Some Examples of the Use of Molecular Markers for Needs of Basic Biology and Modern Society
Abstract
:Simple Summary
Abstract
1. Introduction
2. Estimation of Genetic Introgression: Concept, Terms, and Methods
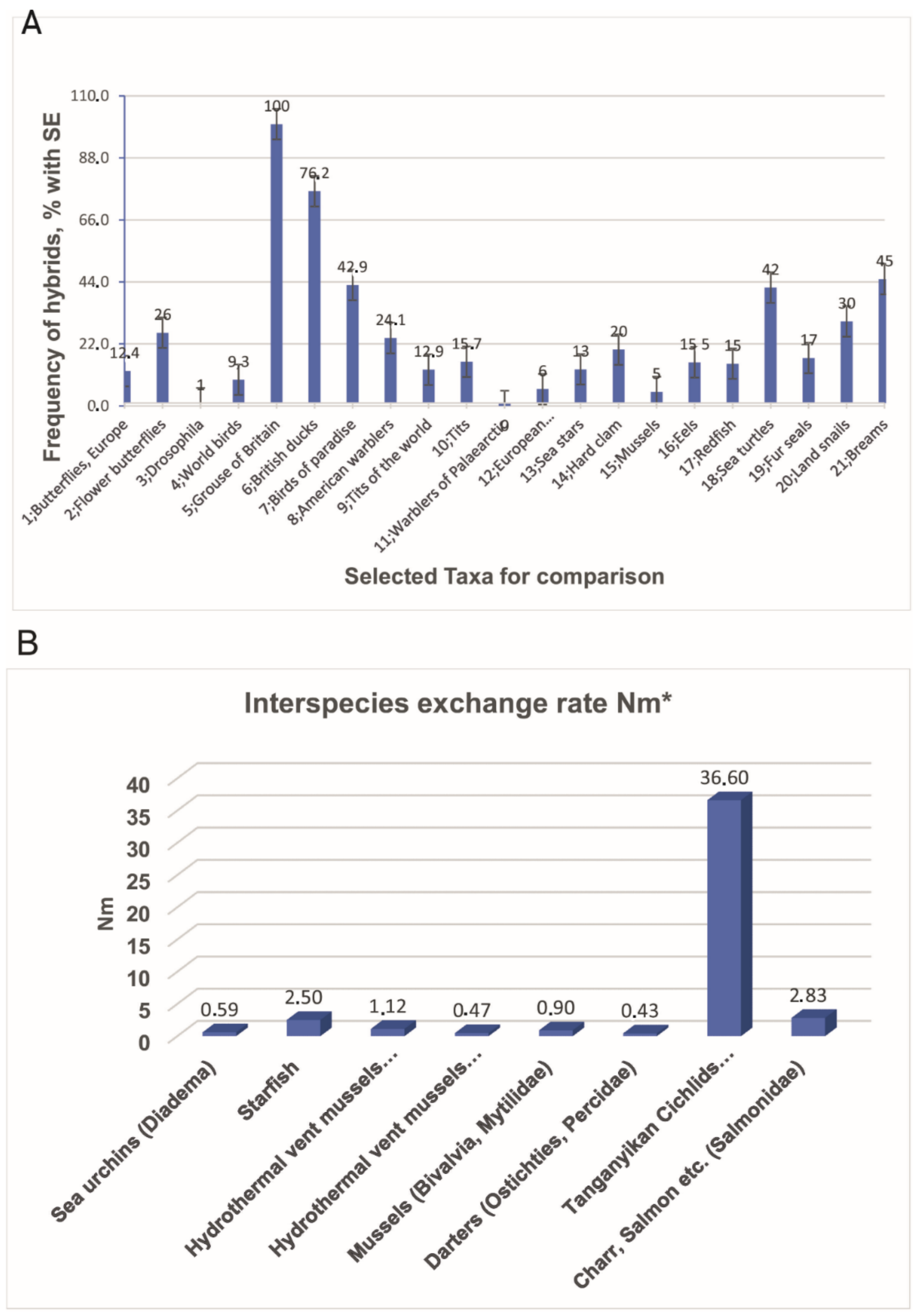
2.1. Notion and Investigation of Hybrids
2.2. Genetic Introgression across Species Boundaries
3. The Topology Mode of Gene Trees, Molecular Diversity between Taxa, and Fit of These Data to the BSC/STE and DNA Barcoding Practice
3.1. Topology of Gene Trees Inferred from Empirical Data
3.2. Congruence between DNA Barcode Data and Conventional Taxonomy Classification
3.3. Molecular Diversity in Taxa of Different Ranks
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F1: F2, Fb | denoted hybrids of first, second generations, and backcrosses, correspondingly |
| MM | biological molecular marker (markers, MMs) |
| iBOL | the international barcode of life project; www.ibol.org (accessed on 7 August 2020) |
| BOLD | barcode of life database; http://boldsystems.org (accessed on 8 July 2015) |
| mtDNA | mitochondrial DNA |
| nDNA | nuclear DNA |
| mitochondrial genome | mitogenome |
| Co-1 (COI, cox-1, etc.) | gene, which encodes subunit 1 of cytochrome c oxidase mtDNA |
| Cyt-b (cytb, etc.) | gene, which encodes cytochrome b mtDNA |
| Bp | base pairs |
| SNPs | single nucleotide substitutions |
References
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, P.D.N.; Ratnasingham, S.; De Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonov, A.S.; Belozzerskii, A.N. Comparative analysis of nucleotide composition of deoxyribonucleic acids of some vertebrates and invertebrates. Dokl. Akad. Nauk SSSR 1961, 138, 1216–1220. [Google Scholar]
- Antonov, A.S.; Miroshnichenko, G.P.; Slyusarenko, A.G. The data on DNA primary structure in the plant systematics. Usp. Sovrem. Biol. 1971, 74, 247–261. [Google Scholar]
- Zuckerkandl, E.; Pauling, L. Molecules as documents of evolutionary history. J. Theor. Biol. 1965, 8, 357–366. [Google Scholar] [CrossRef]
- Hubby, J.L.; Throckmorton, D.H. Protein differences of Drosophila. II. Comparative species genetics and evolutionary problems. Genetics 1965, 52, 203–215. [Google Scholar] [CrossRef]
- Altukhov, Y.P. Populyatsionnaya Genetika Ryb (Fish. Population Genetics); Pishchevaya Promyshlennost: Moscow, Russia, 1974. [Google Scholar]
- Nanney, D.L. Genes and Phenes in Tetrahymena. Bioscience 1982, 32, 783–788. [Google Scholar] [CrossRef]
- Shneyer, V.S.; Rodionov, A.V. Plant DNA Barcodes. Biol. Bull. Rev. 2019, 9, 295–300. [Google Scholar] [CrossRef]
- Zhokhova, E.V.; Rodionov, A.V.; Povydysh, M.N.; Goncharov, M.Y.; Protasova, Y.A.; Yakovlev, G.P. Use of DNA barcoding and DNA fingerprinting for analysis of the quality of medicinal plant materials and medicinal herbal preparations. Biol. Bull. Rev. 2019, 9, 301–314. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P.; Lee, J.-S. Analysis of nucleotide diversity at genes Cyt-b and Co-1 on population, species, and genera levels. Russ. J. Genet. 2006, 42, 341–362, (In Russian, Translated in English). [Google Scholar] [CrossRef]
- Kartavtsev, Y.P. Analysis of sequence diversity at mitochondrial genes on different taxonomic levels. Applicability of DNA based distance data in genetics of speciation and phylogenetics. In Genetic Diversity; Mahoney, C.L., Springer, D.A., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 17–50. ISBN 9781607413042. [Google Scholar]
- Kartavtsev, Y.P. Sequence divergence at mitochondrial genes in animals: Applicability of DNA data in genetics of speciation and molecular phylogenetics. Mar. Genom. 2011, 49, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P. Sequence divergence at Co-1 and Cyt-b mtDNA on different taxonomic levels and genetics of speciation in animals. Mitochondrial DNA 2011, 2, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P. Sequence Diversity at Cyt-b and Co-1 mtDNA Genes in Animal Taxa Proved Neo-Darwinism. J. Phylogenet. Evol. Biol. 2013, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kartavtsev, Y.P. Some current concerns of NeoDarvinism: Gene introgression throughout a species border. J. Phylogenet. Evol. Biol. 2013, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kartavtsev, Y.P. Genetic divergence of species and other taxa. Geographical speciation and the genetic paradigm of Neo-Darwinism in action. Usp. Sovrem. Biol. 2013, 5, 419–451. [Google Scholar]
