, 349–355. [Google Scholar] [CrossRef]
- Van Der Meide, P.H.; Schellekens, H. Cytokines and the immune response. Biotherapy 1996, 8, 243–249. [Google Scholar] [CrossRef] [PubMed]
- El-Hakim, Y.M.A.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.; Moustafa, A.A.; Mohamed, A.A.R. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef]
- Francis, N.O.; Esa, Y.B. A review of production protocols used in producing economically viable monosex tilapia. J. Fish. Aquat. Sci. 2016, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Kim, W.-J.; Park, C.-J.; Park, J.-W.; Noh, G.E.; Lee, S.; Lee, Y.M.; Kim, H.C. Analysis of Manifestation of CC and CXC Chemokine Genes in Olive Flounders (Paralichthys olivaceus) Artificially Infected with VHSV during the Early Developmental Stage. Dev. Reprod. 2018, 22, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Köllner, B.; Wasserrab, B.; Kotterba, G.; Fischer, U. Evaluation of immune functions of rainbow trout (Oncorhynchus mykiss)—how can environmental influences be detected? Toxicol. Lett. 2002, 131, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Kreutz, L.C.; Gil Barcellos, L.J.; Marteninghe, A.; Dos Santos, E.D.; Zanatta, R. Exposure to sublethal concentration of glyphosate or atrazine-based herbicides alters the phagocytic function and increases the susceptibility of silver catfish fingerlings (Rhamdia quelen) to Aeromonas hydrophila challenge. Fish Shellfish. Immunol. 2010, 29, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Neamat-Allah, A.N.; Abd El Hakim, Y.; Mahmoud, E.A. Alleviating effects of β-glucan in Oreochromis niloticus on growth performance, immune reactions, antioxidant, transcriptomics disorders and resistance to Aeromonas sobria caused by atrazine. Aquac. Res. 2020, 51, 1801–1812. [Google Scholar] [CrossRef]

| Ingredients | % |
|---|---|
| Yellow corn | 30 |
| Soybean meal, 48% | 20 |
| Meat meal high fat, 50% | 18 |
| Wheat flour | 10 |
| Fish meal, 60% | 15 |
| Vegetable oil | 5.5 |
| Vitamins and minerals mixture 1 | 1.5 |
| Total | 100 |
| Chemical analysis (%) 2 | |
| DM | 86.02 |
| CP | 32.02 |
| EE | 9.93 |
| CF | 1.74 |
| Ash | 10.13 |
| NFE | 35.97 |
| DE, Kcal/ kg diet 3 | 2867.48 |
| LCT | |||||
|---|---|---|---|---|---|
| Intercept ± S.E. | Slope ± S.E. | 95 % Confidence Limit | Conc (µg/L) | Point | |
| upper | lower | ||||
| −3.446 ± 0.455 | 2.167 ± 0.281 | 0.140 | 0.748 | 0.517 | LC 1 |
| 0.555 | 1.005 | 0.831 | LC 5 | ||
| 0.774 | 1.145 | 0.999 | LC 10 | ||
| 0.920 | 1.241 | 1.112 | LC 15 | ||
| 1.491 | 1.692 | 1.590 | LC 50 | ||
| 1.935 | 2.272 | 2.069 | LC 85 | ||
| 2.030 | 2.418 | 2.182 | LC 90 | ||
| 2.169 | 2.637 | 2.349 | LC 95 | ||
| 2.426 | 3.053 | 2.664 | LC 99 | ||
| Methomyl (MTM) | |||||
| −2.401 ± 0.262 | 0.006 ± 0.001 | 83.386 | −93.678 | 12.670 | LC 1 |
| 181.548 | 51.595 | 128.463 | LC 5 | ||
| 235.159 | 127.758 | 190.192 | LC 10 | ||
| 272.273 | 178.202 | 231.840 | LC 15 | ||
| 448.241 | 372.456 | 407.941 | LC 50 | ||
| 658.665 | 532.254 | 584.042 | LC 85 | ||
