Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head—Preliminary Results
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection
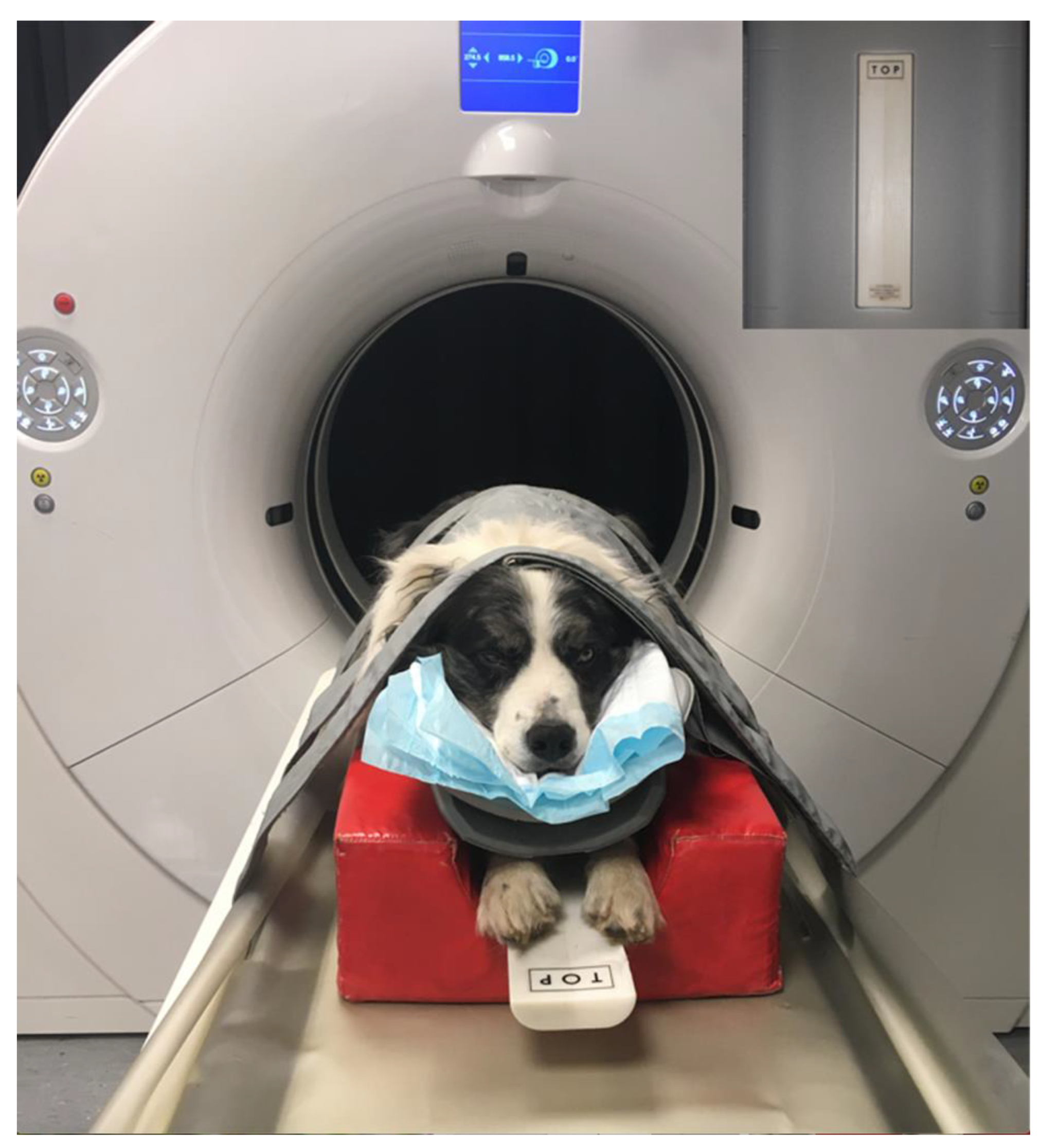
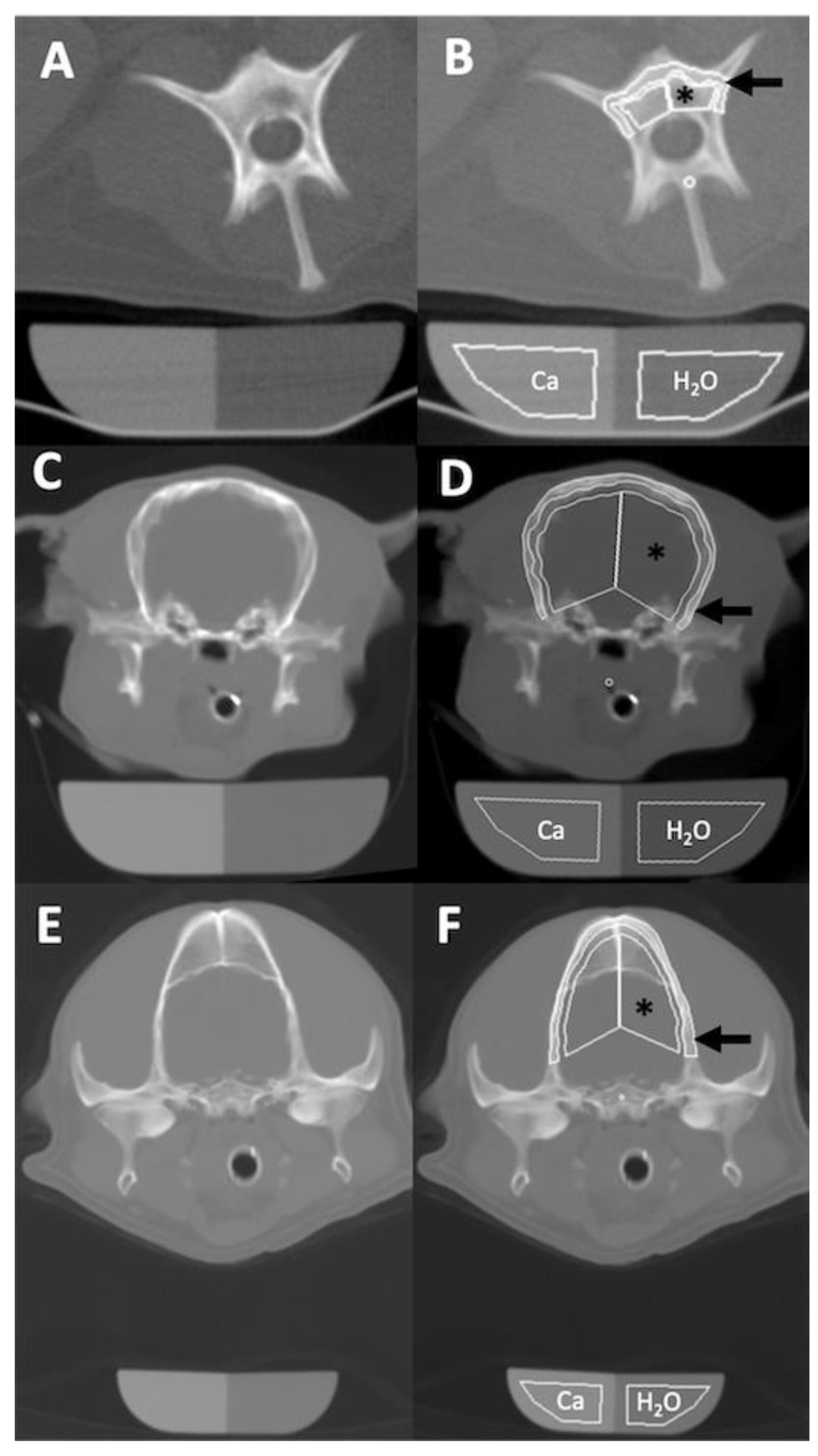
2.2. Quantitative CT Examination
2.3. Statistical Analysis
3. Results
3.1. Population Data
3.2. Osteodensitometry Procedure
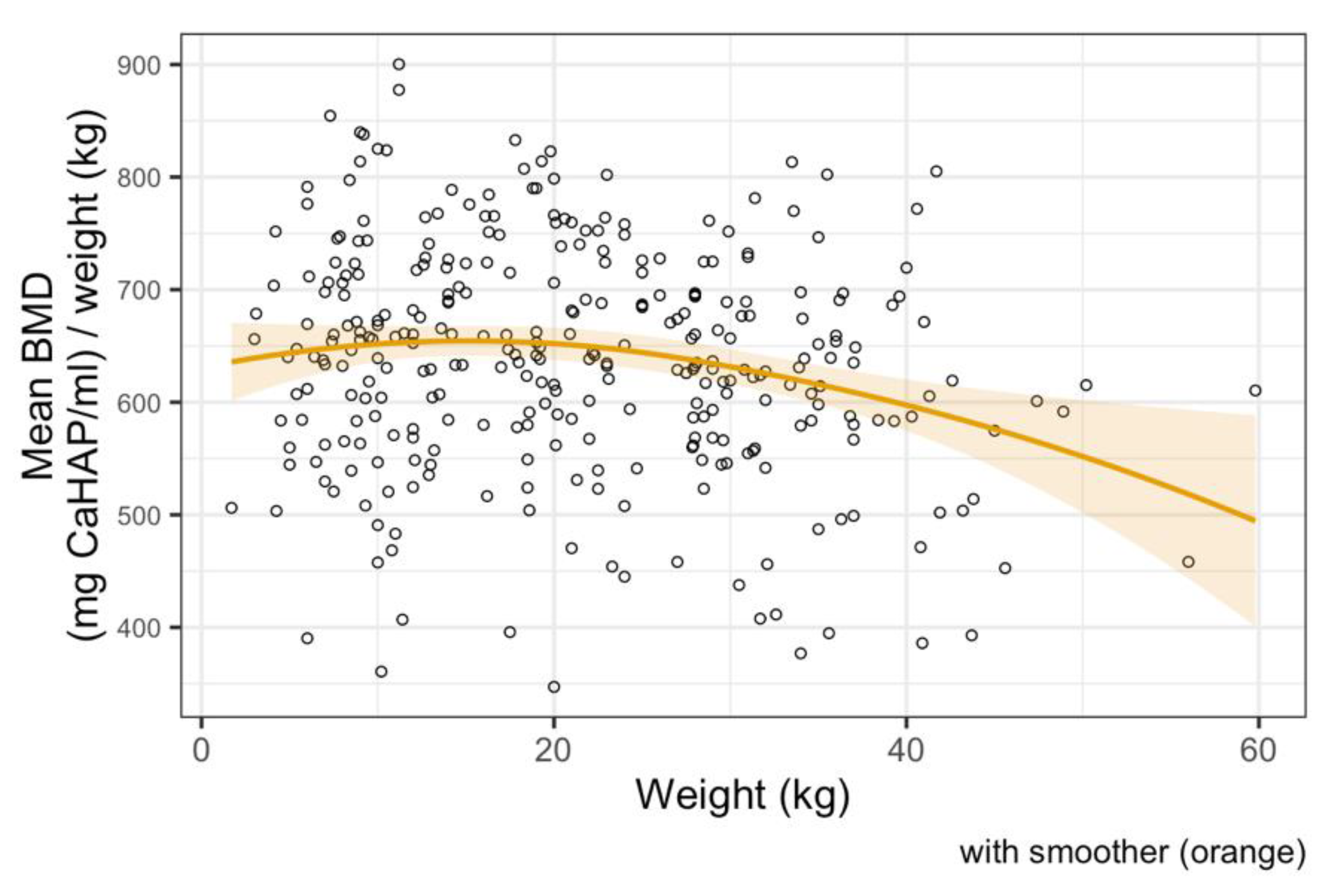
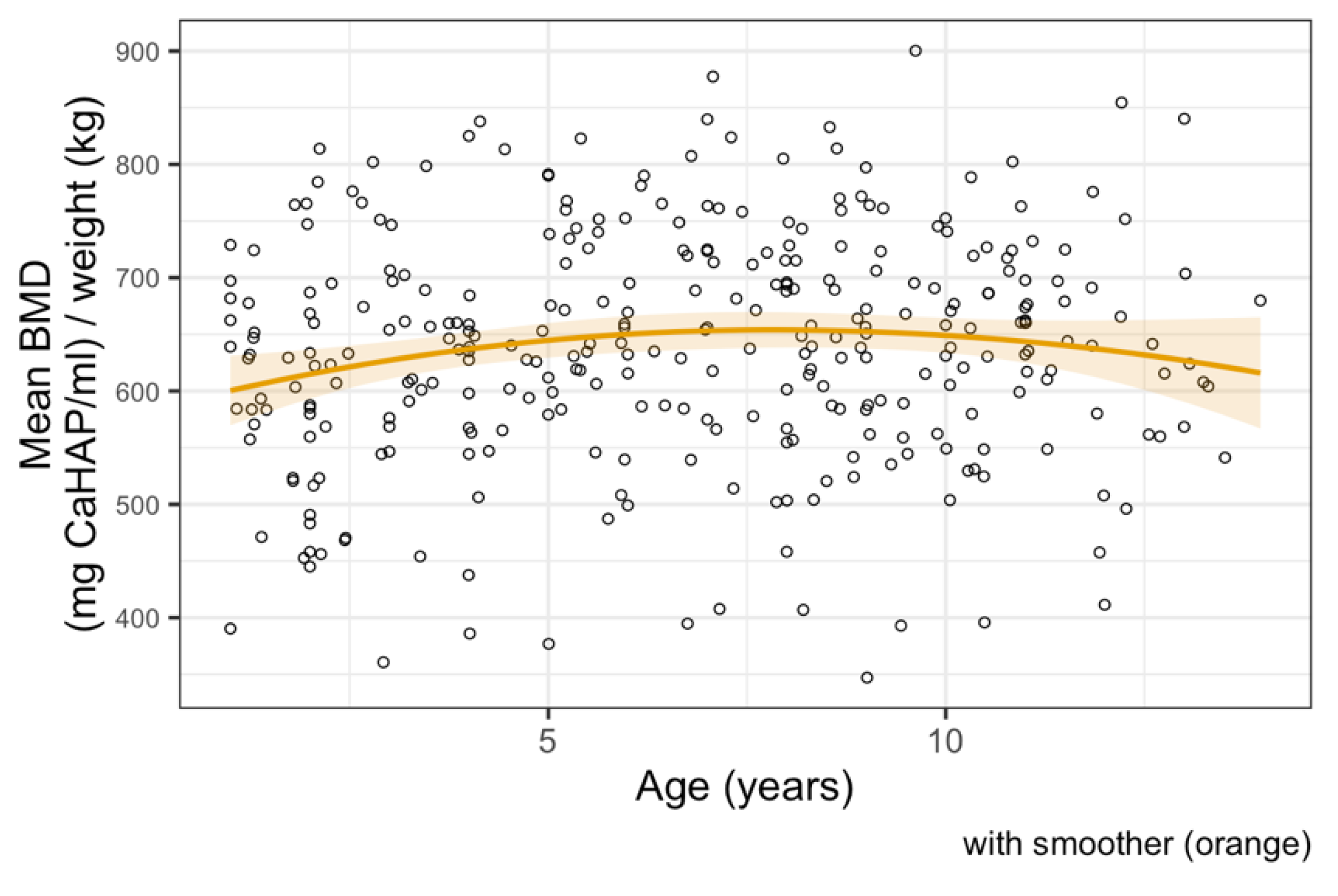
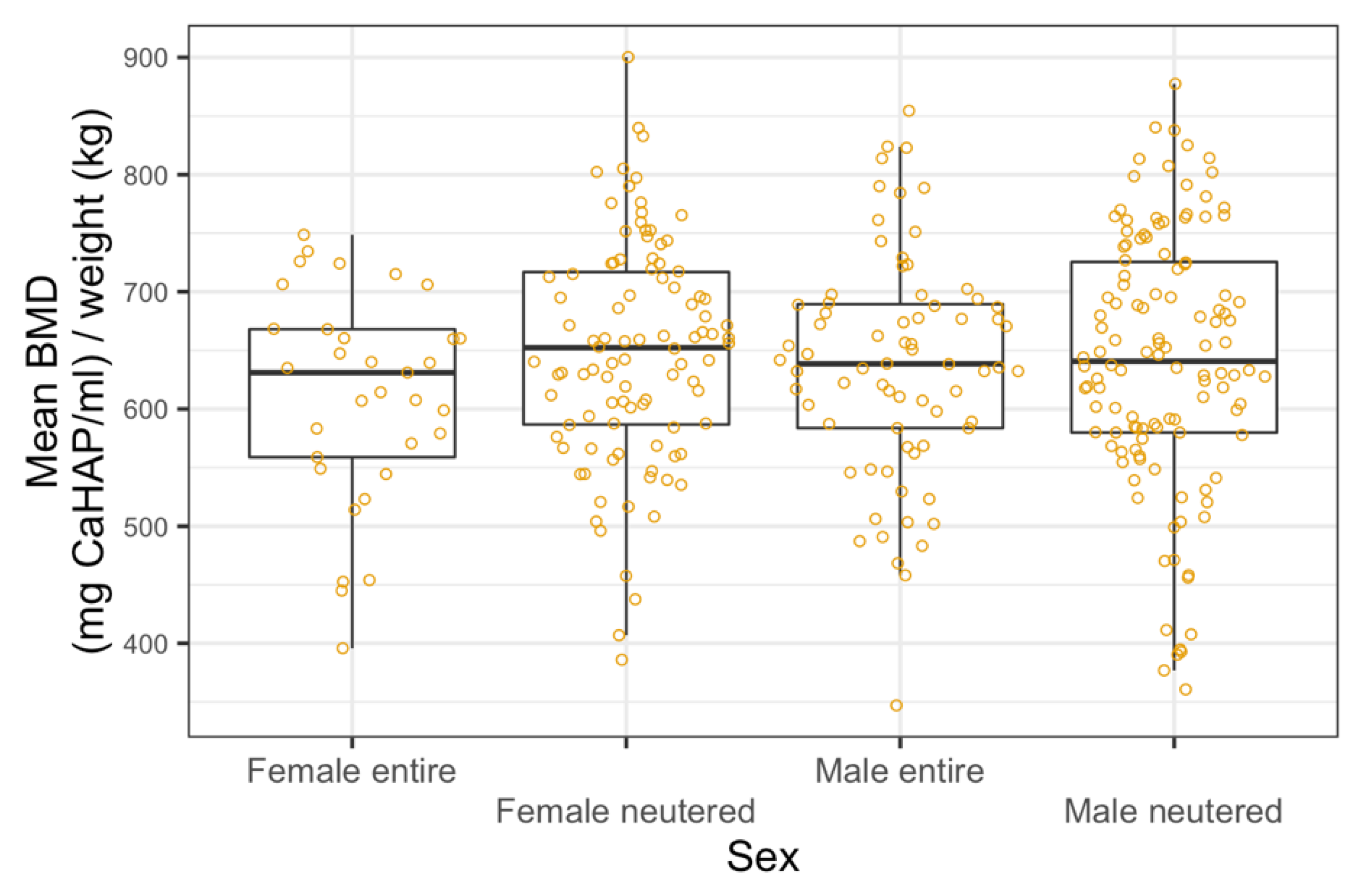
3.3. Bone Mineral Density Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nielsen, S.W.; McSherry, B.J. Renal Hyperparathyroidism (Rubber Jaw Syndrome) in a Dog. J. Am. Vet. Med. Assoc. 1954, 124, 270–274. [Google Scholar]
- Hall, G.; Breheny, C.; Khan, Z.; Schwarz, T.; Mellanby, R.J. Severe Nutritional Deficiencies and Osteopenia in a Dog Fed a Homemade Raw Diet. Vet. Rec. Case Rep. 2020, 8, e001038. [Google Scholar] [CrossRef]
