Changes in Diarrhea Score, Nutrient Digestibility, Zinc Utilization, Intestinal Immune Profiles, and Fecal Microbiome in Weaned Piglets by Different Forms of Zinc
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
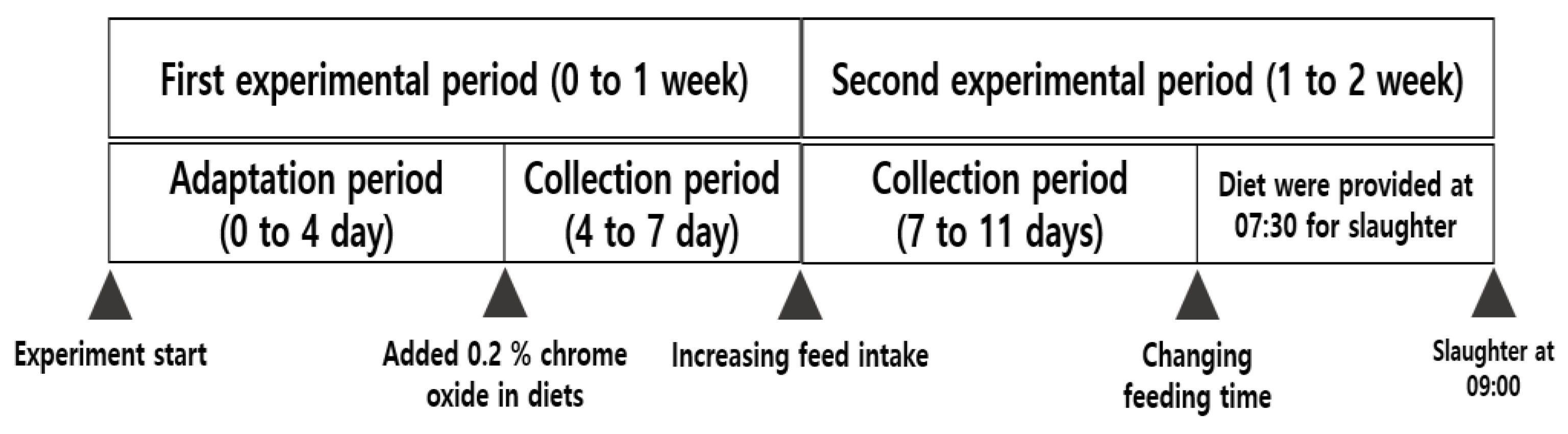
2.1. Animals, Facilities, and Dietary Treatments
2.2. Growth Performance and Diarrhea Scores
2.3. Sample Collections
2.4. Chemical Analysis for Diet and Feces
2.5. Fecal Microbiome Analysis
2.6. Complete Blood Count Analysis
2.7. Flow Cytometry Analysis
2.8. Histological Analysis
2.9. Quantitative RT-PCR
2.10. Statistical Analysis
3. Results
3.1. Effects of Different Forms of Zinc-Containing Diets on Growth Performance and Diarrhea Incidence in Piglets
3.2. Nutrient Digestibility and Zinc Utilization in Piglets Fed Different Forms of Zinc
3.3. Blood Immune Profiles and Zinc Levels in Piglets Fed Different Forms of Zinc
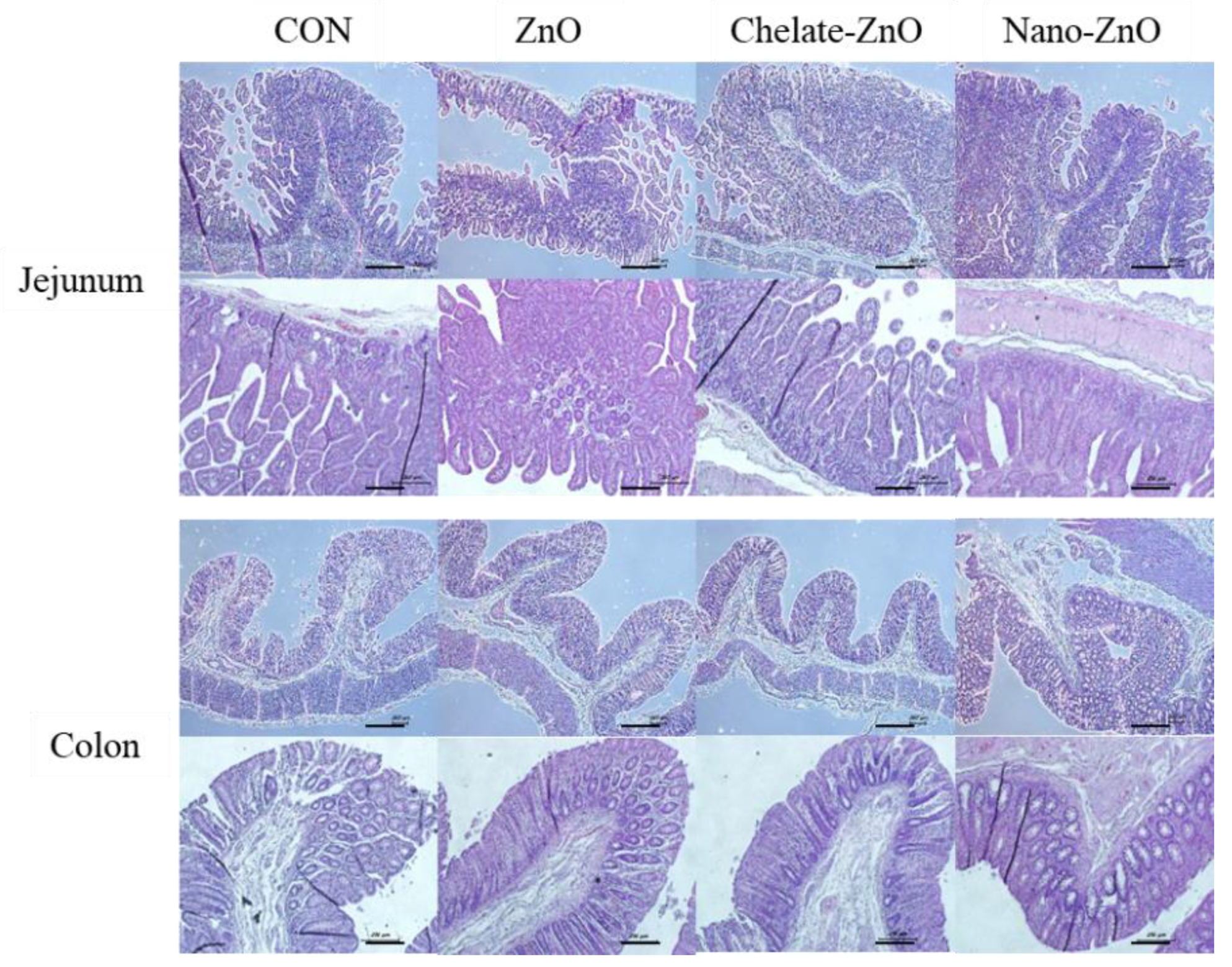
3.4. Changes in Intestinal Morphology in Piglets Fed Different Forms of Zinc
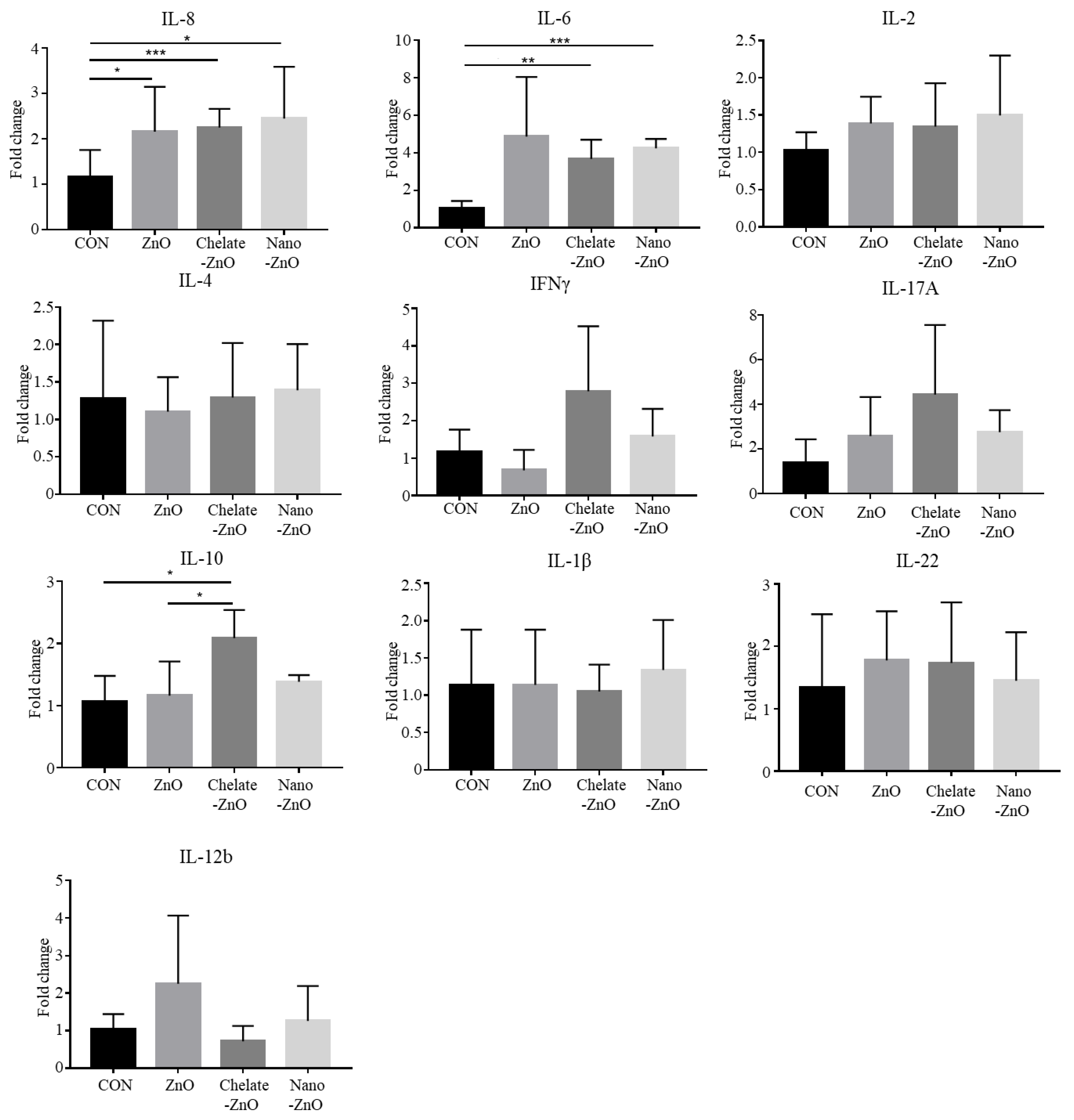
3.5. T Cell Subset and Cytokine Expression in Gut Lymph Node of Piglets Fed Different Forms of Zinc
3.6. mRNA Expression of T Cell Transcription Factors and Cytokine Expression in the Colon Tissues of Piglets Fed Different Forms of Zinc
3.7. mRNA Expression of Tight Junction Proteins and Antimicrobial Peptides in the Colon Tissue of Piglets Fed Different Forms of Zinc
3.8. Changes in the Fecal Microbiomes of Piglets by the Supplementation with Different Forms of Zinc
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Schell, T.C.; Kornegay, E.T. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-Methionine, Zn-Lysine, or ZnSO4. J. Anim. Sci. 1996, 74, 1584–1593. [Google Scholar] [CrossRef]
- King, L.E.; Frentzel, J.W.; Mann, J.J.; Fraker, P.J. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005, 24, 494–502. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Hujanen, E.S.; Seppä, S.T.; Virtanen, K. Polymorphonuclear leukocyte chemotaxis induced by zinc, copper and nickel in vitro. Biochim. Biophys. Acta Gen. Subj. 1995, 1245, 145–152. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Jiang, X.R.; Wang, W.J.; Qiao, J.Y. Effects of Lactobacillus acidophilus and zinc oxide on the growth performance, jejunal morphology and immune function of weaned piglet following an Escherichia coli K88 challenge. Ital. J. Anim. Sci. 2018, 17, 114–120. [Google Scholar] [CrossRef]
- Kloubert, V.; Blaabjerg, K.; Dalgaard, T.S.; Poulsen, H.D.; Rink, L.; Wessels, I. Influence of zinc supplementation on immune parameters in weaned pigs. J. Trace Elem. Med. Biol. 2018, 49, 231–240. [Google Scholar] [CrossRef]