| 710.568 | 567.909 | 625.690 | LC 90 | ||
| 788.096 | 620.155 | 687.419 | LC 95 | ||
| 934.650 | 717.036 | 803.212 | LC 99 | ||
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession No |
|---|---|---|---|
| TNF-α | CCAGAAGCACTAAAGGCGAAGA | CCTTGGCTTTGCTGCTGATC | NC_031985.2 |
| IL-1b | TGGTGACTCTCCTGGTCTGA | GCACAACTTTATCGGCTTCCA | DQ061114.1 |
| IL-10 | CTGCTAGATCAGTCCGTCGAA | GCAGAACCGTGTCCAGGTAA | NC031970.1 |
| CC-chemokine | ACAGAGCCGATCTTGGGTTACTTG | TGAAGGAGAGGCGGTGGATGTTAT | FF279635.1 |
| CXC-chemokine | CTATCCATGGAGCCTCAGGT | CACTCCAGAGATCAAAGCAGTTCC | XM_003452201 |
| GAPDH | CCGATGTGTCAGTGGTGGAT | CTTCTTGAGCGTGGCAATAA | NC_031976.2 |
| Parameter | Control | TUR | LCT | MTM | LCT + MTM | LCT + TUR | MTM + TUR | LCT + MTM + TUR |
|---|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 30.24 ± 0.005 | 30.28 ± 0.0 ns | 30.28 ± 0.1ns | 30.23 ± 0.18 ns | 30.48 ± 0.0 ns | 30.25 ± 0.22 ns | 30.49 ± 0.15 ns | 30.60 ± 0.05 ns |
| Final body weight (g) | 57.12 ± 0.58 | 58.60 ± 0.91 ns | 42.54 ± 0.64 ** | 44.96 ± 1.10 ** | 37.04 ± 0.49 *** | 48.34 ± 0.78 ## | 40.84 ± 0.38 ## | 44.22 ± 0.24 ## |
| Weight gain (g) | 26.88 ± 0.56 | 28.32 ± 0.91 * | 12.26 ± 0.65 *** | 14.66 ± 1.24 ** | 6.70 ± 0.52 *** | 18.06 ± 0.70 ### | 10.38 ± 0.47 ### | 13.62 ± 0.30 ### |
| SGR (%) | 1.13 ± 0.02 | 1.18 ± 0.03 ns | 0.61 ± 0.03 ** | 0.70 ± 0.05 ** | 0.36 ± 0.03 *** | 0.83 ± 0.03 ## | 0.52 ± 0.02 ## | 0.66 ± 0.01 ### |
| K (condition factor) | 0.46 ± 0.02 | 0.43 ± 0.01 ns | 0.35 ± 0.02 * | 0.37 ± 0.01 * | 0.41 ± 0.02 * | 0.43 ± 0.02 # | 0.35 ± 0.01 ns | 0.36 ± 0.01 ns |
| No of mortality | 0/60 | 0/60 | 31/60 | 26/60 | 37/60 | 18/60 | 12/60 | 21/60 |
| Mortality % | 0 | 0 | 51.66 | 43.33 | 61.67 | 30 | 20 | 35 |
| Hematological Indices | ||||||||
| RBCs (106/mm3) | 3.03 ± 0.08 | 3.27 ± 0.06 ns | 1.38 ± 0.25 *** | 1.20 ± 0.04 *** | 0.70 ± 0.04 *** | 2.53 ± 0.06 ## | 2.10 ± 0.04 ## | 1.33 ± 0.13 ## |
| Hb (gm/dL) | 9.43 ± 0.22 | 9.10 ± 0.04 ns | 3.50 ± 0.23 *** | 4.93 ± 0.24 *** | 2.21 ± 0.04 *** | 6.57 ± 0.08 ### | 5.98 ± 0.27 ## | 4.30 ± 0.12 ## |
| PCV (%) | 27.00 ± 0.71 | 28.33 ± 0.85 ns | 14.33 ± 0.62 *** | 19.00 ± 1.08 ** | 8.00 ± 0.41 *** | 21.00 ± 0.41 ## | 18.68 ± 0.24 ns | 14.00 ± 0.41 ## |
| MCV(fl) | 104.6 ± 1.60 | 101.5 ± 1.14 ns | 170.8 ± 2.00 *** | 161.7 ± 1.70 *** | 127.9 ± 2.32 *** | 143.3 ± 1.05 ### | 136.6 ± 1.52 ## | 118.8 ± 0.23 # |
| WBCs (103/mm3) | 6.13 ± 0.08 | 5.47 ± 0.17 ns | 2.50 ± 0.16 *** | 3.87 ± 0.12 ** | 1.78 ± 0.05 *** | 4.67 ± 0.15 ### | 4.70 ± 0.04 ## | 3.47 ± 0.05# |
| Lymphocytes (103/mm3) | 2.8 ± 0.04 | 3.0 ± 0.13 ns | 1.1 ± 0.08 *** | 2.0 ± 0.06 ** | 0.9 ± 0.02 *** | 1.8 ± 0.04 ## | 2.2 ± 0.04 # | 1.4 ± 0.04 ## |
| Heterophils (103/mm3) | 1.82 ± 0.01 | 1.68 ± 0.07 ns | 1.05 ± 0.24 ** | 1.30 ± 0.04 * | 0.70 ± 0.04 *** | 1.70 ± 0.04 ### | 1.43 ± 0.02 # | 1.37 ± 0.02 ## |
| Eosinophils (103/mm3) | 0.35 ± 0.03 | 0.33 ± 0.01 ns | 0.14 ± 0.03 ** | 0.20 ± 0.04 * | 0.13 ± 0.03 ** | 0.23 ± 0.02 # | 0.20 ± 0.04 # | 0.27 ± 0.02 # |
