Near Infrared Reflectance Spectroscopy Analysis to Predict Diet Composition of a Mountain Ungulate Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
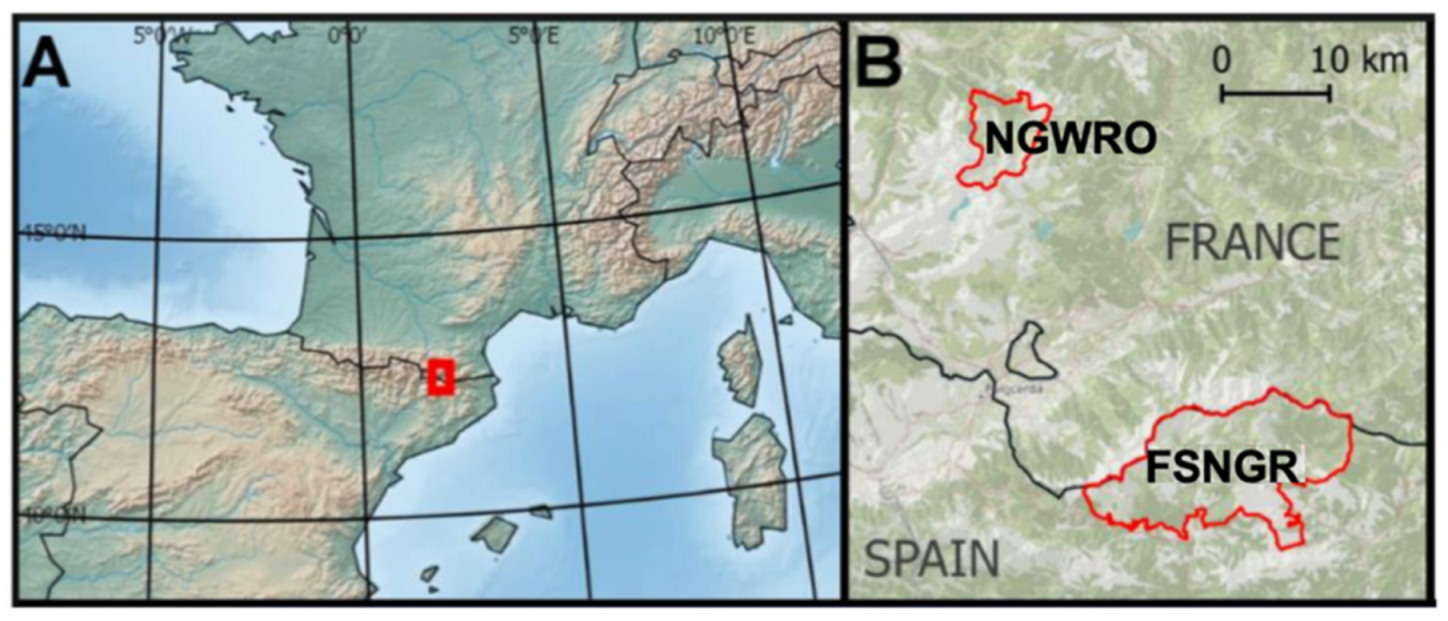
2.1. Sampling Area
2.2. Fecal Sampling Procedure
2.3. Fecal Cuticle Microhistological Analysis
2.4. NIRS Analysis and Spectral Data Analysis
2.5. Relationships between Fecal CMA and NIRS Methods
3. Results and Discussion
3.1. Spectral Characteristics of Samples
3.2. Development of Prediction Models
3.3. Comparison between NIRS Predictions and Microhistologic Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Type | Technique | Pros | Cons | Authors | |
|---|---|---|---|---|---|
| Invasive methods | Rumen content | - Direct sample observation - Good estimation | - Inappropriate for continuous monitoring over time and/or protected species - Some species may be finely masticated and/or highly digested through digestive tract | [68] | |
| Esophageal fistula | [69] | ||||
| Non-invasive methods | Direct grazing animal observation | - Simple - Small material investment - Can determine the species and plant parts that are consumed | - The accuracy strongly depends on the degree of training of the observer - Time consuming | [70,71] | |
| Video recording | [72] | ||||
| Fecal analysis | Cuticle microhistological analysis | - No animal stress infringed - Small material investment | - No quantitative method - Some species are highly digested through digestive tract - Time consuming - Long specialist training period - The accuracy strongly depends on the degree of training of the specialist | [9,10,11,12,13,14] | |
| n-alkane markers (wax components) | - Useful on simple dietary mixtures of up to four components (livestock) | - Not effective enough on complex diets (wild herbivores) - Expensive - Time consuming | [73,74] | ||
| Isotopes | [75] | ||||
| DNA-barcoding | - The most powerful diet assessing method | - Variations in DNA content and different digestibility of several plants limit its accuracy - Long previous work period - Highly expensive | [76] | ||
References
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of global mountain systems to climate warming during the 21st Century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Björk, R.G.; Bjorkman, A.D.; Callaghan, T.V.; Collier, L.S.; Cooper, E.J.; Cornelissen, J.H.C.; Day, T.A.; et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol. Lett. 2012, 15, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yu, J.; Wang, F.; Wang, P.; Zhang, Y.C.; Jin, K. Quantitative contributions of climate change and human activities to vegetation changes over multiple time scales on the Loess Plateau. Sci. Total Environ. 2021, 755, 142419. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Dawson, D.A.; Hipperson, H.; Ishtiaq, F. A diet rich in C3 plants reveals the sensitivity of an alpine mammal to climate change. Mol. Ecol. 2019, 28, 250–265. [Google Scholar] [CrossRef]