- Kreuzer-Redmer, S.; Arends, D.; Schulte, J.N.; Karweina, D.; Korkuc, P.; Wöltje, N.; Hesse, D.; Pieper, R.; Gerdts, V.; Zentek, J.; et al. High dosage of zinc modulates T-cells in a time-dependent manner within porcine gut-associated lymphatic tissue. Br. J. Nutr. 2018, 120, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitabayashi, C.; Fukada, T.; Kanamoto, M.; Ohashi, W.; Hojyo, S.; Atsumi, T.; Ueda, N.; Azuma, I.; Hirota, H.; Hirano, T.; et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010, 22, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, C.; Chen, S.; Gao, L.; Lv, H.; Feng, R.; Zhu, Q.; Xu, J.; Chen, Z.; Jiang, Z. Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets. Front. Microbiol. 2017, 8, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Van Noten, N.; Degroote, J.; Romeo, A.; Vermeir, P.; Michiels, J. Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 231–241. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.Y.; Ma, Y.F.; Lv, M.Y.; Wu, Z.P.; Qian, L.C. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Br. J. Nutr. 2014, 111, 1405–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, C.; Carlson, M. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 2002, 80, 1917–1924. [Google Scholar] [CrossRef]
- Ward, T.L.; Asche, G.L.; Louis, G.F.; Pollmann, D.S. Zinc-methionine improves growth performance of starter pigs. J. Anim. Sci. 1996, 74, 182. [Google Scholar]
- Wang, Y.; Tang, J.W.; Ma, W.Q.; Feng, J. Dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets. Biol. Trace. Elem. Res. 2010, 133, 325–334. [Google Scholar] [CrossRef]
- Mullan, B.; D’Souza, D. The role of organic minerals in modern pig production. In Re-Defining Mineral Nutrition; Nottingham University Press: Nottingham, UK, 2005; pp. 89–106. [Google Scholar]
- Nitrayova, S.; Windisch, W.; Von Heimendahl, E.; Müller, A.; Bartelt, J. Bioavailability of zinc from different sources in pigs. J. Anim. Sci. Biotechnol. 2012, 90, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Nys, Y.; Jondreville, C. Zinc availability and digestive zinc solubility in piglets and broilers fed diets varying in their phytate contents, phytase activity and supplemented zinc source. Animal 2010, 4, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, D. Application of nano minerals in animal production system. Res. J. Biotechnol. 2013, 8, 1–3. [Google Scholar]
- Long, L.; Chen, J.; Zhang, Y.; Liang, X.; Ni, H.; Zhang, B.; Yin, Y. Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS ONE 2017, 12, e0182550. [Google Scholar] [CrossRef] [Green Version]
- Barreto, M.S.; Andrade, C.T.; da Silva, L.C.R.; Cabral, L.M.; Paschoalin, V.M.F.; Del Aguila, E.M. In vitro physiological and antibacterial characterization of ZnO nanoparticle composites in simulated porcine gastric and enteric fluids. BMC Vet. Res. 2017, 13, 181. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Kim, H.B.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2007. [Google Scholar]
- chloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Weber, C.F.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Knight, R.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navas-Molina, J.A.; Peralta-Sánchez, J.M.; González, A.; McMurdie, P.J.; Vázquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Knight, R.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [CrossRef] [Green Version]