- Ward, R.D.; Costa, F.O.; Holmes, B.H.; Steinke, D. DNA barcoding of shared fish species from the North Atlantic and Australasia: Minimal divergence for most taxa, but Zeus faber and Lepidopus caudatus each probably constitute two species. Aquat. Biol. 2008, 3, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.D.; Mariani, S. Smoke, mirrors, and mislabeled cod: Poor transparency in the European seafood industry. Front. Ecol. Environ. 2010, 8, 517–521. [Google Scholar] [CrossRef]
- Hanner, R.; Becker, S.; Ivanova, N.V.; Steinke, D. FISH-BOL and seafood identification: Geographically dispersed case studies reveal systemic market substitution across Canada. Mitochondrial DNA 2011, 22, 106–122. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Colmenero, M.; Blanco, O.; Arias, V.; Martinez, J.L.; Garcia-Vazquez, E. DNA authentication of fish products reveals mislabeling associated with seafood processing. Fisheries 2016, 41, 128–138. [Google Scholar] [CrossRef]
- Naaum, A.M.; Hanner, R. Community engagement in seafood identification using DNA barcoding reveals market substitution in Canadian seafood. DNA Barcodes 2015, 3, 74–79. [Google Scholar] [CrossRef]
- Nedunoori, A.; Turanov, S.V.; Kartavtsev, Y.P. Fish product mislabeling identified in the Russian far east using DNA barcoding. Gene Rep. 2017, 8, 144–149. [Google Scholar] [CrossRef]
- Chauhan, T.; Kumar, R. Molecular markers and their applications in fisheries and aquaculture. Adv. Biosci. A Biotechnol. 2010, 1, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Leray, M.; Ho, S.L.; Lin, I.J.; Machida, R.J. MIDORI server: A webserver for taxonomic assignment of unknown metazoan mitochondrial-encoded sequences using a curated database. Bioinformatics 2018, 34, 3753–3754. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P. Barcode index number, taxonomic rank and modes of speciation: Examples from fish. Mitochondrial DNA Part A 2018, 29, 535–542. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P. Sequence divergence provide a fit between molecular evolution, Neo-Darwinism and DNA barcoding. In Proceedings of the HydromediT 2018, 3rd International Congress on Applied Ichthyology & Aquatic Environment, Volos, Greece, 8–11 November 2018; pp. 463–479. [Google Scholar]
- Kartavtsev, Y.P.; Turanov, S.V.; Zolotova, A.O. Molecular markers and their usage in the biodiversity studies as exemplified on fish. In Studies of Marine Organisms in the Far East: Biodiversity, Monitoring, and Rational Management of Resources; Malakhov, V.V., Chernyshov, A.V., Eds.; Far Eastern Univ. Publisher: Vladivostok, Russia, 2020; Chapter 4; pp. 151–239. ISBN 978-5-7444-4757-1. [Google Scholar]
- Stoeckle, M.Y.; Thaler, D.S. Why should mitochondria define species? BioRxiv 2018, 33, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Kartavtsev, Y.P.; Sviridov, V.V.; Sasaki, T.; Hanzawa, N. Genetic divergence of far eastern dace belonging to the genus Tribolodon (Pisces, Cyprinidae) and closely related taxa: Some insights in taxonomy and speciation. Rus. J. Genet. 2002, 38, 1518–1531. [Google Scholar] [CrossRef]
- Arnold, M.L. Evolution through Genetic Exchange; Oxford Univ. Press: New York, NY, USA, 1997; ISBN 0199229031. [Google Scholar]
- Arnold, M.L. Evolution through Genetic Exchange; Oxford Univ. Press: New York, NY, USA, 2009; ISBN 0-19-857006-6. [Google Scholar]
- Arnold, M.L.; Emms, S.K. Paradigm lost: Natural hybridization and evolutionary innovation. In Endless Forms: Species and Speciation; Howard, D.J., Berlocher, S.H., Eds.; Oxford Univ. Press: New York, NY, USA, 1998; pp. 379–389. [Google Scholar]
- Arnold, M.L.; Fogarty, N.D. Reticulate evolution and marine organisms: The final frontier? Int. J. Mol. Sci. 2009, 10, 3836–3860. [Google Scholar] [CrossRef] [PubMed]
- Borkin, L.J.; Litvinchuk, S.N. Animal hybridization, speciation and systematics. Proc. Zool. Inst. 2013, 317 (Suppl. S2), 83–139. [Google Scholar]
