Seasonal Activity of Urban Bats Populations in Temperate Climate Zone—A Case Study from Southern Poland
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
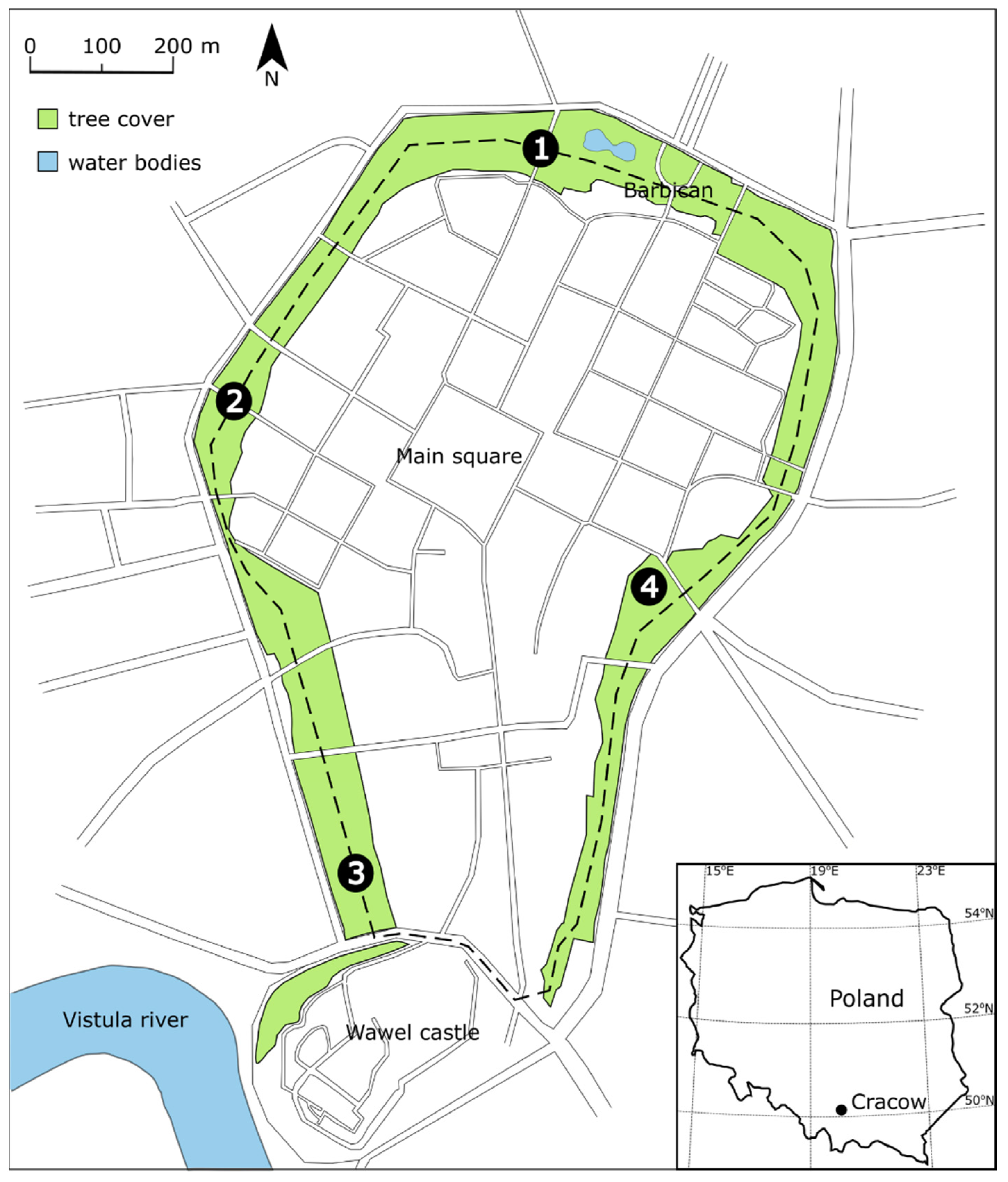
2.1. Study Area
2.2. Ultrasonic Recordings and Sound Analysis
2.3. Statistical Analysis
- −
- At q = 0, the abundances of individual species/taxa are not considered, so the value is simply the species/taxa richness of a given area;
- −
- At q = 1, we obtain the Shannon diversity index, according to the Hill formula; very abundant and less abundant or rare species/taxa all have the same weight, i.e., the value obtained is the most neutral and indicates “true species diversity”;
- −
- At q = 2, we obtain an index which is the reverse of Simpson’s index; Hill’s formula gives greater weight to more numerous and common species and less to rare species.
