Microbial Prevalence and Antimicrobial Sensitivity in Equine Endometritis in Field Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Endometrial Cytology
2.3. Samples Processing
2.4. Culture Media
2.5. Identification of Colonies
2.6. Antimicrobial Susceptibility
2.7. Statistical Analyses
3. Results
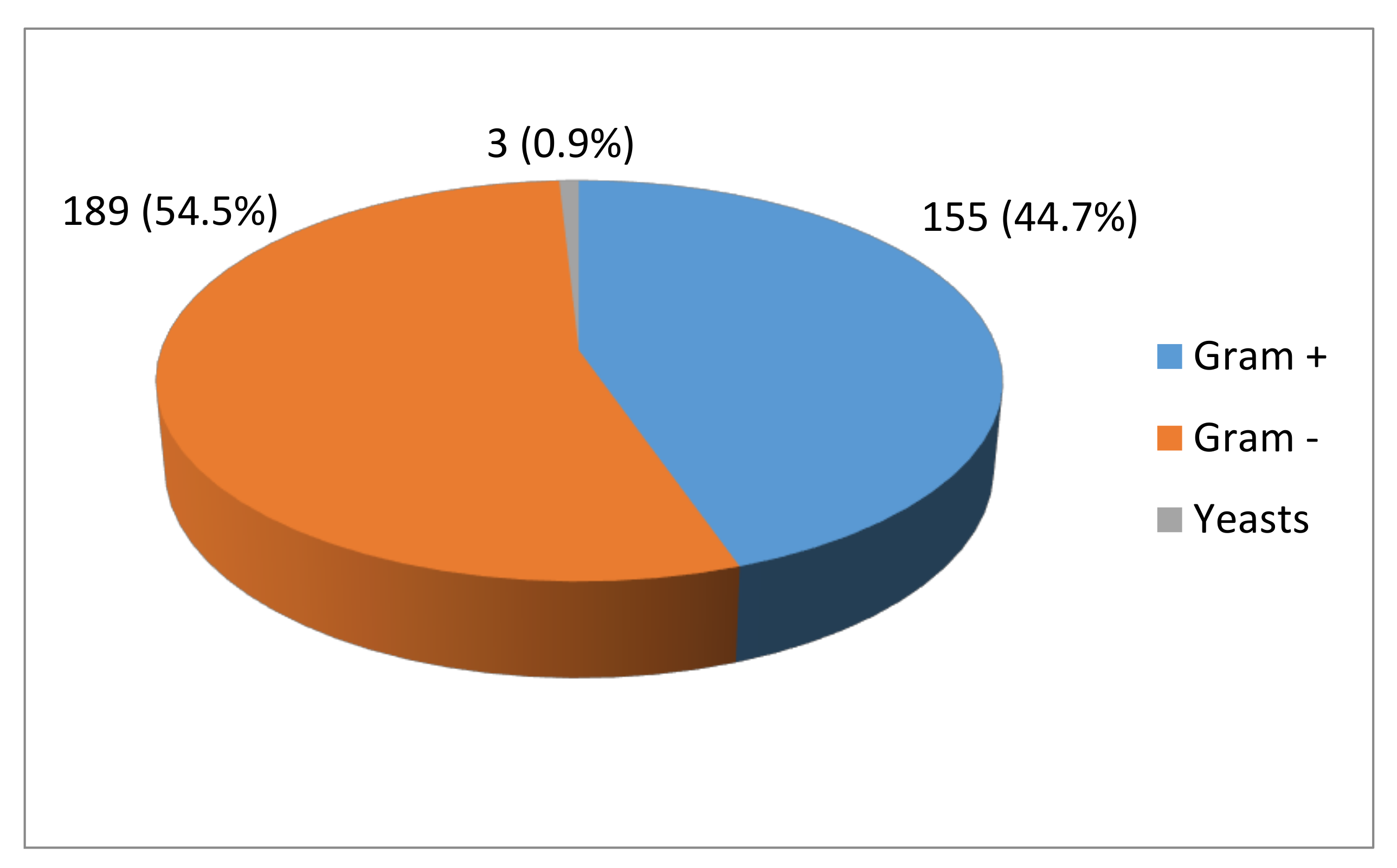
3.1. Identification of Colonies
3.2. Antimicrobial Susceptibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutjahr, S.; Paccamonti, D.; Pycock, J.; Taverne, M.; Dieleman, S.; Van Der Weijden, G. Effect of dose and day of treatment on uterine response to oxytocin in mares. Theriogenology 2000, 54, 447–456. [Google Scholar] [CrossRef]
- Causey, R.C. Making sense of equine uterine infections: The many faces of physical clearance. Vet. J. 2006, 172, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, M.M.; Causey, R.C. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Riddle, W.; LeBlanc, M.; Stromberg, A. Relationships between uterine culture, cytology and pregnancy rates in a Thoroughbred practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Hinrichs, K.; Cummings, M.R.; Sertich, P.L.; Kenney, R.M. Clinical significance of aerobic bacterial flora of the uterus, vagina, vestibule, and clitoral fossa of clinically normal mares. J. Am. Vet. Med. Assoc. 1988, 193, 72–75. [Google Scholar] [PubMed]
- Heil, B.; Thompson, S.; Kearns, T.; Davolli, G.; King, G.; Sones, J. Metagenetic Characterization of the Resident Equine Uterine Microbiome Using Multiple Techniques. J. Equine Vet. Sci. 2018, 66, 111. [Google Scholar] [CrossRef]
- Holyoak, G.R.; Lyman, C.C.; Wieneke, X.; DeSilva, V. The equine endometrial microbiome. Clin. Theriogenol. 2018, 10, 273–277. [Google Scholar]
- Benko, T.; Boldizar, M.; Novotny, F.; Hura, V.; Valocky, I.; Dudrikova, K.; Karamanova, M.; Petrovic, V. Incidence of bacterial pathogens in equine uterine swabs, their antibiotic resistance patterns, and selected reproductive indices in English thoroughbred mares during the foal heat cycle. Vet. Med. 2016, 60, 613–620. [Google Scholar] [CrossRef]
- Pycock, J.F.; Allen, W.E. Inflammatory components in uterine fluid from mares with experimentally induced bacterial endometritis. Equine Vet. J. 1990, 22, 422–425. [Google Scholar] [CrossRef]
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leukocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Katila, T. Onset and duration of uterine inflammatory response of mares after insemination with fresh semen. Biol. Reprod. 1995, 1, 515–517. [Google Scholar] [CrossRef]
- Koskinen, E.; Katila, T. Uterine involution, ovarian activity and fertility in the post-partum mare. J. Reprod. Fertil. 1987, 35, 733–734. [Google Scholar]
- Purswell, B.J.; Ley, W.B.; Sriranganathan, N.; Bowen, J.M. Aerobic and anaerobic bacterial flora in the post-partum mare. Equine Vet. Sci. 1989, 9, 141–144. [Google Scholar] [CrossRef]