- Espunyes, J.; Lurgi, M.; Büntgen, U.; Bartolomé, J.; Calleja, J.A.; Gálvez-Cerón, A.; Peñuelas, J.; Claramunt-López, B.; Serrano, E. Different effects of alpine woody plant expansion on domestic and wild ungulates. Glob. Chang. Biol. 2019, 25, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- López-Olvera, J.R.; Marco, I.; Montané, J.; Lavín, S. Transport stress in Southern chamois (Rupicapra pyrenaica) and its modulation by acepromazine. Vet. J. 2006, 172, 347–355. [Google Scholar] [CrossRef]
- Serrano, E.; Granados, J.E.; Sarasa, M.; González, F.J.; Fandos, P.; Soriguer, R.C.; Pérez, J.M. The effects of winter severity and population density on body stores in the Iberian wild goat (Capra pyrenaica) in a highly seasonal mountain environment. Eur. J. Wildl. Res. 2011, 57, 45–55. [Google Scholar] [CrossRef]
- Serrano, E.; González, F.J.; Granados, J.E.; Moço, G.; Fandos, P.; Soriguer, R.C.; Pérez, J.M. The use of total serum proteins and triglycerides for monitoring body condition in the Iberian wild goat (Capra pyrenaica). J. Zoo Wildl. Med. 2008, 39, 646–649. [Google Scholar] [CrossRef]
- Holechek, J.L.; Vavra, M.; Pieper, R.D. Botanical composition determination of range herbivore diets: A review. J. Range Manag. 1982, 35, 309–315. [Google Scholar] [CrossRef]
- Croker, B.H. A method of estimating the botanical composition of the diet of sheep. N. Z. J. Agric. Res. 1959, 2, 72–85. [Google Scholar] [CrossRef]
- Bartolomé, J.; Plaixats, J.; Piedrafita, J.; Fina, M.; Adrobau, E.; Aixàs, A.; Bonet, M.; Grau, J.; Polo, L. Foraging behavior of Alberes cattle in a Mediterranean forest ecosystem. Rangel. Ecol. Manag. 2011, 64, 319–324. [Google Scholar] [CrossRef]
- Mohammad, A.G.; Pieper, R.D.; Wallace, J.D.; Holechek, J.L.; Murray, L.W. Comparison of fecal analysis and rumen evacuation techniques for sampling diet botanical composition of grazing cattle. J. Range Manag. 1995, 48, 202–205. [Google Scholar] [CrossRef][Green Version]
- Suter, W.; Suter, U.; Krüsi, B.; Schütz, M. Spatial variation of summer diet of red deer Cervus elaphus in the eastern Swiss Alps. Wildlife Biol. 2004, 10, 43–50. [Google Scholar] [CrossRef]
- La Morgia, V.; Bassano, B. Feeding habits, forage selection, and diet overlap in Alpine chamois (Rupicapra rupicapra L.) and domestic sheep. Ecol. Res. 2009, 24, 1043–1050. [Google Scholar] [CrossRef]
- Li, H.; Tolleson, D.R.; Stuth, J.W.; Bai, K.; Mo, F.; Kronberg, S. Faecal near infrared reflectance spectroscopy to predict diet quality for sheep. Small Rumin. Res. 2007, 68, 263–268. [Google Scholar] [CrossRef]