- Hill, G.M.; Cromwell, G.L.; Crenshaw, T.D.; Dove, C.R.; Ewan, R.C.; Knabe, D.A.; Lewis, A.J.; Libal, G.W.; Mahan, D.C.; Veum, T.L.; et al. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J. Anim. Sci. 2000, 78, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holodova, M.; Cobanova, K.; Sefcikova, Z.; Barszcz, M.; Tuśnio, A.; Taciak, M.; Gresakova, L. Dietary zinc and fibre source can influence the mineral and antioxidant status of piglets. Animals 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Guo, Y. Beneficial effects of tetrabasic zinc chloride for weanling piglets and the bioavailability of zinc in tetrabasic form relative to ZnO. Anim. Feed Sci. Technol. 2007, 135, 75–85. [Google Scholar] [CrossRef]
- Hu, C.; You, Z.; Zhu, K.; Luan, Z. Effects of nano zinc oxide on growth performance and intestinal mucosal barrier in weaner piglets. Chin. J. Anim. Nutr. 2012, 24, 285–290. [Google Scholar]
- Sun, Y.B.; Xia, T.; Wu, H.; Zhang, W.J.; Zhu, Y.H.; Xue, J.X.; He, D.T.; Zhang, L.Y. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim. Feed Sci. Technol. 2019, 258, 114312. [Google Scholar] [CrossRef]
- Janczyk, P.; Kreuzer, S.; Assmus, J.; Nöckler, K.; Brockmann, G.A. No protective effects of high-dosage dietary zinc oxide on weaned pigs infected with Salmonella enterica serovar typhimurium DT104. Appl. Environ. Microbiol. 2013, 79, 2914–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madec, F.; Bridoux, N.; Bounaix, S.; Jestin, A. Measurement of digestive disorders in the piglet at weaning and related risk factors. Prev. Vet. Med. 1998, 35, 53–72. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of dietary supplementation with freshwater microalgae on growth performance, nutrient digestibility and gut health in weaned piglets. Animal 2017, 11, 183–192. [Google Scholar] [CrossRef]
- Lei, X.J.; Kim, I.H. Low dose of coated zinc oxide is as effective as pharmacological zinc oxide in promoting growth performance, reducing fecal scores, and improving nutrient digestibility and intestinal morphology in weaned pigs. Anim. Feed Sci. Technol. 2018, 245, 117–125. [Google Scholar] [CrossRef]
- Bąkowski, M.; Kiczorowska, B.; Samolińska, W.; Klebaniuk, R.; Lipiec, A. Silver and zinc nanoparticles in animal nutrition—A review. Ann. Anim. Sci. 2018, 18, 879–898. [Google Scholar] [CrossRef] [Green Version]
- Star, L.; Van der Klis, J.D.; Rapp, C.; Ward, T.L. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 2012, 91, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Huang, J.T.; Tsai, Y.H.; Mao, S.Y.; Fu, C.M.; Lien, T.F. Nanosize of zinc oxide and the effects on zinc digestibility, growth performances, immune response and serum parameters of weanling piglets. Anim. Sci. J. 2016, 87, 1379–1385. [Google Scholar] [CrossRef]