3. Results
3.1. Bats’ Activity
3.2. Richness and Diversity between Seasons
3.3. Seasonal Differences in the Bats’ Activity
3.4. Predictors of Bats’ Activity within the Seasons
4. Discussion
4.1. Species Composition and Activity
4.2. Richness and Diversity of Bats between the Seasons
4.3. Seasonal Differences in Bats’ Activity
4.4. Predictors of Bats’ Activity within the Seasons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, L.W.; VanDruff, L.W.; Luniak, M. Managing urban habitats and wildlife. In Techniques for Wildlife Investigations and Management; Braun, C.E., Ed.; The Wildlife Society: Bethseda, MD, USA, 2005; pp. 714–739. [Google Scholar]
- Jung, K.; Threlfall, C.G. Trait-dependent tolerance of bats to urbanization: A global meta-analysis. Proc. R. Soc. Lond. B 2018, 285, 20181222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinney, M.L. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Jones, G. Sensory ecology: Noise annoys foraging bats. Curr. Biol. 2008, 18, 1098–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, E.L.; Harris, S.; Jones, G. Impacts of artificial lighting on bats: A review of challenges and solutions. Mamm. Biol. 2015, 80, 213–219. [Google Scholar] [CrossRef]
- Tomassini, A.; Colangelo, P.; Agnelli, P.; Jones, G.; Russo, D. Cranial size has increased over 133 years in a common bat, Pipistrellus kuhlii: A response to changing climate or urbanization? J. Biogeogr. 2014, 41, 944–953. [Google Scholar] [CrossRef]
- Russo, D.; Ancillotto, L. Sensitivity of bats to urbanization: A review. Mamm. Biol. 2015, 80, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kalko, E.K.V. Adaptability and vulnerability of high flying Neotropical aerial insectivorous bats to urbanization. Divers. Distrib. 2011, 17, 262–274. [Google Scholar] [CrossRef]
- Duchamp, J.E.; Swihart, R.K. Shifts in bat community structure related to evolved traits and features of human-altered landscapes. Landsc. Ecol. 2008, 23, 849–860. [Google Scholar] [CrossRef]
- Basham, R.; Law, B.; Banks, P. Microbats in a “leafy” urban landscape: Are they persisting, and what factors influence their presence? Austral. Ecol. 2011, 36, 663–678. [Google Scholar] [CrossRef]
- Gaisler, J.; Zukal, J.; Rehak, Z.; Homolka, M. Habitat preference and fight activity of bats in a city. J. Zool. 1998, 244, 439–445. [Google Scholar] [CrossRef]
- Johnson, J.B.; Gates, J.E.; Ford, W.M. Distribution and activity of bats at local and landscape scales within a rural-urban gradient. Urban Ecosyst. 2008, 11, 227–242. [Google Scholar] [CrossRef]
- Avila-Flores, R.; Brock Fenton, M. Use of spatial features by foraging insectivorous bats in a large urban landscape. J. Mammal. 2005, 86, 1193–1204. [Google Scholar] [CrossRef]
- Silva de Araújo, M.L.V.; Bernard, E. Green remnants are hotspots for bat activity in a large Brazilian urban area. Urban Ecosyst. 2016, 19, 287–296. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Williams, N.S.G.; Hahs, A.K.; Livesley, S.J. Approaches to urban vegetation management and the impacts on urban bird and bat assemblages. Landsc. Urban Plan. 2016, 153, 28–39. [Google Scholar] [CrossRef]
- Suarez-Rubio, M.; Ille, C.; Bruckner, A. Insectivorous bats respond to vegetation complexity in urban green spaces. Ecol. Evol. 2018, 8, 3240–3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Threlfall, C.G.; Law, B.; Banks, P.B. Sensitivity of insectivorous bats to urbanization: Implications for suburban conservation planning. Biol. Conserv. 2012, 146, 41–52. [Google Scholar] [CrossRef]
