Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Macroscopic Anatomy
2.3. Histological Process
3. Results
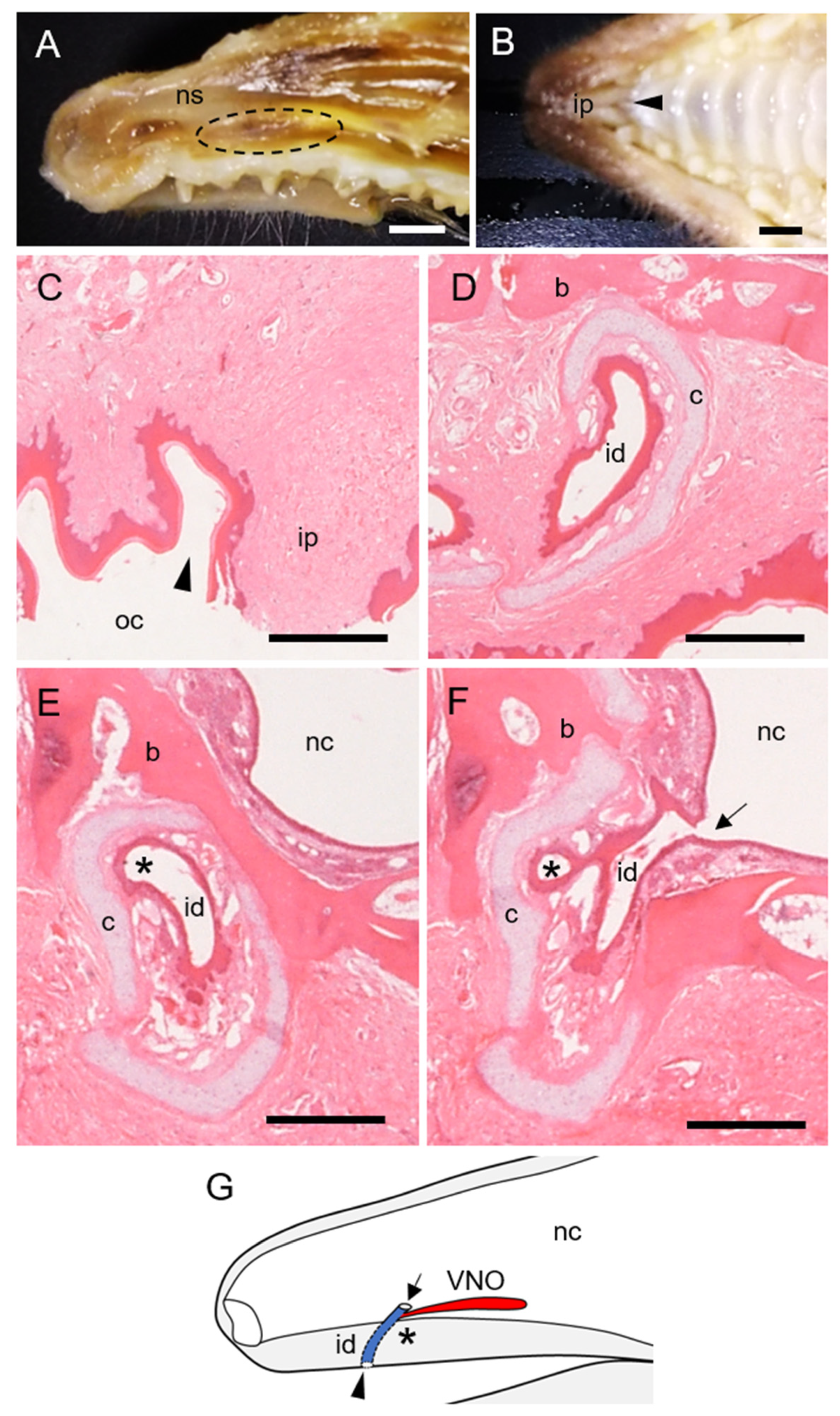
3.1. Morphological Features of Hedgehog VNO
3.2. Cartilage and Soft Tissue Components of Hedgehog VNO
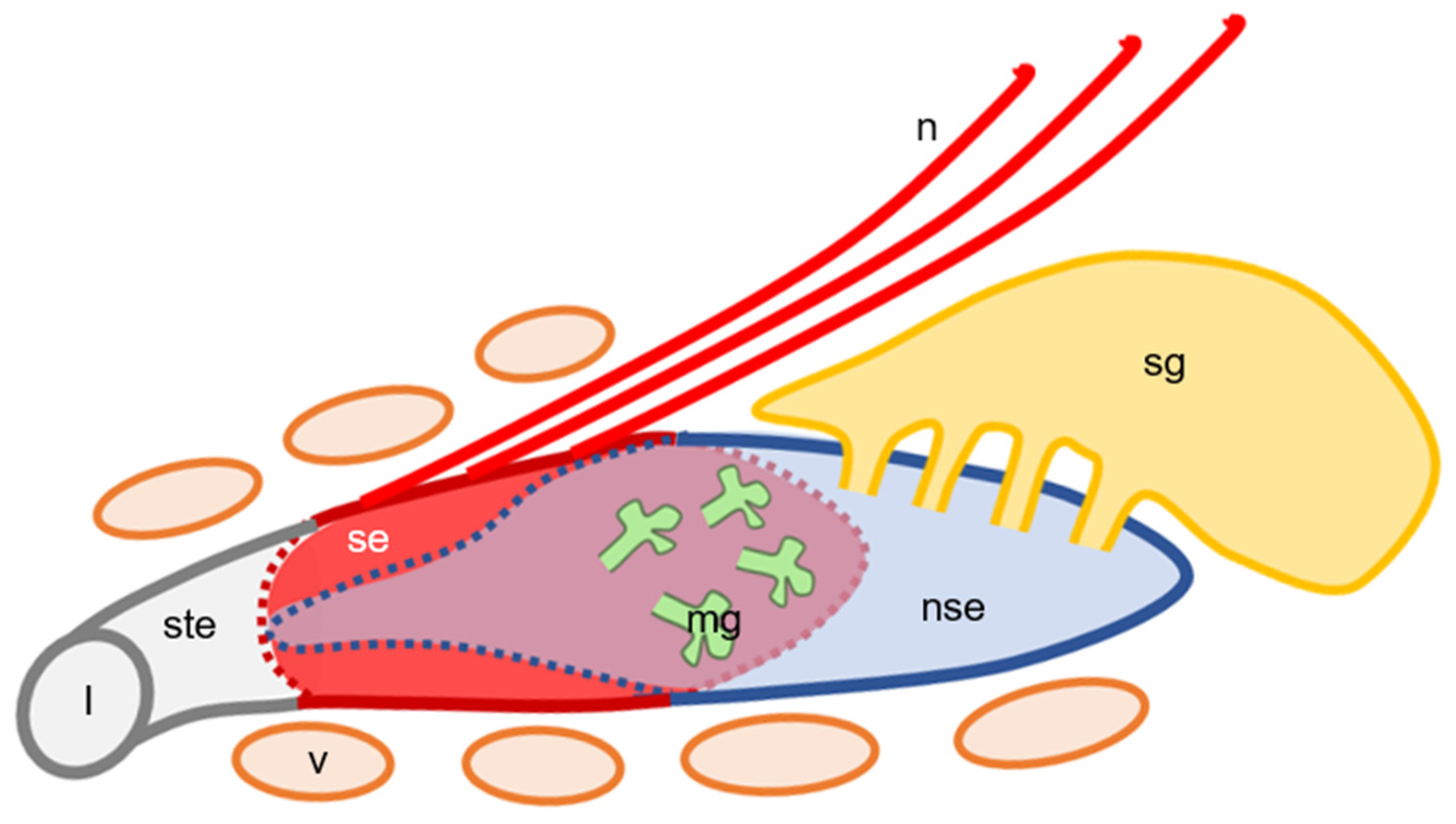
3.3. The Lumen and Epithelial Lining in Hedgehog VNO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fortes-Marco, L.; Lanuza, E.; Martinez-Garcia, F. Of pheromones and kairomones: What receptors mediate innate emotional responses? Anat. Rec. 2013, 296, 1346–1363. [Google Scholar] [CrossRef] [Green Version]
- Allison, A.C. The morphology of the olfactory system in the vertebrates. Biol. Rev. 1953, 28, 195–244. [Google Scholar] [CrossRef]
- Dellmann, H.D. Textbook of Veterinary Histology, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1993; pp. 140–141. [Google Scholar]
- Meredith, M.; O’Connell, R.J. Efferent control of stimulus access to the hamster vomeronasal organ. J. Phygiol. 1979, 286, 301–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, M.; Marques, D.M.; O’Connell, R.O.; Stern, F.L. Vomeronasal pump: Significance for male hamster sexual behavior. Science 1980, 207, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Quinteiro, P.S.; Alemañ, N.; Prieto, D. Anatomical, immunohistochemical and physiological characteristics of the vomeronasal vessels in cows and their possible role in vomeronasal reception. J. Anat. 2008, 212, 686–696. [Google Scholar] [CrossRef]
- Halpern, M. The organization and function of the vomeronasal system. Ann. Rev. Neurosci. 1987, 10, 325–362. [Google Scholar] [CrossRef] [PubMed]
- Upham, N.S.; Esselstyn, J.A.; Jetz, W. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 2019, 17, e3000494. [Google Scholar] [CrossRef] [PubMed]
- Ivey, E.; Carpenter, J.W. African hedgehogs. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Elsevier: St. Louis, MO, USA, 2012; pp. 411–427. [Google Scholar]
- Meisami, E.; Bhatnagar, K.P. Structure and diversity in mammalian accessory olfactory bulb. Microsc. Res. Tech. 1998, 43, 476–499. [Google Scholar] [CrossRef]
- Karimi, H.; Balazadehkoche, F.; Ranjbarsarscanrood, M. Anatomy and histological structure of Jacobson organ in male long-eared hedgehog (Hemiechinus auritus). J. Anim. Res. 2017, 30, 98–109. (In Arabic) [Google Scholar]