- Kartavtsev, Y.P. Molecular Evolution and Population Genetics, Vladivostok: Dal’nevost, 1st ed.; Gos. Univ. (Far Eastern State Univ. Publ.): Vladivostok, Russia, 2005; p. 234, (In Russian). ISBN 5-7444-1680-3. [Google Scholar]
- Kartavtsev, Y.P. Molecular Evolution and Population Genetics, Vladivostok: Dal’nevost, 2nd ed.; Gos. Univ. (Far Eastern State Univ. Publ.): Vladivostok, Russia, 2009; p. 280, (In Russian; Content, Chapter Abstracts, and Headings are in English); ISBN 978-7444-2271-2. [Google Scholar]
- Kartavtsev, Y.P.; Batishcheva, N.M.; Bogutskaya., N.G.; Katugina., A.O.; Hanzawa., N. Molecular Systematics Research, DNA Barcoding of Altai Osmans, Oreoleuciscus (Pisces, Cyprinidae, Leuciscinae), and Nearest Relatives, Inferred from Sequences of Cytochrome b (Cyt-b), Cytochrome Oxidase c (Co-1), and Complete Mitochondrial Genome. Mitochondrial DNA 2017, 28, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Turanov, S.V.; Kartavtsev, Y.P.; Lee, Y.-H.; Jeong, D. Molecular phylogenetic reconstruction and taxonomic investigation of eelpouts (Cottoidei: Zoarcales) based on Co-1 and Cyt-b mitochondrial genes. Mitochondrial DNA Part A 2016. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P.; Redin, A.D. Estimates of Genetic Introgression, Gene Tree Reticulation, Taxon Divergence, and Sustainability of DNA Barcoding Based on Genetic Molecular Markers. Biol. Bull. Rev. 2019, 9, 275–294. [Google Scholar] [CrossRef]
- Lewontin, R.C. The Genetic Basis of Evolutionary Change. Columbia University Press: New York, NY, USA, 1974; p. 13. ISBN 9780231083188. [Google Scholar]
- Bush, G.L. Modes of animal speciation. Ann. Rev. Ecol. Syst. 1975, 6, 339–364. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia Univ. Press: New York, NY, USA, 1987; p. 512. [Google Scholar] [CrossRef]
- Templeton, A.R. Mechanisms of speciation—Population genetic approach. Ann. Rev. Ecol. Syst. 1981, 12, 23–48. [Google Scholar] [CrossRef]
- Templeton, A.R. Species and speciation: Geography, population structure, ecology, and gene trees. In Endless Forms: Species and Speciation; Howard, S.H., Belocher, D.J., Eds.; Oxford University Press on Demand: Oxford, UK, 1998; pp. 32–43. ISBN 0195109007. [Google Scholar]
- Avise, J.C.; Wollenberg, K. Phylogenetics and Origin of Species. Proc. Natl. Acad. Sci. USA 1997, 94, 7748–7755. [Google Scholar] [CrossRef] [Green Version]
- Kondrashov, A.S.; Yampolsky, L.Y.; Shabalina, S.A. On the sympatric origin of species by means of natural selection. In Endless Forms: Species and Speciation; Howarth, D.J., Berlocher, S.H., Eds.; Oxford Univ. Press: Oxford, UK, 1998; pp. 90–98. [Google Scholar]
- Zhuravlev, Y.N.; Avetisov, V.A. The definition of life in the context of its origin. Biogeosciences 2006, 281–291. Available online: www.biogeosciences.net/3/281/2006/ (accessed on 19 May 2021).
- Kartavtsev, Y.P. Molecular Evolution and Population Genetics. In A Course for Marine Biology Students; Johnson, M.S., Ed.; CRS Press, Taylor & Francis Publ. Group: New York, NY, USA, 2015; p. 349. ISBN 978-1-4987-0160-0. [Google Scholar]
- Barton, N.H.; Hewitt, G.M. Analysis of hybrid zones. Ann. Rev. Ecol. Syst. 1985, 16, 113–148. [Google Scholar] [CrossRef]
- Campton, D.E. Natural hybridization and introgression in fishes. Method of detection and genetic interpretation. In Population Genetics & Fishery Management; Ryman, N., Utter, F., Eds.; Washington Sea Grant Publications: London, UK, 1987; pp. 161–192. [Google Scholar]
- Avise, J.C. Phylogeography. The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000; p. 447. ISBN 0-674-66638-0. [Google Scholar]
- Gerber, A.S.; Tibbets, C.A.; Dowling, T.E. The role of introgressive hybridization in the evolution of the Gila robusta complex (Teleostei, Cyprinidae). Evolution 2001, 55, 2028–2039. [Google Scholar] [CrossRef]
- Avise, J. Phylogeography—Retrospect and prospect. J. Biogeogr. 2009, 36, 3–15. [Google Scholar] [CrossRef] [Green Version]
- STATISTICA (Data Analysis Software System). Version 6, StatSoft. 2001. Available online: http://www.statsoft.com (accessed on 8 January 2017).