- Lyons, R.K.; Stuth, J.W. Fecal NIRS equations for predicting diet quality of free-ranging cattle. J. Range Manag. 1992, 45, 238–244. [Google Scholar] [CrossRef]
- Gálvez-Cerón, A.; Serrano, E.; Bartolomé, J.; Mentaberre, G.; Fernández-Aguilar, X.; Fernández-Sirera, L.; Navarro-González, N.; Gassó, D.; López-Olvera, J.R.; Lavín, S.; et al. Predicting seasonal and spatial variations in diet quality of Pyrenean chamois (Rupicapra pyrenaica pyrenaica) using near infrared reflectance spectroscopy. Eur. J. Wildl. Res. 2013, 59, 115–121. [Google Scholar] [CrossRef]
- Kamler, J.; Homolka, M.; Čižmár, D. Suitability of NIRS analysis for estimating diet quality of free-living red deer Cervus elaphus and roe deer Capreolus capreolus. Wildlife Biol. 2004, 10, 235–240. [Google Scholar] [CrossRef]
- Showers, S.E.; Tolleson, D.R.; Stuth, J.W.; Kroll, J.C.; Koerth, B.H. Predicting diet quality of white-tailed deer via NIRS fecal profiling. Rangel. Ecol. Manag. 2006, 59, 300–307. [Google Scholar] [CrossRef]
- Alonso, E.; Igarzabal, A.; Oregui, L.M.; Mandaluniz, N. Estimacion del contenido de nitrogeno en heces de rumiantes mediante estimación esteroscopia en el infrarojo cercano (NIRS). In Proceedings of the XLV Reunión Científica de la Sociedad Española Para el Estudio de Los Pastos (SEEP), Oviedo, Spain, 28 May–3 June 2014; pp. 89–96. [Google Scholar]
- Dixon, R.M.; Coates, D.B. Review: Near infrared spectroscopy of faeces to evaluate the nutrition and physiology of herbivores. J. Near Infrared Spectrosc. 2009, 17, 1–31. [Google Scholar] [CrossRef]
- Villamuelas, M.; Serrano, E.; Espunyes, J.; Fernández, N.; López-Olvera, J.R.; Garel, M.; Santos, J.; Parra-Aguado, M.Á.; Ramanzin, M.; Fernández-Aguilar, X.; et al. Predicting herbivore faecal nitrogen using a multispecies near-infrared reflectance spectroscopy calibration. PLoS ONE 2017, 12, e0176635. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, F.; Dong, S.; Zhao, H.; Hou, X.; Wu, X. Spatiotemporal dynamics of grassland aboveground biomass on the Qinghai-Tibet Plateau based on validated MODIS NDVI. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tolleson, D.R.; Stuth, J.W.; Williams, K. Deterimantion of fecal nitrogen and phosphorus in herbivores via near infrared refrectance spectroscopy. In Proceedings of the Society of Range Management Meeting, Salt Lake City, UT, USA, 24–30 January 2004; pp. 24–30. [Google Scholar]
- Coates, D.B.; Dixon, R.M. Development of near infrared analysis of faeces to estimate non-grass proportions in the diet selected by cattle grazing tropical pastures. J. Near Infrared Spectrocopy 2008, 16, 471–480. [Google Scholar] [CrossRef]