- Waelchli, R.O.; Känzig, M.; Gygax, A.; Corboz, L.; Rüsch, P. The Relationship between Cycle Stage and Results of Uterine Culture in the Mare. J. Vet. Med. 1993, 40, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Langoni, H.; Alvarenga, M.A.; Papa, F.O.; Sakamoto, C.; Baldini, S.; Listoni, F.J.P. Aerobic, microaerobic and anaerobic bacteria in equine endometritis. Pherdeheilkunde 1997, 13, 548. [Google Scholar]
- Frontoso, R.; De Carlo, E.; Pasolini, M.P.; van der Meulen, K.; Pagnini, U.; Iovane, G.; De Martino, L. Retrospective study of bacterial isolates and their antimicrobial susceptibilities in equine uteri during fertility problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef]
- Davis, H.A.; Stanton, M.B.; Thungrat, K.; Boothe, D.M. Uterine bacterial isolates from mares and their resistance to antimicrobials: 8296 cases (2003–2008). J. Am. Vet. Med. Assoc. 2013, 242, 977–983. [Google Scholar] [CrossRef]
- Pycock, J.F.; Newcombe, J.R. Assessment of the effect of three treatments to remove intrauterine fluid on pregnancy rate in the mare. Vet. Rec. 1996, 138, 320–323. [Google Scholar] [CrossRef]
- Ricketts, S.W. Treatment of equine endometritis with intrauterine irrigations of ceftiofur sodium: A comparison with mares treated in a similar manner with a mixture of sodium benzylpenicilin, neomycin sulphate, polymixin B sulphate and furaltadone hydrochloride. Pferdeheilkunde 1997, 13, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.N. Hematologic techniques. In Shalm’s Veterinary Hematology, 4th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 1978; pp. 20–86. [Google Scholar]
- Overbeck, W.; Witte, T.; Heuwieser, W. Comparison of three diagnostic methods to identify subclinical endometritis in mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.G.; Whitcomb, R.F.; Clark, H.F.; Williamson, D.L. Pathogenic mycoplasmas: Cultivation and vertebrate pathogenicity of a new spiroplasma. Science 1977, 195, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Brinsko, S.P.; Blanchard, T.L.; Varner, D.D.; Schumacher, J.; Love, C.C.; Hinrichs, K.; Hartman, D.L. (Eds.) Endometritis. In Manual of Equine Reproduction, 3rd ed.; Mosby-Elsevier: Maryland Heights, MO, USA, 2003; pp. 73–84. [Google Scholar]
- Dimock, W.W.; Edwards, P. Pathology and bacteriology of the reproductive organs of mares in relation to sterility. Ky. Agric. Exp. Stn. Bull. 1928, 286, 157–237. [Google Scholar]
- Collins, A.M. A study of the incidence of cervical and uterine infection in Thoroughbred mares in Ireland. Vet. Rec. 1964, 76, 673–676. [Google Scholar]
- Bain, A.M. The role of infection in infertility in the thoroughbred mare. Vet. Rec. 1966, 78, 168–173. [Google Scholar] [CrossRef]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar]