- Morton, N.E.; Crow, J.F.; Muller, H.J. An Estimate of the Mutational Damage in Man from Data on Consanguineous Marriages. Proc. Natl. Acad. Sci. USA 1956, 42, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Altukhov, Y.P. Genetic Processes in Populations, 2nd ed.; Nauka Publ.: Moscow, Russia, 1989. [Google Scholar]
- Balakirev, E.S.; Romanov, N.S.; Mikheev, P.B.; Ayala, F.J. Mitochondrial DNA variation and introgression in Siberian taimen Hucho taimen. PLoS ONE 2013, 8, e71147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbs, C.L. Hybridization between fish species in nature. Syst. Zool. 1955, 4, 1–20. [Google Scholar] [CrossRef]
- Schwartz, F.J. World literature on to fish hybrids, with an analysis by family, species and hybrid. Publ. Gulf Coast Res. Lab. Amuseum 1972, 3, 1–328. [Google Scholar]
- Schwartz, F.J. World Literature on to Fish Hybrids, with an Analysis by Family, Species and Hybrid: Supplement 1; NOAA Techn. Report NMFS SSRF–750; U.S. Dep. Commerce: Washington, DC, USA, 1981; p. 507. [Google Scholar]
- Altukhov, Y.P.; Salmenkova, E.A.; Omeltchenko, V.T. Salmonid Fishes: Population Biology, Genetics and Management; Blackwell Science: Oxford, UK, 2000; p. 354. [Google Scholar]
- Barley, D.M.; Rana, B.K.; Immink, A.J. The use of inter-specific hybrids in aquaculture and fisheries. Rev. Fish. Biol. Fish. 2001, 10, 325–337. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Kirpichnikov, V.S. Genetic Basis for Fish Breeding; Science Publ.: Leningrad, Russia, 1979; p. 392. [Google Scholar] [CrossRef]
- Podlesnykh, A.V.; Brykov, V.A.; Kukhlevsky, A.D. Unstable linkage of molecular markers with sex determination gene in pacific salmon (Oncorhynchus spp.). J. Hered. 2017, 108, 328–333. [Google Scholar] [CrossRef]
- Avise, J.; Saunders, N.C. Hybridization and introgression among species of sunfish (Lepomis): Analysis by mitochondrial DNA and allozyme markers. Genetics 1984, 108, 237–250. [Google Scholar] [CrossRef]
- Avise, J.C. Cytonuclear genetic signatures of hybridization phenomena: Rationale utility and empirical examples from fishes and other aquatic animals. Rev. Fish. Biol. Fish. 2001, 10, 253–263. [Google Scholar] [CrossRef]
- Hubbs, C.; Kuehne, R.A.; Ball, J.C. The fishes of upper Guadelupe River, Texas. Tex. J. Sci. 1953, 5, 216–244. [Google Scholar]
- Nelson, J.S. Hybridization between two cyprinid fishes, Hybopes plumbea and Rhinichthys cataractae, in Alberta. Can. J. Zool. 1966, 44, 663–968. [Google Scholar] [CrossRef]
- Nelson, J.S. Occurrence of hybrids between longnose sucker (Catastomus catastomus) and white sucker (C. commersoni) in upper Canananaskis Resorvoir, Alberta. J. Fish. Res. Board. Can. 1973, 30, 557–560. [Google Scholar] [CrossRef]
- Stevenson, M.M.; Buchanon, T.M. An analysis of hybridization between the cyprinodont fishes, Cyprinodon variegates and C. elegans. Copeia 1973, 682–692. [Google Scholar] [CrossRef]
- Simon, R.C.; Noble, R.E. Hybridization in Oncorhynchus (Salmonidae). I. Viability and inheritance in artificial crosses of chum and pink salmon. Trans. Amer. Fish. Soc. 1968, 97, 109–118. [Google Scholar] [CrossRef]
- Altukhov, Y.P.; Salmenkova, E.A. The genetic structure of salmon populations. Aquaculture 1991, 98, 11–40. [Google Scholar] [CrossRef]
- Mayr, E. Process of speciation in animals. In Mechanisms of Speciation; Barigozzi, C., Ed.; Alan, R. Liss, Inc.: New York, NY, USA, 1982; pp. 1–20. [Google Scholar]
- Timofeev-Resovsky, A.; Vorontsov, N.N.; Yablokov, A.V. A Short Essay on the Theory of Evolution; Nauka Publ.: Moscow, Russia, 1977; p. 301. [Google Scholar]
- Genovart, M. Natural hybridization and conservation. Biodivers. Conserv. 2008, 18, 1435–1439. [Google Scholar] [CrossRef]
- Seehausen, O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004, 19, 198–207. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, R.B. Hybridization of bird species. Science 1992, 256, 193–197. [Google Scholar] [CrossRef]
- Prager, E.M.; Wilson, A.C. Slow evolutionary loss of the potential for interspecific hybridization in birds: A manifestation of slow regulatory evolution. Proc. Natl. Acad. Sci. USA 1975, 72, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, B.M. Rates of evolution of hybrid unviability in birds and mammals. Evolution 2004, 58, 1865–1870. [Google Scholar] [CrossRef]
- Mallet, J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 2005, 20, 229–237. [Google Scholar] [CrossRef]
- Fong, J.J.; Chen, T.-H. DNA evidence for the hybridization of wild turtles in Taiwan: Possible genetic pollution from trade animals. Conserv. Genet. 2010, 11, 2061–2066. [Google Scholar] [CrossRef] [Green Version]
- Nevado, B.; Fazalova, V.; Backeljau, T.; Hanssens, M.; Verheyen, E. Repeated unidirectional introgression of nuclear and mitochondrial DNA between four congeneric Tanganyikan cichlids. Mol. Biol. Evol. 2011, 28, 2253–2267. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Oohara, I.; Saitoh, K.; Shigenobu, Y.; Hasegawa, N.; Kanamori, M.; Baba, K.; Turon, X.; Bishop, J.D. Molecular and morphological discrimination between an invasive ascidian, Ascidiella aspersa, and its congener A. scabra (Urochordata: Ascidiacea). Zool. Sci. 2014, 31, 180–185. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P.; Chichvarkhin, A.Y.; Kijima, A.; Hanzawa, N.; Park, I. Allozyme and morphometric analysis of two common mussel species of the genus Mytilus (Mollusca, Mytilidae) in Korean, Japanese and Russian waters. Korean J. Genet. 2005, 27, 289–306. [Google Scholar]