- Keli, A.; Andueza, D.; de Vega, A.; Guada, J.A. Validation of the n-alkane and NIRS techniques to estimate intake, digestibility and diet composition in sheep fed mixed lucerne: Ryegrass diets. Livest. Sci. 2008, 119, 42–54. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Carrion, D.; Peña Blanco, F.; Domenech García, V.; Garzón Sigler, A.; Martínez-Marín, A.L. Evaluation of botanical and chemical composition of sheep diet by using faecal near infrared spectroscopy. Anim. Feed Sci. Technol. 2016, 222, 1–6. [Google Scholar] [CrossRef]
- Volesky, J.D.; Coleman, S.W. Estimation of botanical composition of esophageal extrusa samples using near infrared reflectance spectroscopy. J. Range Manag. 1996, 49, 163–166. [Google Scholar] [CrossRef]
- Schiborra, A.; Bulang, M.; Berk, A.; Susenbeth, A.; Schlecht, E. Using faecal near-infrared spectroscopy (FNIRS) to estimate nutrient digestibility and chemical composition of diets and faeces of growing pigs. Anim. Feed Sci. Technol. 2015, 210, 234–242. [Google Scholar] [CrossRef]
- Albanell, E.; Bartolomé, J.; Cristobal, I.; Cassinello, J. Predicción de la Composición Botánica de la Dieta de Herbívoros Silvestres Mediante NIRS; López-Carrasco, C., Rodríguez, M.P., San Miguel, A., Fernández, F., Roig, S., Eds.; Sociedad Española para el Estudio de los Pastos: Toledo, Spain, 2011. [Google Scholar]
- Améztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-use legacies rather than climate change are driving the recent upward shift of the mountain tree line in the Pyrenees. Glob. Ecol. Biogeogr. 2015, 25, 263–273. [Google Scholar] [CrossRef]
- Lasanta, T.; Nadal-Romero, E.; García-Ruíz, J.M. Clearing shrubland as a strategy to encourage extensive livestock farming in the Mediterranean mountains. Geogr. Res. Lett. 2019, 45, 487–513. [Google Scholar] [CrossRef]
- Prévosto, B.; Kuiters, L.; Bernhardt-Römermann, M. Impacts of land abandonment on vegetation: Successional pathways in European habitats. Folia geobot 2011, 46, 303–325. [Google Scholar] [CrossRef]
- Corlatti, L.; Lorenzini, R.; Lovari, S. The conservation of the chamois Rupicapra spp. Mamm. Rev. 2011, 41, 163–174. [Google Scholar] [CrossRef]
- Ninot, J.M.; Carrillo, E.; Font, X.; Carreras, J.; Ferré, A.; Masalles, R.M.; Soriano, I.; Vigo, J. Altitude zonation in the Pyrenees. A geobotanic interpretation. Phytocoenologia 2007, 37, 371–398. [Google Scholar] [CrossRef]
- Vigo, J. L´alta Muntanya Catalana, 2nd ed.; Institut d’Estudis Catalans: Barcelona, Spain, 2008. [Google Scholar]
- Espunyes, J. Effects of global change on the diet of a mountain ungulate: The Pyrenean chamois. Doctoral Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Stewart, D.R.M. Analysis of Plant Epidermis in Faeces: A Technique for Studying the Food Preferences of Grazing Herbivores. J. Appl. Ecol. 1967, 4, 83–111. [Google Scholar] [CrossRef]