- Davda, J.; Labhasetwar, V. Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 2002, 233, 51–59. [Google Scholar] [CrossRef]
- Li, B.T.; Van Kessel, A.G.; Caine, W.R.; Huang, S.X.; Kirkwood, R.N. Small intestinal morphology and bacterial populations in ileal digesta and feces of newly weaned pigs receiving a high dietary level of zinc oxide. Can. J. Anim. Sci. 2001, 81, 511–516. [Google Scholar] [CrossRef]
- Cho, J.H.; Upadhaya, S.D.; Kim, I.H. Effects of dietary supplementation of modified zinc oxide on growth performance, nutrient digestibility, blood profiles, fecal microbial shedding and fecal score in weanling pigs. Anim. Sci. J. 2015, 86, 617–623. [Google Scholar] [CrossRef]
- Umu, Ö.C.; Frank, J.A.; Fangel, J.U.; Oostindjer, M.; da Silva, C.S.; Bolhuis, E.J.; Bosch, G.; Willats, W.G.T.; Pope, P.B.; Diep, D.B. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 2015, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Megahed, A.; Zeineldin, M.; Evans, K.; Maradiaga, N.; Blair, B.; Aldridge, B.; Lowe, J. Impacts of environmental complexity on respiratory and gut microbiome community structure and diversity in growing pigs. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Y.; Yang, Z.; Li, C.; Liang, H.; Wu, Z.; Pu, W. Yeast probiotics shape the gut microbiome and improve the health of early-weaned piglets. Front. Microbiol. 2018, 9, 2011. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Ganzle, M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Young, W.; Moon, C.D.; Thomas, D.G.; Cave, N.J.; Bermingham, E.N. Pre- and post-weaning diet alters the faecal metagenome in the cat with differences in vitamin and carbohydrate metabolism gene abundances. Sci. Rep. 2016, 6, 34668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Thompson, R.; Budinich, M.F.; Broadbent, J.R.; Steele, J.L. Genome sequence and comparative genome analysis of Lactobacillus casei: Insights into their niche-associated evolution. Genome Biol. Evol. 2009, 1, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Pieper, R.; Rieger, J.; Vahjen, W.; Davin, R.; Plendl, J.; Meyer, W.; Zentek, J. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS ONE 2014, 9, e91091. [Google Scholar] [CrossRef] [Green Version]
- Peng, P.; Chen, J.; Yao, K.; Yin, Y.; Long, L.; Fang, R. The effects of dietary supplementation with porous zinc oxide on growth performance, intestinal microbiota, morphology, and permeability in weaned piglets. Anim. Sci. J. 2019, 90, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, Y.; Wang, Z.; Zhou, A.; He, M.; Mao, L.; Zou, H.; Peng, Q.; Xue, B.; Lv, Y. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br. J. Nutr. 2014, 111, 2123–2134. [Google Scholar] [CrossRef]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoell, D.L.; Liu, M.J. Impact of zinc metabolism on innate immune function in the setting of sepsis. Int. J. Vitam. Nutr. Res. 2010, 80, 271. [Google Scholar] [CrossRef]