- Kartavtsev, Y.P.; Masalkova, N.A.; Katolikova, M.V. Genetic-and-morphometric variability in the settlements of two mussel species (Mytilus ex. gr. edulis), M. trossulus and M. galloprovincialis, in North-West Japan Sea. J. Shellfish. Res. 2018, 37, 1–17. [Google Scholar] [CrossRef]
- Katolikova, M.; Khaitov, V.; Väinölä, R.; Gantsevich, M.; Strelkov, P. Genetic, Ecological and Morphological Distinctness of the Blue Mussels Mytilus trossulus Gould and M. edulis L. in the White Sea. PLoS ONE 2016, 11, e0152963. [Google Scholar] [CrossRef] [Green Version]
- Heath, D.A.; Rawson, P.D.; Hilbish, T.J. PCR-based nuclear markers identify alien blue mussel (Mytilus spp.) genotypes on the west coast of Canada. Can. J. Fish. Aquat. Sci. 1995, 52, 2621–2627. [Google Scholar] [CrossRef]
- Inoue, K.; Takeuchi, Y.; Miki, D.; Odo, S. Mussel adhesive plaque protein gene is a novel member of epidermal growth factor like gene family. J. Biol. Chem. 1995, 270, 6698–6701. [Google Scholar] [CrossRef] [Green Version]
- Rawson, P.D.; Hilbish, T.J. Evolutionary relationships among the male and female mitochondrial DNA lineages in the Mytilus edulis species complex. Mol. Biol. Evol. 1995, 12, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Rawson, P.D.; Secor, C.L.; Hilbish, T.J. The effects of natural hybridization on the regulation of doubly uniparental mtDNA inheritance in blue mussels (Mytilus spp.). Genetics 1996, 144, 241–248. [Google Scholar] [PubMed]
- Rawson, P.D.; Argawal, V.; Hilbish, T.J. Hybridization between the blue mussels Mytilus galloprovincialis and M. trossulus along the pacific coast of North America: Evidence for limited introgression. Marine Biol. 1999, 134, 201–211. [Google Scholar] [CrossRef]
- Skurikhina, L.A.; Kartavtsev, Y.F.; Chichvarkhin, A.Y.; Pan’kova, M.V. Study of two species of mussels, Mytilus trossulus and Mytilus galloprovincialis (Bivalvia, Mytilidae), and their hybrids in Peter the Great Bay of the Sea of Japan with the use of PCR markers. Russ. J. Genet. 2001, 37, 1448–1451. [Google Scholar] [CrossRef]
- Väinölä, R.; Strelkov, P. Mytilus trossulus in Northern Europe. Mar. Biol. 2011, 158, 817–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartavtsev, Y.P.; Katolikova, M.V.; Sharina, S.N.; Chichvarkhina, O.V.; Masalkova, N.A. A population genetic study of the hybrid zone of Mytilus trossulus Gould, 1850 and an introduced species, M. galloprovincialis Lamarck, 1819, (Bivalvia: Mytilidae) in peter the great bay in the Sea of Japan. Russ. J. Mar. Biol. 2014, 40, 208–216. [Google Scholar] [CrossRef]
- Structure. Available online: http://pritch.bsd.uchicago.edu/structure.html (accessed on 14 July 2015).
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, M.; Rousset, F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A complete reimplementation of the Genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Beerli, P.; Felsenstein, J. Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics 1999, 152, 763–773. [Google Scholar] [CrossRef]
- Beerli, P. Comparison of Bayesian and maximum likelihood inference of population genetic parameters. Bioinformatics 2006, 22, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Excoffier, L.; Novembre, J.; Schneider, S. SIMCOAL: A general coalescent program for the simulation of molecular data in interconnected populations with arbitrary demography. J. Hered. 2000, 91, 506–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brower, A.V.Z. Delimitation of phylogenetic species with DNA sequences: A critique of Davis and Nixon’s population aggregation analysis. Syst. Biol. 1999, 48, 199–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sites, J.W.; Marshall, J.C. Operational criteria for delimiting species. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 199–227. [Google Scholar] [CrossRef] [Green Version]
- Kafanov, A.I. Subfamily Mytilinae Rafinisque, 1815 (Bivalvia, Mytilidae) in Cainozoe of North Pacific. In Fauna and Distribution of Mollusks: North Pacific and Polar Basin; Far East Center Publisher: Vladivostok, Russia, 1987; pp. 65–103. (In Russian) [Google Scholar]
- Yonekawa, H.; Moriwaki, K.; Gotoh, O.; Hayashi, J.I.; Watanabe, J.; Miyashita, N.; Petras, M.L.; Tagashira, Y. Evolutionary relationships among five subspecies of Mus musculus based on restriction enzyme cleavage patterns of mitochondrial DNA. Genetics 1981, 98, 801–816. [Google Scholar] [CrossRef]
- Yonekawa, H.; Tsuda, K.; Tsuchiya, K.; Suzuki, H.; Yakimenko, L. Genetic diversity, geographic distribution and evolutionary relationships of Mus musculus subspecies based on polymorphism of mitochondrial DNA. In Problems of Evolution; Kryukov, A.P., Yakimenko, L.V., Eds.; Dalnauka: Vladivostok, Russia, 2000; pp. 90–108. [Google Scholar]
- Ferris, S.D.; Sage, R.D.; Huang, C.M.; Nielsen, J.T.; Ritte, U.; Wilson, A.C. Flow of mitochondrial DNA across a species boundary. Proc. Natl. Acad. Sci. USA 1983, 80, 2290–2294. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.R. Introgression in Fishes: Significance for Paleontology, Cladistics, and Evolutionary Rates. Syst. Biol. 1992, 41, 41–57. [Google Scholar] [CrossRef]
- Suzuki, H.; Yasuda, S.P.; Sakaizumi, M.; Wakana, S.; Motokawa, M.; Tsuchiya, K. Differential geographic patterns of mitochondrial DNA variation in two sympatric species of Japanese wood mice. Genes Genet. Syst. 2004, 79, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Filippucci, M.G.; Chelomina, G.N.; Sato, J.J.; Serizawa, K.; Nevo, E. A biogeographic view of Apodemus in Asia and Europe inferred from nuclear and mitochondrial gene sequences. Bioch. Genet.s 2008, 46, 329–346. [Google Scholar] [CrossRef]