- Rodier, D.; Ponthier, J.; Parrilla-Hernández, S.; Deleuze, S. Effect of artificial insemination site on post-mating endometritis in the mares. Reprod. Domest. Anim. 2012, 47 (Suppl. S5), 103. [Google Scholar]
- Bourke, M.; Mills, J.N.; Barnes, A.L. Collection of endometrial cells in the mare. Aust. Vet. J. 1997, 75, 755–758. [Google Scholar] [CrossRef]
- Nielsen, J.M. Endometritis in the mare: A diagnostic study comparing cultures from swab and biopsy. Theriogenology 2005, 64, 510–518. [Google Scholar] [CrossRef]
- Buczkowska, J.; Kozdrowski, R.; Nowak, M.; Raś, A.; Staroniewicz, Z.; Siemieniuch, M.J. Comparison of the biopsy and cytobrush techniques for diagnosis of subclinical endometritis in mares. Reprod. Biol. Endocrinol. 2014, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Ball, B.A.; Shin, S.J.; Patten, V.H.; Lein, D.H.; Woods, G.L. Use of a low-volume uterine flush for microbiologic and cytologic examination of the mares endometrium. Theriogenology 1988, 298, 1269–1283. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelly, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. S1), S1–S25. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, M.M. Advances in the Diagnosis and Treatment of Chronic Infectious and Post-Mating-Induced Endometritis in the Mare. Reprod. Domest. Anim. 2010, 45, 21–27. [Google Scholar] [CrossRef]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Glapa, K.E.; Martin, K.H.; Mangalea, M.R.; Hennet, M.L.; Wolfe, L.M.; Broeckling, C.D.; Borlee, B.R. Model of Chronic Equine Endometritis Involving a Pseudomonas aeruginosa Biofilm. Infect. Immun. 2017, 85, e00332-17. [Google Scholar] [CrossRef] [Green Version]
- Digby, N.J.W.; Ricketts, S.W. Results of concurrent bacteriological and cytological examinations of the endometrium of mares in routine stud farm practice 1978–1981. J. Reprod. Fertil. 1982, 32, 181–185. [Google Scholar]
- Pyörälä, S.; Taponen, J.; Katila, T. Use of Antimicrobials in the Treatment of Reproductive Diseases in Cattle and Horses. Reprod. Domest. Anim. 2014, 49, 16–26. [Google Scholar] [CrossRef]
- Pisello, L.; Rampacci, E.; Stefanetti, V.; Beccati, F.; Hyatt, D.R.; Coletti, M.; Passamonti, F. Temporal efficacy of antimicrobials against aerobic bacteria isolated from equine endometritis: An Italian retrospective analysis (2010–2017). Vet. Rec. 2019, 185, 598. [Google Scholar] [CrossRef] [Green Version]
- Christoffersen, M.; Söderlind, M.; Rudefalk, S.R.; Pedersen, H.G.; Allen, J.; Krekeler, N. Risk factors associated with uterine fluid after breeding caused by Streptococcus zooepidemicus. Theriogenology 2015, 84, 1283–1290. [Google Scholar] [CrossRef]
- Shin, S.J.; Lein, D.H.; Aronson, A.K.; Nusbaum, S.R. The bacteriological culture of equine uterine contents, in vivo sensitivity of organisms isolated and interpretation. J. Reprod. Fertil. 1979, 27, 307–315. [Google Scholar]