- Rothkötter, H.J.; Sowa, E.; Pabst, R. The pig as a model of developmental immunology. Hum. Exp. Toxicol. 2002, 21, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Van Heugten, E.; Spears, J.W.; Kegley, E.B.; Ward, J.D.; Qureshi, M.A. Effects of organic forms of zinc on growth performance, tissue zinc distribution, and immune response of weanling pigs. J. Anim. Sci. 2003, 81, 2063–2071. [Google Scholar] [CrossRef] [Green Version]
- El Hendy, H.A.; Yousef, M.I.; El-Naga, N.I.A. Effect of dietary zinc deficiency on hematological and biochemical parameters and concentrations of zinc, copper, and iron in growing rats. Toxicology 2001, 167, 163–170. [Google Scholar] [CrossRef]
- Campo, C.A.; Wellinghausen, N.; Faber, C.; Fischer, A.; Rink, L. Zinc inhibits the mixed lymphoyte culture. Biol. Trace Elem. Res. 2001, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Rink, L. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Z.; Xu, Z.R.; Lin, W.X.; Huang, H.Q.; Wang, Z.Q. Developmental gene expression of antimicrobial peptide PR-39 and effect of zinc oxide on gene regulation of PR-39 in piglets. Asian-Australas. J. Anim. Sci. 2004, 17, 1635–1640. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc signals and immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.L.; Medina, E.A.; Hubbard, N.E. Micronutrients and innate immunity. J. Infect. Dis. 2000, 182, S5–S10. [Google Scholar] [CrossRef]
- Talukder, P.; Satho, T.; Irie, K.; Sharmin, T.; Hamady, D.; Nakashima, Y.; Kashige, N.; Miake, F. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int. Immunopharmacol. 2011, 11, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2017; p. 840. [Google Scholar]
- Dardenne, M. Zinc and immune function. Eur. J. Clin. Nutr. 2002, 56, S20–S23. [Google Scholar] [CrossRef] [Green Version]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2021, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.A.; Perdue, N.R.; Killebrew, J.R.; Urdahl, K.B.; Campbell, D.J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009, 10, 595. [Google Scholar] [CrossRef]
- O’Connor, R.A.; Floess, S.; Huehn, J.; Jones, S.A.; Anderton, S.M. F oxp3+ T reg cells in the inflamed CNS are insensitive to IL-6-driven IL-17 production. Eur. J. Immunol. 2012, 42, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Senda, T.; Dogra, P.; Granot, T.; Furuhashi, K.; Snyder, M.E.; Carpenter, D.J.; Szabo, P.A.; Thapa, P.; Miron, M.; Farber, D.L. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. 2019, 12, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef]
- Sargeant, H.R.; McDowall, K.J.; Miller, H.M.; Shaw, M.A. Dietary zinc oxide affects the expression of genes associated with inflammation: Transcriptome analysis in piglets challenged with ETEC K88. Vet. Immunol. Immunopathol. 2010, 137, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Masters, A.R.; Fortner, K.A.; Champagne, D.P.; Yanguas-Casás, N.; Silberger, D.J.; Weaver, C.T.; Haynes, L.; Rincon, M. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21–producing B helper CD8+ T cells. J. Exp. Med. 2016, 213, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef] [Green Version]