- Plank, J.; Rychlo, A. A method for quick decalcification. Zentralbl. Allg. Pathol. 1952, 89, 252–254. [Google Scholar]
- Kondoh, D.; Tomiyasu, J.; Itakura, R.; Sugahara, M.; Yanagawa, M.; Watanabe, K.; Alviola, P.A.; Yap, S.A.; Cosico, E.A.; Cruz, F.A.; et al. Comparative histological studies on properties of polysaccharides secreted by vomeronasal glands of eight Laurasiatheria species. Acta Histochem. 2020, 122, 151515. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.R.; Wiekamp, M.D. The canine vomeronasal organ. J. Anat. 1984, 138, 771–787. [Google Scholar] [PubMed]
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M.; Caballero, T.G. The vomeronasal organ of the cat. J. Anat. 1996, 188, 445–454. [Google Scholar]
- Tomiyasu, J.; Kondoh, D.; Sakamoto, H.; Matsumoto, N.; Sasaki, M.; Kitamura, N.; Haneda, S.; Matsui, M. Morphological and histological features of the vomeronasal organ in the brown bear. J. Anat. 2017, 231, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; López-Beceiro, A.; Sanchez-Quinteiro, P. The vomeronasal organ of wild canids: The fox (Vulpes vulpes) as a model. J. Anat. 2020, 237, 890–906. [Google Scholar] [CrossRef]
- Kratzing, J. The structure of the vomeronasal organ in the sheep. J. Anat. 1971, 108, 247–260. [Google Scholar]
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M. The soft-tissue components of the vomeronasal organ in pigs, cows and horses. Anat. Histol. Embryol. 1997, 26, 179–186. [Google Scholar] [CrossRef]
- Smith, T.D.; Garrett, E.C.; Bhatnagar, K.P.; Bonar, C.J.; Bruening, A.E.; Dennis, J.C.; Kinznger, J.H.; Johnson, E.W.; Morrison, E.E. The vomeronasal organ of New World monkeys (platyrrhini). Anat. Rec. 2011, 294, 2158–2178. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.L.; Hultgren, B. Gross and microscopicanatomy of the vomeronasal organ in the Asian elephant, Elephas maximus. In Chemical Signals in Vertebrates 5; Mcdonald, D., Muller-Schwarze, D., Natynczuk, S.E., Eds.; Oxford University Press: Oxford, UK, 1990; pp. 154–161. [Google Scholar]
- Göbbel, L.; Fischer, M.S.; Smith, T.D.; Wible, J.R.; Bhatnagar, L.P. The vomeronasal organ and associated structures of the fetal African elephant, Loxodonta africana (Proboscidea, Elephantidae). Acta Zool. 2004, 85, 41–52. [Google Scholar] [CrossRef]