- Oleinik, A.G.; Skurikhina, L.A. Mitochondrial DNA diversity and relationships of endemic charrs of the genus Salvelinus from lake Kronotskoye (Kamchatka Penisula). Hydrobiologia 2010, 650, 145–159. [Google Scholar] [CrossRef]
- Hewitt, G.M. Quaternary phylogeography: The roots of hybrid zones. Genetica 2011, 139, 617–638. [Google Scholar] [CrossRef]
- Oleinik, A.G. Molecular evolution of char of Salvelinus genus: Phylogenetic and phylogeographic aspects. In Abstract of Dissertation of Doctor of Biological Science Degree; Zhirmunsky, A.V., Ed.; Institute of Marine Biology: Vladivostok, Russia, 2013; p. 47. [Google Scholar]
- Saarman, N.P.; Pogson, G.H. Introgression between invasive and native blue mussels (genus Mytilus) in the central California hybrid zone. Mol. Ecol. 2015, 24, 4723–4738. [Google Scholar] [CrossRef] [PubMed]
- Panov, E.N. Hybridization and Ethological Isolation; Nauka Publ.: Moscow, Russia, 1989; p. 510. [Google Scholar]
- Aliabadian, M.; Nijman, V. Avian hybrids: Incidence and geographic distribution of hybridization in birds. Contrib. Zool. 2007, 76, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.V.; Potter, S.; Schmitt, C.J.; Bragg, J.G.; Moritz, C. Reticulation, divergence, and the phylogeography-phylogenetics continuum. In The Light of Evolution, Vol. X: Comparative Phylogeography; Part II; Avise, J.C., Ayala, F.J., Eds.; The National Academies Press: Washington, DC, USA, 2017; Chapter 9; pp. 171–189. ISBN 13 978-0-309-44422-4. [Google Scholar] [CrossRef]
- Lecaudey, L.A.; Schliewen, U.K.; Osinov, A.G.; Taylor, E.B.; Bernatchez, L.; Weiss, S.J. Inferring phylogenetic structure, hybridization and divergence times within Salmoninae (Teleostei: Salmonidae) using RAD-sequencing. Mol. Phys. Evol. 2018, 124, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Plötner, J.; Uzzell, T.; Beerli, P.; Spolsky, C.; Ohst, T.; Litvinchuk, S.N.; Guex, G.-D.; Reyer, H.-U.; Hotz, H. Widespread unidirectional transfer of mitochondrial DNA: A case in western Palaearctic water frogs. J. Evol. Biol. 2008, 21, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.R. Interspecific cytoplasmic gene flow in the absence of nuclear gene flow: Evidence from Drosophila. Proc. Natl. Acad. Sci. USA 1983, 80, 492–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahata, N.; Slatkin, M. Mitochondrial gene flow. Proc. Nat. Acad. Sci. USA 1984, 81, 1764–1767. [Google Scholar] [CrossRef] [Green Version]
- Birky, C.W., Jr. Species Detection and Identification in Sexual Organisms Using Population Genetic Theory and DNA Sequences. PLoS ONE 2013, 8, e52544. [Google Scholar] [CrossRef]
- Hedges, S.B.; Marin, J.; Suleski, M.; Paymer, M.; Kumar, S. Tree of Life Reveals Clock-Like Speciation and Diversification. Mol. Biol. Evol. 2015, 32, 835–845. [Google Scholar] [CrossRef]
- Redin, A.D.; Kartavtsev, Y.P. Phylogenetic relationships of flounders from the family Pleuronectidae (Ostichties: Pleuronectiformes) based on 16S rRNA gene. Russ. J. Genet. 2021, 57, 348–360. [Google Scholar] [CrossRef]
- Hall, B. Phylogenetic Trees Made Easy: A How-To Manual for Molecular Biologists, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2011; p. 282. [Google Scholar]
- Miya, M.; Takeshima, H.; Endo, H.; Ishiguro, N.B.; Inoue, J.G.; Mukai, T.; Satoh, T.P.; Yamaguchi, M.; Kawaguchi, A.; Mabuchi, K.; et al. Major Patterns of Higher Teleostean Phylogenies: A New Perspective Based on 100 complete mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2003, 26, 121–138. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P.; Sharina, S.N.; Saitoh, K.; Imoto, J.M.; Hanzawa, N.; Redin, A.D. Phylogenetic relationships of Russian Far Eastern flatfish (Pleuronectiformes, Pleuronectidae) based on two mitochondrial gene sequences, Co-1 and Cyt-b, with inferences in order phylogeny using complete mitogenome data. Mitochodrial DNA 2016, 27, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Imoto, J.M.; Saitoh, K.; Sasaki, T.; Yonezawa, T.; Adachi, J.; Kartavtsev, Y.P.; Miya, M.; Nishida, M.; Hanzawa, N. Phylogeny and biogeography of highly diverged freshwater fish species (Leuciscinae, Cyprinidae, Teleostei) inferred from mitochondrial genome analysis. Gene 2013, 514, 112–124. [Google Scholar] [CrossRef]
- Berendzen, P.B.; Dimmick, W.W. Phylogenetic relationships of Pleuronectiformes based on molecular evidence. Copeia 2002, 3, 642–652. [Google Scholar] [CrossRef]
- Pardo, B.G.; Machordom, A.; Foresti, F.; Porto-Foresti, F.; Azevedo, M.F.C.; Bañón, R.; Sanchez, L.; Martinez, P. Phylogenetic analysis of flatfish (Order Pleuronectiformes) based on mitochondrial 16s rDNA sequences. Sci. Mar. 2005, 69, 531–543. [Google Scholar] [CrossRef]
- Saitoh, K.; Miya, M.; Nishida, M. Mitochondrial genomics of ostariophysan fishes: Perspectives on phylogeny and biogeography. J. Mol. Evol. 2003, 56, 464–472. [Google Scholar] [CrossRef]