- Gallo, T.; Lehrer, E.W.; Fidino, M.; Kilgour, R.J.; Wolff, P.J.; Magle, S.B. Need for multiscale planning for conservation of urban bats. Conserv. Biol. 2018, 32, 638–647. [Google Scholar] [CrossRef]
- Tena, E.; Fandos, G.; de Paz, Ó.; de la Peña, R.; Tellería, J.L. Size does matter: Passive sampling in urban parks of a regional bat assemblage. Urban Ecosyst. 2020, 23, 227–234. [Google Scholar] [CrossRef]
- Tzortzakaki, O.; Papadatou, E.; Kati, V.; Giokas, S. Winners and losers in an urban bat community: A case study from southeastern Europe. Hystrix Ital. J. Mammal. 2019. [Google Scholar] [CrossRef]
- Lintott, P.R.; Bunnefeld, N.; Park, K.J. Opportunities for improving the foraging potential of urban waterways for bats. Biol. Conserv. 2015, 191, 224–233. [Google Scholar] [CrossRef]
- Bokwa, A.; Hajto, M.J.; Walawender, J.P.; Szymanowski, M. Influence of diversified relief on the urban heat island in the city of Kraków, Poland. Theor. Appl. Climatol. 2015, 122, 365–382. [Google Scholar] [CrossRef] [Green Version]
- Barataud, M. Acoustic Ecology of European Bats: Species Identification and Studies of Their Habitats and Foraging Behaviour; Biotope: Mèze, France; National Museum of Natural History: Paris, France, 2015; 340p. [Google Scholar]
- Middleton, N.; Froud, A.; French, K. Social Calls of the Bats of Britain and Ireland; Pelagic Publishing: Exeter, UK, 2014; 200p. [Google Scholar]
- Pfalzer, G.; Kusch, J. Structure and variability of bat social calls: Implications for specificity and individual recognition. J. Zool. 2003, 261, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey-Ehrenbold, A.; Bontadina, F.; Arlettaz, R.; Obrist, M.K. Landscape connectivity, habitat structure and activity of bat guilds in farmland-dominated matrices. J. Appl. Ecol. 2013, 50, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Godlevska, L.V. Northward expansion of the winter range of Nyctalus noctula (Chiroptera: Vespertilionidae) in Eastern Europe. Mammalia 2015, 79, 315–324. [Google Scholar] [CrossRef]
- Lesiński, G.; Janus, K. A mass wintering of the common noctule Nyctalus noctula (Schreber, 1774) (Chiroptera: Vespertilionidae) in a town of south-eastern Poland. Acta Zool. Bulg. 2020, 72, 409–412. [Google Scholar]
- Boston, E.S.M.; Roué, S.G.; Montgomery, W.I.; Prodöhl, P.A. Kinship, parentage, and temporal stability in nursery colonies of Leisler’s bat (Nyctalus leisleri). Behav. Ecol. 2012, 23, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Roche, N.; Langton, S.; Aughney, T.; Russ, J.M.; Marnell, F.; Lynn, D.; Catto, C. A car-based monitoring method reveals new information on bat populations and distributions in Ireland. Anim. Conserv. 2011, 14, 642–651. [Google Scholar] [CrossRef]
- Stoycheva, S.; Georgiev, D.; Pandourski, I.; Tilova, E. Bat diversity in two large towns of the Upper Thrace, Bulgaria (Chiroptera). Lynx New Ser. 2009, 93, 83–93. [Google Scholar]
- Kravchenko, K.; Vlaschenko, A.; Prylutska, A.; Rodenko, O.; Hukov, V.; Shuvaev, V. Year-round monitoring of bat records in an urban area: Kharkiv (NE Ukraine), 2013, as a case study. Turk. J. Zool. 2017, 41, 530–548. [Google Scholar] [CrossRef]
- Haupt, M.; Menzler, S.; Schmidt, S. Flexibility of habitat use in Eptesicus nilssonii: Does the species profit from anthropogenically altered habitats? J. Mammal. 2006, 87, 351–361. [Google Scholar] [CrossRef]