- Broom, R. On the organ of Jacobson in the hyrax. J. Anat. Physiol. 1898, 32, 709–713. [Google Scholar]
- Poran, N.S. Vomeronasal organ and its associated structures in the opossum Monodelphis domestica. Microsc. Res. Tech. 1998, 43, 500–510. [Google Scholar] [CrossRef]
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. The vomeronasal organ of the tammar wallaby. J. Anat. 2008, 213, 93–105. [Google Scholar] [CrossRef]
- Vaccarezza, O.L.; Sepich, L.N.; Tramezzani, J.H. The vomeronasal organ of the rat. J. Anat. 1981, 132, 167–185. [Google Scholar] [PubMed]
- Taniguchi, K.; Mochizuki, K. Comparative morphological studies on the vomeronasal organ in rats, mice, and rabbits. Jpn. J. Vet. Sci. 1983, 45, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.S. Morphological studies on the rodent main and accessory olfactory system: The regio olfactoria and vomeronasal organ. Ann. Anat. 1993, 175, 425–446. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Cifuentes, J.M.; Fdz.-de-Troconiz, P.; Sanchez-Quinteiro, P. Morphological and immunohistochemical study of the rabbit vomeronasal organ. J. Anat. 2018, 233, 814–827. [Google Scholar] [CrossRef]
- Carmanchahi, P.D.; Aldana Marcos, H.J.; Ferrari, C.C.; Affanni, J.M. The vomeronasal organ of the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia): Anatomy, histology and ultrastructure. J. Anat. 1999, 195, 587–604. [Google Scholar] [CrossRef]
- Cooper, J.G.; Bhatnagar, K.P. Comparative anatomy of the vomeronasal organ complex in bats. J. Anat. 1976, 122, 571–601. [Google Scholar]
- Oikawa, T.; Shimamura, K.; Saito, T.R.; Taniguchi, K. Fine structure of the vomeronasal organ in the house musk shrew (Suncus murinus). Exp. Anim. 1993, 42, 411–419. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Besoluk, K.; Eken, E.; Boydak, M. The vomeronasal organ in Angora goats (Capra hircus). Vet. Arh. 2001, 71, 11–18. [Google Scholar]