- Mayden, R.L.; Chen, W.-J.; Bart, H.L.; Doosey, M.H.; Simons, A.M.; Tang, K.L.; Wood, R.M.; Agnew, M.K.; Yang, L.; Hirt, M.V.; et al. Reconstructing the phylogenetic relationships of the earth’s most diverse clade of freshwater fishes—Order Cypriniformes (Actinopterygii: Ostariophysi): A case study using multiple nuclear loci and the mitochondrial genome. Mol. Phylogenet. Evol. 2009, 51, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Betancur-R, R.; Orti, G. Molecular evidence for the monophyly of flatfishes (Carangimorpharia: Pleuronectiformes). Mol. Phylogenet. Evol. 2014, 73, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Vinnikov, K.A.; Thomson, R.C.; Munroe, T.A. Revised classification of the righteye flounders (Teleostei: Pleuronectidae) based on multilocus phylogeny with complete taxon sampling. Mol. Phylogenet. Evol. 2018, 125, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Bringloe, T.T.; Cottenie, K.; Martin, G.K.; Adamowicz, S.J. The importance of taxonomic resolution for additive beta diversity as revealed through DNA barcoding. Genome 2016, 59, 1130–1140. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Barraclough, T.G. Delimiting Species Using Single-Locus Data and the Generalized Mixed Yule Coalescent Approach: A Revised Method and Evaluation on Simulated Data Sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, S. JML: Testing hybridization from species trees. Mol. Ecol. Resour. 2012, 12, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P.; Park, T.-J.; Lee, J.-S.; Vinnikov, K.A.; Ivankov, V.N.; Sharina, S.N.; Ponomarev, A.S. Phylogenetic inferences introduced on cytochrome b gene sequences data for six flatfish species (Teleostei, Pleuronectidae) and species synonymy between representatives of genera Pseudopleuronectes and Hippoglossoides from far eastern seas. Russ. J. Genet. 2008, 44, 451–458. [Google Scholar] [CrossRef]
- Bayne, B.L.; Ahrens, M.J.; Allen, S.K.; D’Auriac, M.A.; Backeljau, T.; Beninger, P.; Bohn, R.; Boudry, P.; Davis, J.; Green, T.; et al. The Proposed Dropping of the Genus Crassostrea for All Pacific Cupped Oysters and Its Replacement by a New Genus Magallana: A Dissenting View. J. Shellfish. Res. 2017, 36, 545–547. [Google Scholar] [CrossRef] [Green Version]
- Wiens, J.J.; Kuczynski, C.A.; Stephens, P.R. Discordant mitochondrial and nuclear gene phylogenies in emydid turtles: Implications for speciation and conservation. Biol. J. Linn. Soc. 2010, 99, 445–461. [Google Scholar] [CrossRef] [Green Version]
- Drummond, A.J.; Bouckaert, R.R. Bayesian Evolutionary Analysis with BEAST; Cambridge University Press: Cambridge, UK, 2015; pp. 98–125. [Google Scholar] [CrossRef]
- Bravo, G.A.; Antonelli, A.; Bacon, C.D.; Bartoszek, K.; Blom, M.P.K.; Huynh, S.; Jones, G.; Knowles, L.L.; Lamichhaney, S.; Marcussen, T.; et al. Embracing heterogeneity: Coalescing the tree of life and the future of phylogenomics. PeerJ 2019, 21, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Steinke, D.; Zemlak, T.S.; Boutillier, J.A.; Hebert, P.D.N. DNA barcoding of Pacific Canada’s fishes. Mar. Biol. 2009, 156, 2641–2647. [Google Scholar] [CrossRef]
- Turanov, S.; Kartavtsev, Y.P.; Lipinsky, V.V.; Zemnukhov, V.V.; Balanov, A.A.; Lee, Y.-H.; Jeong, D. DNA-barcoding of perch-like fishes (Actinopterygii: Perciformes) from far-eastern seas of Russia with taxonomic remarks for some groups. Mitochondrial DNA 2014, 27, 1–22. [Google Scholar] [CrossRef]
- McCusker, M.R.; Denti, D.; Van Guelpen, L.; Kenchington, E.; Bentzen, P. Barcoding Atlantic Canada’s commonly encountered marine fishes. Mol. Ecol. Resour. 2013, 13, 177–188. [Google Scholar] [CrossRef]
- Sharina, S.N.; Kartavtsev, Y.P. Phylogenetic and taxonomic analysis of flatfish species (Teleostei, Pleuronectiformes) inferred from the primary nucleotide sequence of cytochrome oxidase 1 gene (Co-1). Russ. J. Genet. 2010, 46, 356–361. [Google Scholar] [CrossRef]
- Roje, D.M. Incorporating molecular phylogenetics with larval morphology while mitigating the effects of substitution saturation on phylogeny estimation: A new hypothesis of relationships for the flatfish family Pleuronectidae (Percomorpha: Pleuronectiformes). J. Mol. Phys. Evol. 2010, 56, 586–600. [Google Scholar] [CrossRef]
- Kijewska, A.; Burzyński, A.; Wenne, R. Molecular identification of European flounder (Platichthys flesus) and its hybrids with European plaice (Pleuronectes platessa). ICES J. Mar. Sci. 2009, 66, 902–906. [Google Scholar] [CrossRef] [Green Version]
- Zbawicka, M.; Gardner, J.P.A.; Wenne, R. Cryptic diversity in smooth-shelled mussels on Southern Ocean islands: Connectivity, hybridisation and a marine invasion. Front. Zoöl. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Hanzawa, N.; Taniguchi, N.; Shinzawa, H. Genetic markers of the artificial hybrids between Tribolodon hakonensis and T. sp. (Ukeguchi-Ugui). Otsuchi Mar. Res. Cent. Rep. Univ. Tokyo 1984, 10, 11–17. [Google Scholar]
- Miya, M.; Saitoh, K.; Wood, R.; Nishida, M.; Mayden, R.L. New primers for amplifying and sequencing the mitochondrial ND4/ND5 gene region of the Cypriniformes (Actinopterygii: Ostariophysi). Ichthyol. Res. 2006, 53, 75–81. [Google Scholar] [CrossRef]
- Saitoh, K.; Sado, T.; Mayden, R.L.; Hanzawa, N.; Nakamura, K.; Nishida, M.; Miya, M. Mitogenomic Evolution and Interrelationships of the Cypriniformes (Actinopterygii: Ostariophysi): The First Evidence Toward Resolution of Higher-Level Relationships of the World’s Largest Freshwater Fish Clade Based on 59 Whole Mitogenome Sequences. J. Mol. Evol. 2006, 63, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, K.; Chen, W.-J.; Mayden, R.L. Extensive hybridization and tetrapolyploidy in spined loach fish. Mol. Phylogenet. Evol. 2010, 56, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Ito, Y.; Shedko, S.V.; Safronov, S.N.; Frolov, S.V.; Chereshnev, I.A.; Jeon, S.-R.; Goto, A. Phylogenetic and Taxonomic Relationships of Northern Far Eastern Phoxinin Minnows, Phoxinus and Rhynchocypris (Pisces, Cyprinidae), as Inferred from Allozyme and Mitochondrial 16S rRNA Sequence Analyses. Zoöl. Sci. 2006, 23, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Semina, A.V.; Polyakova, N.E.; Brykov, V.A. Genetic analysis identifies a cryptic species of Far Eastern daces of the genus Tribolodon. Dokl. Biol. Sci. 2006, 407, 173–175. [Google Scholar] [CrossRef]