- Shipov, A.; Shahar, R.; Sugar, N.; Segev, G. The Influence of Chronic Kidney Disease on the Structural and Mechanical Properties of Canine Bone. J. Vet. Intern. Med. 2018, 32, 280–287. [Google Scholar] [CrossRef]
- Saitta, J.; Ott, S.; Sherrard, D.; Walden, C.; Lipkin, E. Metabolic Bone Disease in Adults Receiving Long-Term Parenteral Nutrition: Longitudinal Study with Regional Densitometry and Bone Biopsy. J. Parenter. Enter. Nutr. 1993, 17, 214–219. [Google Scholar] [CrossRef]
- Högberg, U.; Andersson, J.; Högberg, G.; Thiblin, I. Metabolic Bone Disease Risk Factors Strongly Contributing to Long Bone and Rib Fractures during Early Infancy: A Population Register Study. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Lauten, S.; Cox, N.; Brawner, W.; Baker, H. Use of Dual Energy X-ray Absorptiometry for Noninvasive Body Composition Measurements in Clinically Normal Dogs. Am. J. Vet. Res. 2001, 62. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.; Nolte, I.; Galindo-Zamora, V.; Lerch, M.; Stukenborg-Colsman, C.; Behrens, B.A.; Bouguecha, A.; Betancur, S.; Almohallami, A.; Wefstaedt, P. Comparative Measurements of Bone Mineral Density and Bone Contrast Values in Canine Femora Using Dual-Energy X-ray Absorptiometry and Conventional Digital Radiography. BMC Vet. Res. 2017, 13, 130. [Google Scholar] [CrossRef]
- Rezaeifar, P.; Fattahi, I.; Khoshbaten, M.; Ahmadzadeh, M.; Shirazi, K.; Somi, M. Bone Density and Bone Metabolism in Patients with Inflammatory Bowel Disease. Saudi J. Gastroenterol. 2012, 18, 241. [Google Scholar] [CrossRef]
- Wu, T.; Song, H.Y.; Ji, G. Abnormal Bone Metabolism in Crohn’s Disease. Front. Biosci. 2012, 17, 2675–2683. [Google Scholar]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-Energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Clin. Densitom. 2017, 20, 334–345. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Hsu, W.K. Use of Computed Tomography for Assessing Bone Mineral Density. Neurosurg. Focus 2014, 37, E4. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J. Diagnostic Radiology of the Dog and Cat; Pedersen, D., Ed.; WB Saunders: Philadelphia, PA, USA, 1987; p. 312. [Google Scholar]
- Schwarz, T.; Störk, C.; Mellor, D.; Sullivan, M. Osteopenia and Other Radiographic Signs in Canine Hyperadrenocorticism. J. Small Anim. Pract. 2000, 41. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J.; Lee, H.; Kim, B.; Han, H.; Oh, H.; Kim, M.; Yoon, H.; Lee, B.; Eom, K. Quantitative Computed Tomographic Evaluation of Bone Mineral Density in Beagle Dogs: Comparison with Dual-Energy x-Ray Absorptiometry as a Gold Standard. J. Vet. Med. Sci. 2018, 80, 620–628. [Google Scholar] [CrossRef]
- Ziemlewicz, T.J.; Maciejewski, A.; Binkley, N.; Brett, A.D.; Brown, J.K.; Pickhardt, P.J. Opportunistic Quantitative CT Bone Mineral Density Measurement at the Proximal Femur Using Routine Contrast-Enhanced Scans: Direct Comparison with DXA in 355 Adults. J. Bone Miner. Res. 2016, 31, 1835–1840. [Google Scholar] [CrossRef]
- Cheon, H.; Choi, W.; Lee, Y.; Lee, D.; Kim, J.; Kang, J.-H.; Na, K.; Chang, J.; Chang, D. Assessment of Trabecular Bone Mineral Density Using Quantitative Computed Tomography in Normal Cats. J. Vet. Med. Sci. 2012, 74, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D. Nutrition and Bone Disease in the Dog and Cat. Vet. Rec. 1976, 98, 313–321. [Google Scholar] [CrossRef]
- Bandeira, F.; Cusano, N.E.; Silva, B.C.; Cassibba, S.; Almeida, C.B.; Machado, V.C.C.; Bilezikian, J.P. Bone Disease in Primary Hyperparathyroidism. Arq. Bras. Endocrinol. Metabol. 2014, 58, 553. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, F.; Griz, L.; Chaves, N.; Carvalho, N.; Borges, L.; Lazaretti-Castro, M.; Borba, V.; Castro, L.; Borges, J.L.; Bilezikian, J. Diagnosis and Management of Primary Hyperparathyroidism—A Scientific Statement from the Department of Bone Metabolism, the Brazilian Society for Endocrinology and Metabolism. Arq. Bras. Endocrinol. Metabol. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Drüeke, T.; Lameire, N.; Eknoyan, G. Chronic Kidney Disease–Mineral-Bone Disorder: A New Paradigm. Adv. Chronic Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef]
- Christodoulou, S.; Goula, T.; Ververidis, A.; Drosos, G. Vitamin D and Bone Disease. Biomed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Kyle, M.G.; Davis, G.B.; Thompson, K.G. Renal Osteodystrophy with Facial Hyperostosis and ‘rubber Jaw’ in an Adult Dog. N. Z. Vet. J. 1985, 33, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. A Language and Environment for Statistical Computing. Available online: https://www.r-project.org (accessed on 8 October 2020).