- Bartolomé, J.; López-Garrido, O. Atles D’epidermis. Available online: https://ddd.uab.cat/collection/atlepi (accessed on 15 January 2021).
- Williams, P.C.; Sobering, D.C. How do we do it: A brief summary of the methods we use in developing near infrared calibrations. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P.C., Eds.; NIR Publications: Chichester, UK, 1996; pp. 185–188. [Google Scholar]
- Williams, P. The RPD Statistic: A Tutorial Note. NIR News 2014, 25, 22–26. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Zaki, R.; Bulgiba, A.; Ismail, R.; Ismail, N.A. Statistical methods used to test for agreement of medical instruments measuring continuous variables in method comparison Studies: A systematic review. PLoS ONE 2012, 7, e37908. [Google Scholar] [CrossRef]
- Datta, D. blandr: A Bland-Altman Method Comparison package for R 2017. Available online: https://mran.microsoft.com/snapshot/2017-08-06/web/packages/blandr/index.html (accessed on 20 October 2017).
- Team R. R: A Language and Environment for Statistical Computing. Found. Stat. Comput. 2019. Available online: https://cran.microsoft.com/snapshot/2014-09-08/web/packages/dplR/vignettes/xdate-dplR.pdf (accessed on 20 October 2017).
- Shenk, J.S.; Workman, J.J.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of Near-infrared Analysis; CRC Press: Boca Raton, FL, USA, 2001; pp. 347–386. [Google Scholar]
- Cozzolino, D.; La Manna, A.; Vaz Martins, D. Use of near infrared reflectance spectroscopy to analyse bovine faecal samples. J. Near Infrared Spectrosc. 2002, 10, 309–314. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, R.; Cuartas, P. Trophic utilization of a montane/subalpine forest by chamois (Rupicapra pyrenaica) in the Central Pyrenees. For. Ecol. Manage. 1996, 88, 15–23. [Google Scholar] [CrossRef]
- Espunyes, J.; Bartolomé, J.; Garel, M.; Gálvez-Cerón, A.; Aguilar, X.F.; Colom-Cadena, A.; Calleja, J.A.; Gassó, D.; Jarque-Bascuñana, L.; Lavín, S.; et al. Seasonal diet composition of Pyrenean chamois is mainly shaped by primary production waves. PLoS ONE 2019, 14, e0210819. [Google Scholar] [CrossRef]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification, 2nd ed.; NIR Publications: Chichester, UK, 2002; ISBN 978-1-906715-25-0. [Google Scholar]
- Williams, P.C. Implementation of near-infrared technology. In Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2001; pp. 143–167. [Google Scholar]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolaï, B. NIR spectroscopy applications for internal and external quality analysis of citrus fruit-A review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Anthony, R.G.; Smith, N.S. Comparison of rumen and fecal analysis to describe deer diets. J. Wildl. Manage. 1974, 38, 535–540. [Google Scholar] [CrossRef]