- Rydell, J. Exploitation of insects around streetlamps by bats in Sweden. Funct. Ecol. 1992, 6, 744–750. [Google Scholar] [CrossRef]
- Rydell, J. Seasonal use of illuminated areas by foraging northern bats Eptesicus nilssoni. Ecography 1991, 14, 203–207. [Google Scholar] [CrossRef]
- Lesinski, G.; Fuszara, E.; Fuszara, M.; Kowalski, M.; Wojtowicz, B. The parti-coloured bat Vespertilio murinus in Warsaw, Poland. Myotis 2001, 39, 21–25. [Google Scholar]
- Uhrin, M.; Hüttmeir, U.; Kipson, M.; Estók, P.; Sachanowicz, K.; Bücs, S.; Karapandža, B.; Paunović, M.; Presetnik, P.; Bashta, A.T.; et al. Status of Savi’s pipistrelle Hypsugo savii (Chiroptera) and range expansion in Central and south-eastern Europe: A review. Mamm. Rev. 2016, 46, 1–16. [Google Scholar] [CrossRef]
- Ancillotto, L.; Budinski, I.; Nardone, V.; Di Salvo, I.; Della Corte, M.; Bosso, L.; Conti, P.; Russo, D. What is driving range expansion in a common bat? Hints from thermoregulation and habitat selection. Behav. Process. 2018, 157, 540–546. [Google Scholar] [CrossRef]
- Popczyk, B.; Lesiński, G.; Baumann, A.; Wojtowicz, B. Kuhl’s pipistrelle, Pipistrellus kuhlii (Kuhl, 1817) or Pipistrellus lepidus Blyth, 1845, in Central Poland—Accidental record or a result of expansion? Nyctalus 2008, 13, 279–281. [Google Scholar]
- Sachanowicz, K.; Wower, A.; Bashta, A. Further range extension of Pipistrellus kuhlii (Kuhl, 1817) in central and eastern Europe. Acta Chiropterol. 2006, 8, 543–548. [Google Scholar] [CrossRef]
- Ancillotto, L.; Santini, L.; Ranc, N.; Maiorano, L.; Russo, D. Extraordinary range expansion in a common bat: The potential roles of climate change and urbanisation. Sci. Nat. 2016, 103. [Google Scholar] [CrossRef] [PubMed]
- Nusová, G.; Uhrin, M.; Kaňuch, P. Go to the city: Urban invasions of four pipistrelle bat species in eastern Slovakia. Eur. J. Ecol. 2019, 5, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Sachanowicz, K.; Ciechanowski, M.; Tryjanowski, P.; Kosicki, J.Z. Wintering range of Pipistrellus nathusii (Chiroptera) in Central Europe: Has the species extended to the north-east using urban heat islands. Mammalia 2019, 83, 260–271. [Google Scholar] [CrossRef]
- Gili, F.; Newson, S.E.; Gillings, S.; Chamberlain, D.E.; Border, J.A. Bats in urbanising landscapes: Habitat selection and recommendations for a sustainable future. Biol. Conserv. 2020, 241, 108343. [Google Scholar] [CrossRef]
- Legakis, A.; Papadimitriou, C.; Gaethlich, M.; Lazaris, D. Survey of the bats of the Athens metropolitan area. Myotis 2000, 38, 41–46. [Google Scholar]
- Ślęzak, J.; (Jagiellonian University, Cracow, Poland). Personal communication, 2016.
- Bonnet-Lebrun, A.S.; Manica, A.; Rodrigues, A.S.L. Effects of urbanization on bird migration. Biol. Conserv. 2020, 244, 108423. [Google Scholar] [CrossRef]
- Ciechanowski, M.; Zając, T.; Zielińska, A.; Dunajski, R. Seasonal activity patterns of seven vespertilionid bat species in Polish lowlands. Acta Theriol. 2010, 55, 301–314. [Google Scholar] [CrossRef]
- Heim, O.; Schröder, A.; Eccard, J.; Jung, K.; Voigt, C.C. Seasonal activity patterns of European bats above intensively used farmland. Agric. Ecosyst. Environ. 2016, 233, 130–139. [Google Scholar] [CrossRef]
- Dietz, C.; von Helversen, O.; Nill, D. Bats of Europe and Northwest Africa; Multico Press: Warsaw, Poland, 2009; pp. 76–79. [Google Scholar]