- Turner, A.S.; Maillet, J.M.; Mallinckrodt, C.; Cordain, L. Bone Mineral Density of the Skull in Premenopausal Women. Calcif. Tissue Int. 1997, 61, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Rico, H.; Revilla, M.; Hernandez, E.R.; Villa, L.F.; del Buergo, M. Sex Differences in the Acquisition of Total Bone Mineral Mass Peak Assessed through Dual-Energy X-ray Absorptiometry. Calcif. Tissue Int. 1992, 51, 251–254. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Hasserius, R.; Nilsson, J.A.; Obrant, K.J. European Journal of Experimental Musculoskeletal Research; Scandinavian University Press: Oslo, Norway, 1992; Volume 4. [Google Scholar]
- Brismar, T.; Ringertz, J. Effect of Bone Density of the Head on Total Body DEXA Measurements in 100 Healthy Swedish Women. Acta Radiol. 1996, 37. [Google Scholar] [CrossRef] [PubMed]
- Glüer, C.C.; Steiger, P.; Selvidge, R.; Elliesen-Kliefoth, K.; Hayashi, C.; Genant, H.K. Comparative Assessment of Dual-Photon Absorptiometry and Dual-Energy Radiography. Radiology 1990, 174, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mazess, R.; Chesnut, C.H.; McClung, M.; Genant, H. Enhanced Precision with Dual-Energy x-Ray Absorptiometry. Calcif. Tissue Int. 1992, 51, 14–17. [Google Scholar] [CrossRef]
- Nuti, R.; Martini, G. Measurements of Bone Mineral Density by DXA Total Body Absorptiometry in Different Skeletal Sites in Postmenopausal Osteoporosis. Bone 1992, 13, 173–178. [Google Scholar] [CrossRef]
- Sartoris, D.J.; Resnick, D. Innovative Approaches to Noninvasive Bone Densitometry. Crit. Rev. Diagn. Imaging 1990, 30, 19–39. [Google Scholar]
- Lorinson, K.; Loebcke, S.; Skalicky, M.; Grampp, S.; Lorinson, D. Signalment Differences in Bone Mineral Content and Bone Mineral Density in Canine Appendicular Bones. A Cadaveric Study. Vet. Comp. Orthop. Traumatol. 2008, 21, 147–151. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARgamma Insufficiency Enhances Osteogenesis through Osteoblast Formation from Bone Marrow Progenitors. J. Clin. Investig. 2004, 113. [Google Scholar] [CrossRef]
- Palermo, A.; Tuccinardi, D.; Defeudis, G.; Watanabe, M.; D’Onofrio, L.; Pantano, A.L.; Napoli, N.; Pozzilli, P.; Manfrini, S. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int. J. Environ. Res. Public Health 2016, 13, 544. [Google Scholar] [CrossRef]
- Ostlere, S.J.; Gold, R.H. Osteoporosis and Bone Density Measurement Methods. Clin. Orthop. Relat. Res. 1991, 271, 149–163. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Nguyen, N.D.; Nguyen, T.V. Quantification of the Relative Contribution of Estrogen to Bone Mineral Density in Men and Women. BMC Musculoskelet. Disord. 2013, 14, 366. [Google Scholar] [CrossRef]
- Salmeri, K.R.; Bloomberg, M.S.; Scruggs, S.L.; Shille, V. Gonadectomy in Immature Dogs: Effects on Skeletal, Physical, and Behavioral Development. J. Am. Vet. Med. Assoc. 1991, 198, 1193–1203. [Google Scholar]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone Mechanical Properties and Changes with Osteoporosis. Injury 2016, 47 (Suppl. 2), S11–S20. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.; Choi, W.; Chang, J.; Kang, J.-H.; Na, K.-J.; Chang, D.-W. Quantitative CT Assessment of Bone Mineral Density in Dogs with Hyperadrenocorticism. J. Vet. Sci. 2015, 16, 531. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.A.; Dempster, D.W.; Baron, R. Anatomy and Ultrastructure of Bone—Histogenesis, Growth and Remodeling; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ahmed, L.A.; Shigdel, R.; Joakimsen, R.M.; Eldevik, O.P.; Eriksen, E.F.; Ghasem-Zadeh, A.; Bala, Y.; Zebaze, R.; Seeman, E.; Bjørnerem, Å. Measurement of Cortical Porosity of the Proximal Femur Improves Identification of Women with Nonvertebral Fragility Fractures. Osteoporos. Int. 2015, 26, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Lee, L.J.; del Rio, A.M.; Lauder, T.; Bruce, R.J.; Summers, R.M.; Pooler, B.D.; Binkley, N. Simultaneous Screening for Osteoporosis at CT Colonography: Bone Mineral Density Assessment Using MDCT Attenuation Techniques Compared with the DXA Reference Standard. J. Bone Miner. Res. 2011, 26, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Watts, N.B. Fundamentals and Pitfalls of Bone Densitometry Using Dual-Energy X-ray Absorptiometry (DXA). Osteoporos. Int. 2004, 15, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.; Behrens, B.-A.; Nolte, I.; Galindo-Zamora, V.; Betancur, S.; Almohallami, A.; Bouguecha, A.; Mostafa, A.; Lerch, M.; Stukenborg-Colsman, C.; et al. Comparative Investigation of Bone Mineral Density Using CT and DEXA in a Canine Femoral Model. J. Orthop. Res. 2017, 35, 2667–2672. [Google Scholar] [CrossRef]
- Armstrong, J.; da Costa, R.C.; Martin-Vaquero, P. Cervical Vertebral Trabecular Bone Mineral Density in Great Danes with and without Osseous-Associated Cervical Spondylomyelopathy. J. Vet. Intern. Med. 2014, 28, 1799–1804. [Google Scholar] [CrossRef]
- De Decker, S.; Lam, R.; Packer, R.M.A.; Gielen, I.M.V.L.; Volk, H.A. Thoracic and Lumbar Vertebral Bone Mineral Density Changes in a Natural Occurring Dog Model of Diffuse Idiopathic Skeletal Hyperostosis. PLoS ONE 2015, 10, e0124166. [Google Scholar] [CrossRef][Green Version]
- Zotti, A.; Isola, M.; Sturaro, E.; Menegazzo, L.; Piccinini, P.; Bernardini, D. Vertebral Mineral Density Measured by Dual-Energy X-ray Absorptiometry (DEXA) in a Group of Healthy Italian Boxer Dogs. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2004, 51, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Labruyère, J.; Schwarz, T. CT and MRI in Veterinary Patients: An Update on Recent Advances. Practice 2013, 35, 546–563. [Google Scholar] [CrossRef]
- Kastl, S.; Sommer, T.; Klein, P.; Hohenberger, W.; Engelke, K. Accuracy and Precision of Bone Mineral Density and Bone Mineral Content in Excised Rat Humeri Using Fan Beam Dual-Energy X-ray Absorptiometry. Bone 2002, 30, 243–246. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeong, E.S.; Yang, M.H.; Yang, S.-O. Bone Mineral Density Assessment for Research Purpose Using Dual Energy X-ray Absorptiometry. Osteoporos. Sarcopenia 2018, 4, 79–85. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woods, G.; Israeliantz Gunz, N.; Handel, I.; Liuti, T.; Mellanby, R.J.; Schwarz, T. Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head—Preliminary Results. Animals 2021, 11, 1413. https://doi.org/10.3390/ani11051413
Woods G, Israeliantz Gunz N, Handel I, Liuti T, Mellanby RJ, Schwarz T. Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head—Preliminary Results. Animals. 2021; 11(5):1413. https://doi.org/10.3390/ani11051413
Chicago/Turabian StyleWoods, Glynn, Nicolas Israeliantz Gunz, Ian Handel, Tiziana Liuti, Richard J. Mellanby, and Tobias Schwarz. 2021. "Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head—Preliminary Results" Animals 11, no. 5: 1413. https://doi.org/10.3390/ani11051413
APA StyleWoods, G., Israeliantz Gunz, N., Handel, I., Liuti, T., Mellanby, R. J., & Schwarz, T. (2021). Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head—Preliminary Results. Animals, 11(5), 1413. https://doi.org/10.3390/ani11051413