- Sasaki, T.; Kartavtsev, Y.P.; Chiba, S.N.; Uematsu, T.; Sviridov, V.V.; Hanzawa, N. Genetic divergence and phylogenetic independence of Far Eastern species in subfamily Leuciscinae (Pisces: Cyprinidae) inferred from mitochondrial DNA analyses. Genes Genet. Syst. 2007, 82, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Sado, T.; Hirt, M.V.; Pasco-Viel, E.; Arunachalam, M.; Li, J.; Wang, X.; Freyhof, J.; Saitoh, K.; Simons, A.M.; et al. Phylogeny and polyploidy: Resolving the classification of cyprinine fishes (Teleostei: Cypriniformes). Mol. Phylogenet. Evol. 2015, 85, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Śmietanka, B.; Burzyński, A.; Hummel, H.; Wenne, R. Glacial history of the European marine mussels Mytilus, inferred from distribution of mitochondrial DNA lineages. Heredity 2014, 113, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zbawicka, M.; Wenne, R.; Burzyński, A. Mitogenomics of recombinant mitochondrial genomes of Baltic Sea Mytilus mussels. Mol. Genet. Genom. 2014, 289, 1275–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zbawicka, M.; Trucco, M.I.; Wenne, R. Single nucleotide polymorphisms in native South American Atlantic coast populations of smooth shelled mussels: Hybridization with invasive European Mytilus galloprovincialis. Genet. Sel. Evol. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wenne, R.; Bach, L.; Zbawicka, M.; Strand, J.; McDonald, J.H. A first report on coexistence and hybridization of Mytilus trossulus and M. edulis mussels in Greenland. Polar Biol. 2016, 39, 343–355. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.H.G.; Hanner, R.; Foresti, F.; Oliveira, C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC Genet. 2013, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Mayr, E. Zoological Species and Evolution; Mir Publ.: Mowcow, Russia, 1968; p. 398. (In Russian) [Google Scholar]
- Simpson, G.G. Organisms and molecules in evolution. Science 1964, 146, 1535–1538. [Google Scholar] [CrossRef]
- Simpson, G.G. Principles of Animal Taxonomy. The Species and Lower Categories; Columbia Univ. Press: New York, NY, USA, 1961. [Google Scholar]
- Paterson, H.E.H. More evidence against speciation by reinforcement. S. Afr. J. Sci. 1978, 74, 369–371. [Google Scholar]
- Paterson, H.E.H. The recognition concept of species. In Species and Speciation; Vrba, E.S., Ed.; Transvaal Museum Monograf: Pretoria, South Africa, 1985; pp. 21–29. [Google Scholar]
- Wiley, E.O. Phylogenetics. In The Theory and Practice of Phylogenetic Systematics; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Cracraft, J. Species concepts and speciation analysis. Curr. Ornithol. 1983, 1, 159–187. [Google Scholar]
- Van Valen, L. Ecological species, multispecies, and oaks. Taxon 1976, 25, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Dobzhansky, T. Evolution, Genetics and Man; John Wiley & Sons. Inc.: New York, NY, USA; Chapman & Hall, Limited: London, UK, 1955; p. 398. [Google Scholar]
- Edwards, S.V.; Xi, Z.; Janke, A.; Faircloth, B.C.; McCormack, J.E.; Glenn, T.C.; Zhong, B.; Wu, S.; Lemmon, E.M.; Lemmon, A.R.; et al. Implementing and testing the multispecies coalescent model: A valuable paradigm for phylogenomics. Mol. Phylogenetics Evol. 2016, 94, 447–462. [Google Scholar] [CrossRef]
- Martsikalis, P.V.; Gkafas, G.A.; Palaiokostas, C.; Exadactylos, A. Genomics Era on Breeding Aquaculture Stocks. In Organic Aquaculture; Springer Science and Business Media LLC: Berlin, Germany, 2019; pp. 65–77. [Google Scholar]
- Avise, J.C.; Aquadro, C.F. A Comparative Summary of Genetic Distances in the Vertebrates. Evol. Biol. 1982, 15, 151–185. [Google Scholar] [CrossRef]
- Ladoukakis, E.D.; Zouros, E. Evolution and inheritance of animal mitochondrial DNA: Rules and exceptions. J. Biol. Res. 2017, 24, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.H. Evolution, systematics, and the unnatural history of mitochondrial DNA. Mitochondrial DNA Part A 2021, 32, 126–151. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Ayala, F.J. Pseudogenes: Are they “junk” or functional DNA? Annu. Rev. Genet. 2003, 37, 123–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartavtsev, Y.P. Some Examples of the Use of Molecular Markers for Needs of Basic Biology and Modern Society. Animals 2021, 11, 1473. https://doi.org/10.3390/ani11051473
Kartavtsev YP. Some Examples of the Use of Molecular Markers for Needs of Basic Biology and Modern Society. Animals. 2021; 11(5):1473. https://doi.org/10.3390/ani11051473
Chicago/Turabian StyleKartavtsev, Yuri Phedorovich. 2021. "Some Examples of the Use of Molecular Markers for Needs of Basic Biology and Modern Society" Animals 11, no. 5: 1473. https://doi.org/10.3390/ani11051473
APA StyleKartavtsev, Y. P. (2021). Some Examples of the Use of Molecular Markers for Needs of Basic Biology and Modern Society. Animals, 11(5), 1473. https://doi.org/10.3390/ani11051473