- El Ayadi, A.; Herndon, D.N.; Finnerty, C.C. Biomarkers in burn patient care. In Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 232–235. [Google Scholar]








| Items | Ingredient, % |
|---|---|
| Corn | 34.43 |
| Extruded corn | 15.00 |
| Lactose | 10.00 |
| Dehulled soybean meal, 51% CP 1 | 13.50 |
| Soy protein concentrate, 65% CP 1 | 10.00 |
| Plasma powder | 6.00 |
| Whey | 5.00 |
| Soy oil | 2.20 |
| Monocalcium phosphate | 1.26 |
| Limestone | 1.40 |
| L-Lysine-HCl, 78% | 0.06 |
| DL-Methionine, 50% | 0.15 |
| Choline chloride, 25% | 0.10 |
| Vitamin premix 2 | 0.25 |
| Trace mineral premix 3 | 0.25 |
| Salt | 0.40 |
| Total | 100.00 |
| Calculated value 4 | |
| ME, kcal/kg | 3433 |
| CP, % | 20.76 |
| Lysine, % | 1.35 |
| Metionine, % | 0.39 |
| Ca | 0.82 |
| P | 0.65 |
| Primer | Sequence | Size (bp) |
|---|---|---|
| GAPDH | Forward: ACATCATCCCTGCTTCTACCGG | 126 |
| Reverse: CTCGGACGCCTGCTTCAC | ||
| TBX21 | Forward: TGGACTGAGATCACCCCCAT | 103 |
| Reverse: TGTCCCCACTGGAGGGATAG | ||
| GATA3 | Forward: TCTAGCAAATCCAAAAAGTGCAAA | 74 |
| Reverse: GGGTTGAACGAGCTGCTCTT | ||
| RORC | Forward: TTCAGTACGTGGTGGAGTTC | 141 |
| Reverse: TGTGGTTGTCAGCGTTGTAG | ||
| FoxP3 | Forward: CGCATGTTCGCCTTCTTCA | 68 |
| Reverse: AGGCTCAAGTTGTGGCGAAT | ||
| IL-1α | Forward: GCAGTGGAGAAGCCGATGAAG | 142 |
| Reverse: GCACGTTGGCATCACAGACA | ||
| IL-2 | Forward: ACAGTTGCTTTTGAAGGAAGTTAAGAA | 86 |
| Reverse: CCTGCTTGGGCATGTAAAATTT | ||
| IL-4 | Forward: CCAACCCTGGTCTGCTTACTG | 119 |
| Reverse: TCCTTCTCCGTCGTGTTCTCT | ||
| IL-6 | Forward: ACAAAGCCACCACCCCTAAC | 185 |
| Reverse: CGTGGACGGCATCAATCTCA | ||
| IL-8 | Forward: TGGACCCCAAGGAAAAGTGG | 132 |
| Reverse: TGCAGCAGCAGCTGGAAATTTAT | ||
| IL-10 | Forward: TGAGAACAGCTGCATCCACTTC | 104 |
| Reverse: TCTGGTCCTTCGTTTGAAAGAAA | ||
| IL-12β | Forward: TCAGGGACATCATCAAACCA | 141 |
| Reverse: GAACACCAAACATCAGGGAAA | ||
| IL-17A | Forward: CGGCTGGAGAAAGTGATGGT | 138 |
| Reverse: GAAATGGGGCTGGGTCTACTC | ||
| IL-22 | Forward: CTGGGAGCCCTTTCCTTCTG | 143 |
| Reverse: GTTGGTGATGTAGGGCTGCT | ||
| IFNα | Forward: CCATTCAAAGGAGCATGGAT | 146 |
| Reverse: GAGTTCACTGATGGCTTTGC | ||
| ZO-1 | Forward: AAGGATGTTTACCGTCGCATT | 253 |
| Reverse: ATTGGACACTGGCTAACTGCT | ||
| OCLN | Forward: CAGGTGCACCCTCCAGATTG | 167 |
| Reverse: ATGTCGTTGCTGGGTGCATA | ||
| Claudin 4 | Forward: CAACTGCGTGGATGATGAGA | 140 |
| Reverse: CCAGGGGATTGTAGAAGTCG | ||
| pBD1 | Forward: CCTGGAAGCAGGAGGTCAAA | 194 |
| Reverse: AAGGGCTATGGATTGTGCGG | ||
| Reg3α | Forward: CCACCGAGGGCTTGGAA | 70 |
| Reverse: GCAACGTAATTGAGCACATCAGA |
| Zn, Status and Level | CON | ZnO | Chelate -ZnO | Nano -ZnO | SE | p-Value |