- Park, C.; Ahn, M.; Lee, J.Y.; Lee, S.; Yun, Y.; Lim, Y.; Taniguchi, K.; Shin, T. A morphological study of the vomeronasal organ and the accessory olfactory bulb in the Korean roe deer, Capreolus pygargus. Acta Histochem. 2014, 116, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Nakamura, K.G.; Ono, Y.S.; Yuhara, K.; Bando, G.; Watanabe, K.; Horiuchi, N.; Kobayashi, Y.; Sasaki, M.; Kitamura, N. Histological features of the vomeronasal organ in the giraffe, Giraffa camelopardalis. Microsc. Res. Tech. 2017, 80, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Mochizuki, K. Morphological studies on the vomeronasal organ in the golden hamster. Jpn. J. Vet. Sci. 1982, 44, 419–426. [Google Scholar] [CrossRef]
- Torres, M.V.; Ortiz-Leal, I.; Villamayor, P.R.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. The vomeronasal system of the newborn capybara: A morphological and immunohistochemical study. Sci. Rep. 2020, 10, 13304. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.C.; Stilwell, N.K.; Smith, T.D.; Park, T.J.; Bhatnagar, K.P.; Morrison, E.E. Is the Mole Rat Vomeronasal Organ Functional? Anat. Rec. 2020, 303, 318–329. [Google Scholar] [CrossRef]
- Ciges, M.; Lavella, T.; Gayaso, M.; Sachez, G. Ultrastructure of the organ of Jacobson and comparative study with olfactory mucosa. Acta Otolaryngol. 1977, 83, 47–58. [Google Scholar] [CrossRef]
- Mendoza, A.S. The mouse vomeronasal glands: A light and electron microscopical study. Chem. Senses 1986, 4, 541–555. [Google Scholar] [CrossRef]
- Takigami, S.; Mori, Y.; Tanioka, Y.; Ichikawa, M. Morphological evidence for two types of mammalian vomeronasal system. Chem. Senses 2004, 29, 301–310. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondoh, D.; Tanaka, Y.; Kawai, Y.K.; Mineshige, T.; Watanabe, K.; Kobayashi, Y. Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris). Animals 2021, 11, 1462. https://doi.org/10.3390/ani11051462
Kondoh D, Tanaka Y, Kawai YK, Mineshige T, Watanabe K, Kobayashi Y. Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris). Animals. 2021; 11(5):1462. https://doi.org/10.3390/ani11051462
Chicago/Turabian StyleKondoh, Daisuke, Yusuke Tanaka, Yusuke K. Kawai, Takayuki Mineshige, Kenichi Watanabe, and Yoshiyasu Kobayashi. 2021. "Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris)" Animals 11, no. 5: 1462. https://doi.org/10.3390/ani11051462
APA StyleKondoh, D., Tanaka, Y., Kawai, Y. K., Mineshige, T., Watanabe, K., & Kobayashi, Y. (2021). Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris). Animals, 11(5), 1462. https://doi.org/10.3390/ani11051462