| Monocytes (103/mm3) | 0.62 ± 0.01 | 0.62 ± 0.01 ns | 0.41 ± 0.03 ** | 0.51 ± 0.01 * | 0.19 ± 0.04 ** | 0.37 ± 0.02 ns | 0.20 ± 0.04 ns | 0.10 ± 0.00 ns |
| Parameter | Control | TUR | LCT | MTM | LCT + MTM | LCT + TUR | MTM + TUR | LCT + MTM + TUR |
|---|---|---|---|---|---|---|---|---|
| AchE | 9.43 ± 0.22 | 9.10 ± 0.04 ns | 3.50 ± 0.23 *** | 4.93 ± 0.24 *** | 2.25 ± 0.06 *** | 6.57 ± 0.08 ### | 5.98 ± 0.27 ## | 4.30 ± 0.12 ### |
| 8OHDG | 26.67 ± 1.5 | 30.67 ± 1.03 ns | 85.33 ± 0.62 *** | 76.67 ± 0.62 *** | 106.5 ± 0.65 *** | 44.0 ± 0.41 ## | 56.0 ± 0.41 ## | 64.0 ± 1.08 ### |
| Immunoglobulin M (mg/dL) | 121.3 ± 0.62 | 125.7 ± 2.46 ns | 58.33 ± 1.02 *** | 64.07 ± 4.28 *** | 41.13 ± 0.29 *** | 65.93 ± 1.94 ## | 76.73 ± 0.56 ## | 53.43 ± 1.02 ### |
| Lyzozyme activity | 30.60 ± 0.37 | 29.57 ± 0.37 ns | 13.67 ± 0.21 *** | 17.10 ± 0.53 *** | 10.94 ± 0.20 *** | 19.60 ± 0.51 ### | 21.40 ± 0.39 ### | 15.61 ± 0.24 ### |
| Complement 3 (ug/mL) | 78.6 ± 0.45 | 75.9 ± 0.72 ns | 48.8 ± 1.13 *** | 56.53 ± 0.34 *** | 36.26 ± 0.73 *** | 61.67 ± 0.58 ### | 66.9 ± 0.19 ### | 45.23 ± 0.41 ### |
| Nitric oxide (μmol/L) | 66.47 ± 0.31 | 67.50 ± 0.60 ns | 38.77 ± 0.51 ** | 43.03 ± 0.89 ** | 29.69 ± 0.15 *** | 56.43 ± 0.27 ## | 54.27 ± 1.38 ### | 42.63 ± 0.82 # |
| Total protein (g/dL) | 5.40 ± 0.08 | 5.40 ± 0.16 ns | 4.60 ± 0.08 ** | 4.80 ± 0.08 ** | 5.00 ± 0.16 ** | 4.67 ± 0.17 ns | 5.13 ± 0.21 # | 4.87 ± 0.12 # |
| Albumin (A)(g/dL) | 2.63 ± 0.06 | 2.53 ± 0.05 ns | 2.27 ± 0.08 * | 2.47 ± 0.05 ns | 2.61 ± 0.15 ns | 2.23 ± 0.08 ns | 2.57 ± 0.17 ns | 2.40 ± 0.08 ns |
| Globulin (G)(g/dL) | 2.77 ± 0.02 | 2.87 ± 0.12 ns | 2.27 ± 0.02 * | 2.23 ± 0.05 * | 2.11 ± 0.04 * | 2.30 ± 0.04 # | 2.43 ± 0.05 # | 2.33 ± 0.08 # |
| A/G | 0.95 ± 0.02 | 0.89 ± 0.03 ns | 1.00 ± 0.03 ns | 1.11 ± 0.02 * | 1.24 ± 0.08 ** | 0.97 ± 0.03 ns | 1.05 ± 0.05 ns | 1.04 ± 0.06 # |
| α-globulin−1(g/dL) | 0.79 ± 0.01 | 0.77 ± 0.01 ns | 0.30 ± 0.04 ** | 0.40 ± 0.04 ** | 0.14 ± 0.02 *** | 0.44 ± 0.02 # | 0.40 ± 0.04 ns | 0.25 ± 0.02 ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahman Mohamed, A.; Abdel Rahman, A.N.; Salem, G.A.; Deib, M.M.E.; Nassan, M.A.; Rhouma, N.R.; Khater, S.I. The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus. Animals 2021, 11, 1318. https://doi.org/10.3390/ani11051318
Abdel-Rahman Mohamed A, Abdel Rahman AN, Salem GA, Deib MME, Nassan MA, Rhouma NR, Khater SI. The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus. Animals. 2021; 11(5):1318. https://doi.org/10.3390/ani11051318
Chicago/Turabian StyleAbdel-Rahman Mohamed, Amany, Afaf N. Abdel Rahman, Gamal A. Salem, Maha M.El Deib, Mohamed A. Nassan, Nasreddin R. Rhouma, and Safaa I. Khater. 2021. "The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus" Animals 11, no. 5: 1318. https://doi.org/10.3390/ani11051318
APA StyleAbdel-Rahman Mohamed, A., Abdel Rahman, A. N., Salem, G. A., Deib, M. M. E., Nassan, M. A., Rhouma, N. R., & Khater, S. I. (2021). The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus. Animals, 11(5), 1318. https://doi.org/10.3390/ani11051318