|---|---|---|---|---|---|---|
| Items | 0 | 2500 | 200 | 200 | Trt | |
| Initial BW, kg | 6.4 | 6.4 | 6.4 | 6.4 | 0.2 | 0.997 |
| 1 week BW, kg | 7.5 | 7.4 | 7.4 | 7.4 | 0.2 | 0.978 |
| Final BW, kg | 8.9 | 8.9 | 8.8 | 8.8 | 0.2 | 0.988 |
| 0 to 7 days | ||||||
| ADG, g | 153.6 | 142.9 | 139.3 | 142.9 | 20.2 | 0.958 |
| ADFI, g | 229.6 | 227.1 | 245.2 | 234.0 | 6.6 | 0.253 |
| G:F, g/g | 0.672 | 0.632 | 0.568 | 0.602 | 0.083 | 0.827 |
| Diarrhea score 1,x | 1.545 b | 2.454 a | 2.250 a | 2.242 a | 0.078 | 0.001 |
| 7 to 14 days | ||||||
| ADG, g | 201.4 a | 210.7 | 204.3 | 204.8 | 11.0 | 0.937 |
| ADFI, g | 297.5 | 297.1 | 300.0 | 300.0 | 1.7 | 0.492 |
| G:F, g/g | 0.677 | 0.710 | 0.681 | 0.683 | 0.038 | 0.918 |
| Diarrhea score 1,x,y,z,w | 1.958 c | 2.417 a | 1.833 c | 2.222 b | 0.052 | 0.001 |
| Overall period, 0 to 14 days | ||||||
| ADG, g | 177.5 | 176.8 | 171.8 | 173.8 | 12.0 | 0.984 |
| ADFI, g | 263.6 | 262.1 | 272.6 | 267.0 | 3.1 | 0.126 |
| G:F, g/g | 0.674 | 0.674 | 0.630 | 0.649 | 0.044 | 0.852 |
| Diarrhea score 1,x,y | 1.758 c | 2.435 a | 2.033 bc | 2.232 ab | 0.076 | 0.001 |
| Zn, Status, and Level | CON | ZnO | Chelate -ZnO | Nano -ZnO | SE | p-Value |
|---|---|---|---|---|---|---|
| Items | 0 | 2500 | 200 | 200 | trt | |
| One week intake | ||||||
| Feed intake, g | 232.5 | 242.5 | 250.0 | 250.0 | 9.5 | 0.557 |
| Dry matter, g | 211.8 | 220.4 | 225.5 | 228.0 | 8.6 | 0.611 |
| Crude protein, g | 48.3 | 50.3 | 52.3 | 52.1 | 2.0 | 0.487 |
| Energy, kcal | 914.2 | 971.9 | 1016.4 | 989.0 | 36.9 | 0.309 |
| Excretion | ||||||
| Fresh fecal | 220.0 | 207.5 | 210.0 | 213.3 | 10.6 | 0.852 |
| Dry matter, g | 34.2 | 30.3 | 32.6 | 30.8 | 1.3 | 0.225 |
| Crude protein, g | 9.5 | 9.0 | 9.4 | 9.4 | 0.5 | 0.919 |
| Energy, kcal | 184.7 | 161.1 | 176.6 | 163.4 | 9.8 | 0.349 |
| One week ATTD, % | ||||||
| Dry matter x | 83.8 b | 86.2 a | 85.5 a | 86.5 a | 0.5 | 0.014 |
| Crude protein | 80.3 | 82.0 | 82.1 | 81.9 | 0.8 | 0.333 |
| Gross energy x | 79.8 b | 83.4 a | 82.6 a | 83.5 a | 0.7 | 0.013 |
| Two weeks intake | ||||||
| Feed intake, g | 300.0 | 300.0 | 300.0 | 300.0 | 0.0 | 1.000 |
| Dry matter, g | 273.3 | 272.7 | 270.6 | 273.6 | 0.2 | 0.977 |
| Crude protein, g | 62.3 | 62.3 | 62.3 | 62.3 | 0.1 | 0.995 |
| Energy, kcal | 1177.5 | 1203.3 | 1223.1 | 1186.8 | 7.6 | 0.632 |
| Excretion | ||||||
| Fresh fecal, g | 266.5 | 282.5 | 263.8 | 258.3 | 8.0 | 0.254 |
| Dry matter, g | 42.8 | 40.2 | 42.2 | 41.0 | 1.1 | 0.361 |
| Crude protein, g | 12.6 | 11.2 | 12.3 | 11.5 | 0.4 | 0.096 |
| Energy, kcal y | 237.5 ab | 209.1 b | 247.1 a | 219.5 ab | 8.4 | 0.035 |
| Two week ATTD, % | ||||||
| Dry matter | 84.3 | 85.3 | 84.4 | 85.0 | 0.4 | 0.337 |
| Crude protein | 79.7 | 82.1 | 80.3 | 81.6 | 0.7 | 0.113 |
| Gross energy y | 79.8 b | 82.6 a | 79.8 b | 81.5 ab | 0.7 | 0.032 |
| Zn, Status, and Level | CON | ZnO | Chelate -ZnO | Nano -ZnO | SE | p-Value |
|---|---|---|---|---|---|---|