- Ciechanowski, M.; Zaja̧c, T.; Biłas, A.; Dunajski, R. Spatiotemporal variation in activity of bat species differing in hunting tactics: Effects of weather, moonlight, food abundance, and structural clutter. Can. J. Zool. 2007, 85, 1249–1263. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.; Roche, N.; Aughney, T.; Jones, N.; Day, J.; Baker, J.; Langton, S. Barriers and benefits: Implications of artificial night-lighting for the distribution of common bats in Britain and Ireland. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sonar Type/Taxon | BP | FB | SC |
|---|---|---|---|
| Long-range echolocators | |||
| N. noctula | 470 | 2 | 45 |
| N. leisleri | 54 | 0 | 1 |
| Nyctalus sp. | 57 | 0 | 2 |
| E. serotinus | 85 | 1 | 0 |
| E. nilssoniS, Es, Ls | 16 | 0 | 0 |
| V. murinusA | 1 | 0 | 5 |
| Unidentified | 222 | 1 | 2 |
| TOTAL | 905 | 4 | 55 |
| Medium-range echolocators | |||
| P. kuhlii | 19 | 0 | 1 |
| P. nathusiiLs | 3 | 0 | 3 |
| P. kuhlii/P.nathusii | 338 | 0 | 0 |
| P. pygmaeusS, Ls | 4 | 0 | 0 |
| P. pipistrellusS | 1 | 1 | 0 |
| H. saviiS, Ls | 5 | 0 | 0 |
| H. savii/P. kuhlii | 12 | 0 | 0 |
| Unidentified | 63 | 8 | 2 |
| TOTAL | 445 | 9 | 6 |
| Short-range echolocators | |||
| Myotis sp. | 21 | 0 | 0 |
| Overall bat activity | 1371 | 13 | 61 |
| Spring | Early Summer | Late Summer | Autumn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | b | p | β | b | p | β | b | p | β | b | p | ||
| Long-range echolocators | Intercept | - | −1.774 | 0.005 | - | −0.650 | 0.461 | - | - | - | - | 2.614 | <0.001 |
| Temperature | 0.846 | 0.213 | 0.00004 | 0.654 | 0.132 | 0.006 | nq | ns | |||||
| Cloud cover | ns | nq | nq | −0.598 | −0.001 | 0.01 | |||||||
| Humidity | nq | nq | nq | nq | |||||||||
| Moon phase | nq | nq | nq | nq | |||||||||
| Wind speed | nq | nq | nq | nq | |||||||||
| Regression | Stepwise backward | Linear | - | Linear | |||||||||
| R2 adjusted | 0.696 | 0.387 | - | 0.312 | |||||||||
| Medium-range echolocators | Intercept | - | 0.558 | 0.239 | - | 3.973 | 0.0002 | - | 3.691 | 0.00006 | - | 0.822 | 0.01 |
| Temperature | 0.575 | 0.080 | 0.02 | −0.564 | −0.098 | 0.02 | −0.554 | −0.072 | 0.03 | 0.658 | 0.075 | 0.006 | |
| Cloud cover | ns | nq | nq | ns | |||||||||
| Humidity | nq | nq | nq | a | |||||||||
| Moon phase | nq | nq | nq | nq | |||||||||
| Wind speed | nq | nq | nq | nq | |||||||||
| Regression | Linear | Linear | Linear | Stepwise backward | |||||||||
| R2 adjusted | 0.282 | 0.270 | 0.257 | 0.392 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohyt, J.; Pierzchała, E.; Pereswiet-Soltan, A.; Piksa, K. Seasonal Activity of Urban Bats Populations in Temperate Climate Zone—A Case Study from Southern Poland. Animals 2021, 11, 1474. https://doi.org/10.3390/ani11051474
Kohyt J, Pierzchała E, Pereswiet-Soltan A, Piksa K. Seasonal Activity of Urban Bats Populations in Temperate Climate Zone—A Case Study from Southern Poland. Animals. 2021; 11(5):1474. https://doi.org/10.3390/ani11051474
Chicago/Turabian StyleKohyt, Joanna, Ewa Pierzchała, Andrea Pereswiet-Soltan, and Krzysztof Piksa. 2021. "Seasonal Activity of Urban Bats Populations in Temperate Climate Zone—A Case Study from Southern Poland" Animals 11, no. 5: 1474. https://doi.org/10.3390/ani11051474
APA StyleKohyt, J., Pierzchała, E., Pereswiet-Soltan, A., & Piksa, K. (2021). Seasonal Activity of Urban Bats Populations in Temperate Climate Zone—A Case Study from Southern Poland. Animals, 11(5), 1474. https://doi.org/10.3390/ani11051474