- Heggberget, T.M.; Gaare, E.; Ball, J.P. Reindeer (Rangifer tarandus) and climate change: Importance of winter forage. Rangifer 2002, 22, 13–31. [Google Scholar] [CrossRef]
- Marinas, A.; García-González, R. Preliminary data on nutritional value of abundant species in supraforestal pyrenean pastures. Pirineos 2006, 161, 85–109. [Google Scholar] [CrossRef]
- Landau, S.Y.; Glasser, T.; Dvash, L.; Perevolotsky, A. Faecal NIRS to monitor the diet of Mediterranean goats. S. Afr. J. Anim. Sci. 2004, 34, 76–80. [Google Scholar]
- Glasser, T.; Landau, S.Y.; Ungar, E.D.; Perevolotsky, A.; Dvash, L.; Muklada, H.; Kababya, D.; Walker, J.W. A fecal near-infrared reflectance spectroscopy-aided methodology to determine goat dietary composition in a Mediterranean shrubland. J. Anim. Sci. 2008, 86, 1345–1356. [Google Scholar] [CrossRef]
- Walker, J.W.; McCoy, S.D.; Launchbaugh, K.L.; Fraker, M.J.; Powell, J. Calibrating fecal NIRS equations for predicting botanical composition of diets. J. Range Manag. 2002. [Google Scholar] [CrossRef]
- Jean, P.O.; Bradley, R.L.; Giroux, M.A.; Tremblay, J.P.; Côté, S.D. Near infrared spectroscopy and fecal chemistry as predictors of the diet composition of white-tailed deer. Rangel. Ecol. Manag. 2014, 67, 154–159. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to be included in a report on a near infrared spectroscopy project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Bartolomé, J.; Franch, J.; Gutman, M.; Seligman, N.G. Physical factors that influence fecal analysis estimates of herbivore diets. J. Range Manag. 1995, 48, 267–270. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Jung, G.A.; Shenk, J.S.; Abrams, S.M. Estimation of botanical composition in alfalfa/ryegrass mixtures by near infrared spectroscopy. Agron. J. 1990, 82, 669–673. [Google Scholar] [CrossRef]
- Bartolomé, J.; Franch, J.; Plaixats, J.; Seligman, N.G. Diet selection by sheep and goats on Mediterranean heath-woodland range. J. Range Manag. 1998. [Google Scholar] [CrossRef]
- Vavra, M.; Holechek, J.L. Factors influencing microhistological analysis of herbivore diets. J. Range Manag. 1980, 33, 371. [Google Scholar] [CrossRef]
- Leslie, D.M.; Vavra, M.; Starkey, E.E.; Slater, R.C. Correcting for differential digestibility in microhistological analyses involving common coastal forages of the Pacific Northwest. J. Range Manag. 1983, 36, 730. [Google Scholar] [CrossRef]