| Items | 0 | 2500 | 200 | 200 | trt | |
| One week | ||||||
| Feed intake, g | 232.5 | 242.5 | 250.0 | 250.0 | 9.5 | 0.557 |
| Zinc intake, mg x,y,z | 23.3 c | 524.6 a | 89.0 b | 87.3 b | 8.0 | 0.001 |
| Zinc excretion, mg x,y,z,w | 21.3 d | 461.6 a | 36.8 c | 69.5 b | 4.3 | 0.001 |
| ATTD of Zinc, % x,y,z,w | 8.3 c | 11.9 c | 58.4 a | 20.4 b | 2.6 | 0.001 |
| Two week | ||||||
| Feed intake, g | 300.0 | 300.0 | 300.0 | 300.0 | 1.7 | 1.000 |
| Zinc intake, mg x,y,z | 30.0 c | 663.58 a | 106.8 b | 107.6 b | 2.8 | 0.001 |
| Zinc excretion, mg x,y,z,w | 28.1 d | 612.1 a | 55.2 c | 76.8 b | 6.1 | 0.001 |
| ATTD of Zinc, % x,y,z,w | 6.4 c | 7.8 c | 48.4 a | 28.7 b | 1.9 | 0.001 |
| AID of Zinc, % x,y,z,w | 5.0 c | 5.3 c | 39.2 a | 23.0 b | 0.6 | 0.001 |
| Zn, Status, and Level | CON | ZnO | Chelate -ZnO | Nano -ZnO | SE | p-Value |
|---|---|---|---|---|---|---|
| Items | 0 | 2500 | 200 | 200 | trt | |
| WBC, 103/μL | 27.5 | 20.1 | 23.5 | 23.0 | 2.4 | 0.253 |
| RBC, 106/μL | 7.9 | 7.8 | 8.2 | 7.9 | 0.3 | 0.806 |
| Lymphocyte, % | 73.4 | 62.9 | 62.2 | 61.5 | 3.7 | 0.160 |
| Monocyte, % | 0.5 | 0.6 | 2.15 | 0.6 | 0.4 | 0.461 |
| Neutrophil, % | 23.7 | 24.7 | 31.0 | 37.6 | 3.0 | 0.518 |
| IgG, mg/dL | 189.0 | 229.0 | 191.0 | 219.0 | 13.0 | 0.128 |
| IgM, mg/dL | 19.3 | 20.0 | 19.3 | 23.0 | 2.6 | 0.663 |
| Zinc, ug/dL y,z | 164.9 b | 235.5 a | 165.3 b | 175.8 b | 9.6 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.-J.; Park, Y.-J.; Cho, J.H.; Song, M.-H.; Gu, B.-H.; Yun, W.; Lee, J.-H.; An, J.-S.; Kim, Y.-J.; Lee, J.-S.; et al. Changes in Diarrhea Score, Nutrient Digestibility, Zinc Utilization, Intestinal Immune Profiles, and Fecal Microbiome in Weaned Piglets by Different Forms of Zinc. Animals 2021, 11, 1356. https://doi.org/10.3390/ani11051356
Oh H-J, Park Y-J, Cho JH, Song M-H, Gu B-H, Yun W, Lee J-H, An J-S, Kim Y-J, Lee J-S, et al. Changes in Diarrhea Score, Nutrient Digestibility, Zinc Utilization, Intestinal Immune Profiles, and Fecal Microbiome in Weaned Piglets by Different Forms of Zinc. Animals. 2021; 11(5):1356. https://doi.org/10.3390/ani11051356
Chicago/Turabian StyleOh, Han-Jin, Yei-Ju Park, Jae Hyoung Cho, Min-Ho Song, Bon-Hee Gu, Won Yun, Ji-Hwan Lee, Ji-Seon An, Yong-Ju Kim, Jun-Soeng Lee, and et al. 2021. "Changes in Diarrhea Score, Nutrient Digestibility, Zinc Utilization, Intestinal Immune Profiles, and Fecal Microbiome in Weaned Piglets by Different Forms of Zinc" Animals 11, no. 5: 1356. https://doi.org/10.3390/ani11051356
APA StyleOh, H.-J., Park, Y.-J., Cho, J. H., Song, M.-H., Gu, B.-H., Yun, W., Lee, J.-H., An, J.-S., Kim, Y.-J., Lee, J.-S., Kim, S., Kim, H., Kim, E. S., Lee, B.-K., Kim, B.-W., Kim, H. B., Cho, J.-H., & Kim, M.-H. (2021). Changes in Diarrhea Score, Nutrient Digestibility, Zinc Utilization, Intestinal Immune Profiles, and Fecal Microbiome in Weaned Piglets by Different Forms of Zinc. Animals, 11(5), 1356. https://doi.org/10.3390/ani11051356