- Espunyes, J.; Espunya, C.; Chaves, S.; Calleja, J.A.; Bartolomé, J.; Serrano, E. Comparing the accuracy of PCR—Capillary electrophoresis and cuticle microhistological analysis for assessing diet composition in ungulates: A case study with Pyrenean chamois. PLoS ONE 2019, 14, e0216345. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, E.; Roncoroni, C.; Gusmeroli, F.; Marianna, G.D.; Giacometti, G.; Heroldová, M.; Barbieri, S.; Mattiello, S. Feeding ecology of alpine chamois living in sympatry with other ruminant species. Wildlife Biol. 2016, 22, 78–85. [Google Scholar] [CrossRef]
- Vavra, M.; Rice, R.W.; Hansen, R.M. A comparison of esophageal fistula and fecal material to determine steer diets. J. Range Manag. 1978, 31, 11–13. [Google Scholar] [CrossRef]
- Takada, H.; Minami, M. Food habits of the Japanese serow (Capricornis crispus) in an alpine habitat on Mount Asama, central Japan. Mammalia 2019, 83, 455–460. [Google Scholar] [CrossRef]
- Perle, A.; Hamr, J. Food habits of chamois (Rupicapra rupicapra L.) in Northern Tyrol. In The Biology and Management of Mountain Ungulates; Springer: Berlin/Heidelberg, Germany, 1985; pp. 77–84. ISBN 07099-1688-4. [Google Scholar]
- Newmaster, S.G.; Thompson, I.D.; Steeves, R.A.D.; Rodgers, A.R.; Fazekas, A.J.; Maloles, J.R.; McMullin, R.T.; Fryxell, J.M. Examination of two new technologies to assess the diet of woodland caribou: Video recorders attached to collars and DNA barcoding. Can. J. For. Res. 2013, 43, 897–900. [Google Scholar] [CrossRef]
- Andriarimalala, J.H.; Dubeux, J.C.B., Jr.; DiLorenzo, N.; Jaramillo, D.M.; Rakotozandriny, J.D.N.; Salgado, P. Use of n-alkanes to estimate feed intake in ruminants: A meta-analysis. J. Anim. Sci. 2020, 98, 1–10. [Google Scholar] [CrossRef]
- Wright, M.M.; Lewis, E.; Garry, B.; Galvin, N.; Dunshea, F.R.; Hannah, M.C.; Auldist, M.J.; Wales, W.J.; Dillon, P.; Kennedy, E. Evaluation of the n-alkane technique for estimating herbage dry matter intake of dairy cows offered herbage harvested at two different stages of growth in summer and autumn. Anim. Feed Sci. Technol. 2019, 247, 199–209. [Google Scholar] [CrossRef]
- Codron, D.; Lee-Thorp, J.A.; Sponheimer, M.; Codron, J. Stable carbon isotope reconstruction of ungulate diet changes through the seasonal cycle. Afr. J. Wildl. Res. 2007, 37, 117–125. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]

| Calibration Set | Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Range | Mean | SD | n | Range | Mean | SD | |
| Woody | 150 | 0.5–95.0 | 50.42 | 28.17 | 42 | 3.5–87.5 | 46.26 | 26.83 |
| Herbaceous | 150 | 5.0–99.5 | 48.90 | 27.68 | 42 | 12.5–96.5 | 53.11 | 26.55 |
| Graminoids | 150 | 5.0–91.5 | 32.04 | 21.47 | 42 | 10.0–75.0 | 33.39 | 19.71 |
| Fabaceae | 150 | 0.0–70.0 | 22.69 | 15.16 | 42 | 1.5–55.0 | 22.69 | 13.49 |
| Calibration | Cross Validation | External Validation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Math Treatment a | Scatter b Correction | R2CAL | SEC | R2cv | SECV | R2VAL | SEP | Bias | Slope | RPD | RER | |
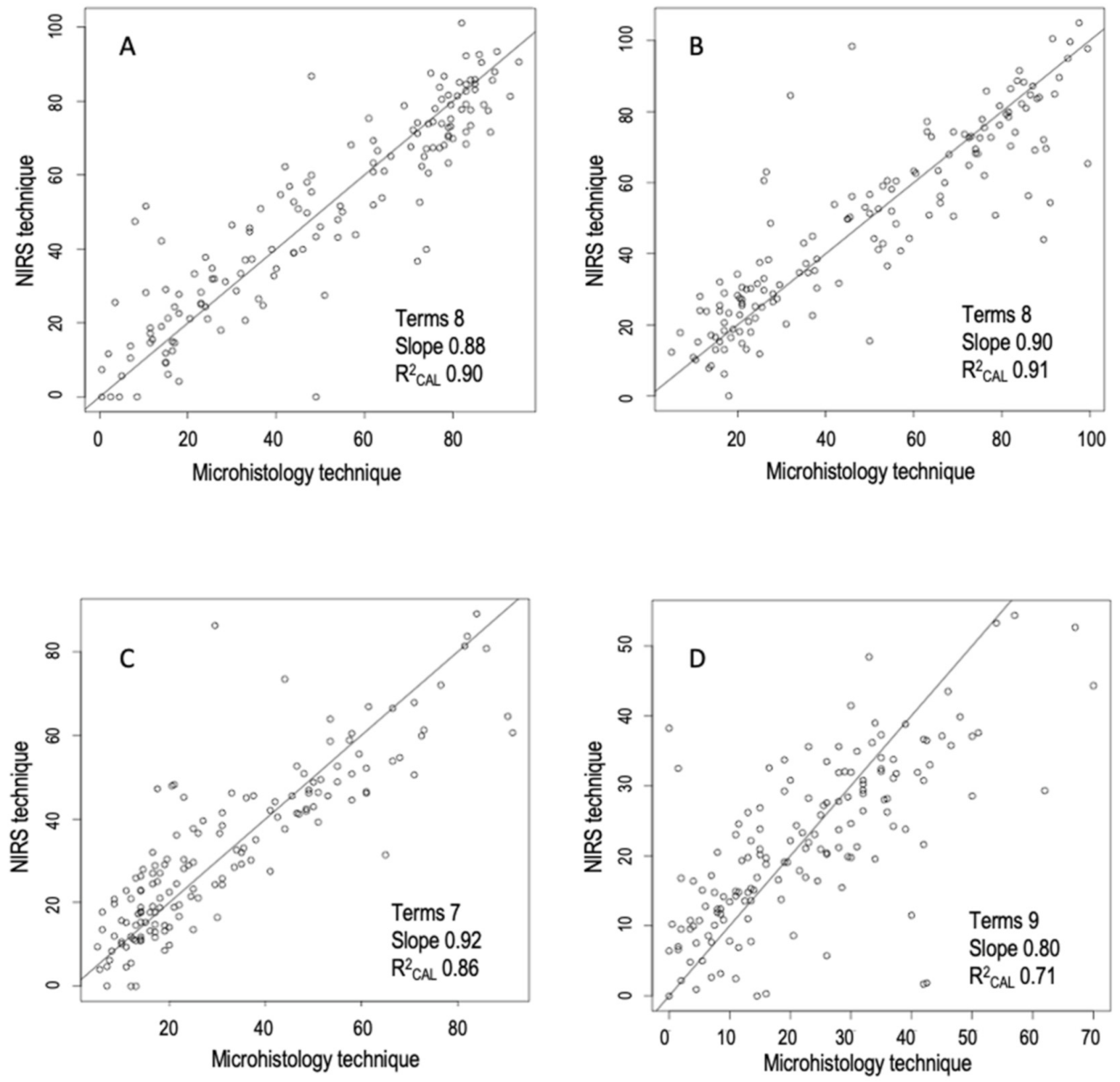
| Woody | 2,5,5,1 | MSC | 0.90 | 9.39 | 0.85 | 11.10 | 0.83 | 11.29 | −0.94 | 0.88 | 2.38 | 7.44 |
| Herbaceous | 2,4,4,1 | none | 0.91 | 8.49 | 0.82 | 11.48 | 0.81 | 11.88 | 2.82 | 0.90 | 2.24 | 7.07 |
| Graminoids | 2,4,4,1 | DT | 0.86 | 7.70 | 0.71 | 11.24 | 0.70 | 11.03 | 0.74 | 0.92 | 1.79 | 5.89 |
| Fabaceae | 1,4,4,1 | none | 0.71 | 7.81 | 0.52 | 9.79 | 0.55 | 9.20 | 1.39 | 0.80 | 1.47 | 5.82 |
| Selected Model | K | AIC | Δi | ωi |
|---|---|---|---|---|
| NIRS method | 3 | 4616.2 | 0.00 | 0.852 |
| NIRS method + Group plant | 6 | 4619.7 | 3.49 | 0.148 |
| NIRS method * Group plant | 9 | 4625.0 | 10.08 | 0.005 |
| Group plant | 5 | 5420.7 | 804.93 | 0.000 |
| Functional Group | Parameter | Mean Value | CI at 95% | |
|---|---|---|---|---|
| Minimum | Maximum | |||
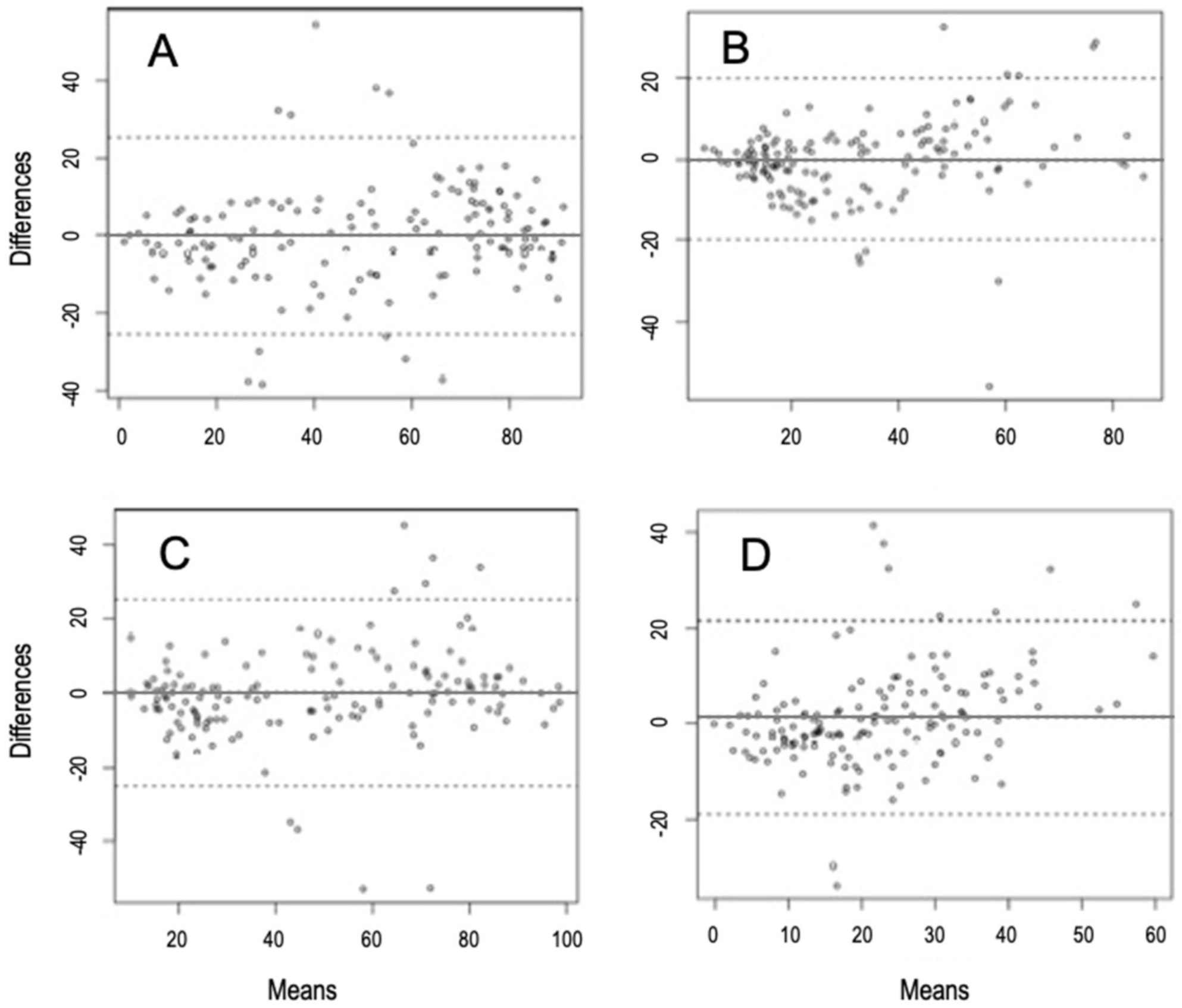
| Woody | Mean differences (bias) | −0.05 | −2.1 | 2.04 |
| ULoA | 25.46 | 21.87 | 29.07 | |
| LLoA | −25.57 | −29.17 | −21.97 | |
| Herbaceous | Mean differences (bias) | 0.14 | −1.91 | 2.21 |
| ULoA | 25.18 | 21.65 | 28.71 | |
| LLoA | −25.01 | −28.42 | −21.36 | |
| Graminoids | Mean differences (bias) | −0.05 | −1.69 | 1.58 |
| ULoA | 19.87 | 17.17 | 22.79 | |
| LLoA | −19.98 | −17.17 | −22.79 | |
| Fabaceae | Mean differences (bias) | 1.28 | −0.37 | 2.94 |
| ULoA | 21.46 | 18.61 | 24.31 | |
| LLoA | −18.88 | −21.721 | −16.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarque-Bascuñana, L.; Bartolomé, J.; Serrano, E.; Espunyes, J.; Garel, M.; Calleja Alarcón, J.A.; López-Olvera, J.R.; Albanell, E. Near Infrared Reflectance Spectroscopy Analysis to Predict Diet Composition of a Mountain Ungulate Species. Animals 2021, 11, 1449. https://doi.org/10.3390/ani11051449
Jarque-Bascuñana L, Bartolomé J, Serrano E, Espunyes J, Garel M, Calleja Alarcón JA, López-Olvera JR, Albanell E. Near Infrared Reflectance Spectroscopy Analysis to Predict Diet Composition of a Mountain Ungulate Species. Animals. 2021; 11(5):1449. https://doi.org/10.3390/ani11051449
Chicago/Turabian StyleJarque-Bascuñana, Laia, Jordi Bartolomé, Emmanuel Serrano, Johan Espunyes, Mathieu Garel, Juan Antonio Calleja Alarcón, Jorge Ramón López-Olvera, and Elena Albanell. 2021. "Near Infrared Reflectance Spectroscopy Analysis to Predict Diet Composition of a Mountain Ungulate Species" Animals 11, no. 5: 1449. https://doi.org/10.3390/ani11051449
APA StyleJarque-Bascuñana, L., Bartolomé, J., Serrano, E., Espunyes, J., Garel, M., Calleja Alarcón, J. A., López-Olvera, J. R., & Albanell, E. (2021). Near Infrared Reflectance Spectroscopy Analysis to Predict Diet Composition of a Mountain Ungulate Species. Animals, 11(5), 1449. https://doi.org/10.3390/ani11051449