- Albihn, A.; Båverud, V.; Magnusson, U. Uterine Microbiology and Antimicrobial Susceptibility in Isolated Bacteria from Mares with Fertility Problems. Acta Vet. Scand. 2003, 44, 121–129. [Google Scholar] [CrossRef]
- Mitchell, A.; De Amorim, M.D.; Thachil, A.; Altier, C.; Cheong, S. Uterine Bacterial Isolates from Mares and Their Resistance to Antimicrobials. J. Equine Vet. Sci. 2018, 66, 114. [Google Scholar] [CrossRef]
- Leblanc, M.M. The current status of antibiotic use in equine reproduction. Equine Vet. Educ. 2009, 21, 156–167. [Google Scholar] [CrossRef]
- Scofield, D.; Black, J.; Wittenburg, L.; Gustafson, D.; Ferris, R.; Hatzel, J.; Traub-Dargatz, J.; McCue, P. Endometrial tissue and blood plasma concentration of ceftiofur and metabolites following intramuscular administration of ceftiofur crystalline free acid to mares. Equine Vet. J. 2014, 46, 606–610. [Google Scholar] [CrossRef] [PubMed]


| Microorganisms Combination | Number of Mares (out of 22) |
|---|---|
| Staphylococcus + Escherichia coli | 8 |
| Staphylococcus + Pseudomonas | 4 |
| Staphylococcus + Klebsiella | 3 |
| Staphylococcus + Aeromonas | 1 |
| Staphylococcus + Proteus | 1 |
| Staphylococcus + Serratia | 1 |
| Staphylococcus + Streptococcus | 1 |
| Staphylococcus + Myroides | 1 |
| Enterococcus + Klebsiella | 1 |
| Micrococcus + Proteus | 1 |
| Microorganism | Number of Isolates | Frequency (%) |
|---|---|---|
| Escherichia coli | 60 | 17.3 |
| Staphylococcus spp. | 54 | 15.6 |
| Streptococcus spp. | 47 | 13.5 |
| Pseudomonas aeruginosa | 23 | 6.6 |
| Pseudomonas spp. | 19 | 5.5 |
| Klebsiella pneumoniae | 16 | 4.6 |
| Enterobacter aerogenes | 12 | 3.5 |
| Staphylococcus xylosus | 11 | 3.2 |
| Aerococcus viridans | 8 | 2.3 |
| Klebsiella ornithinolytica | 8 | 2.3 |
| Proteus spp. | 8 | 2.3 |
| Serratia spp. | 8 | 2.3 |
| Enterococcus faecalis | 7 | 2.0 |
| Enterobacter spp. | 6 | 1.7 |
| Staphylococcus epidermidis | 6 | 1.7 |
| Klebsiella spp. | 5 | 1.4 |
| Staphylococcus haemolyticus | 5 | 1.4 |
| Citrobacter spp. | 4 | 1.2 |
| Staphylococcus capitis | 4 | 1.2 |
| Staphylococcus lentus | 4 | 1.2 |
| Aeromonas hydrophila | 3 | 0.9 |
| Kluveria spp. | 3 | 0.9 |
| Micrococcus spp. | 3 | 0.9 |
| Proteus mirabilis | 3 | 0.9 |
| Agrobacterium radiobacter | 2 | 0.6 |
| Bordetella spp. | 2 | 0.6 |
| Candida spp. | 2 | 0.6 |
| Myroides spp. | 2 | 0.6 |
| Ochrobactrum anthropi | 2 | 0.6 |
| Staphylococcus intermedius | 2 | 0.6 |
| Streptococcus equi zooepidemicus | 2 | 0.6 |
| Candida tropicalis | 1 | 0.3 |
| Enterobacter sakazakii | 1 | 0.3 |
| Proteus panneri | 1 | 0.3 |
| Serratia odorifera | 1 | 0.3 |
| Staphylococcus lugdunensis | 1 | 0.3 |
| Vibrio parahaemolyticus | 1 | 0.3 |
| Antibiotic | Sensitive (%) | Intermediate (%) | Resistant (%) |
|---|---|---|---|
| Amikacin | 57.3 a | 22.7 b | 20.0 c |
| Cefoxitin | 48.6 a | 24.5 a | 26.9 b |
| Gentamicin | 48.3 a | 17.4 b | 34.3 b |
| Trimethoprim/sulphonamide | 38.7 ab | 20.3 a | 41.0 b |
| Kanamycin | 37.8 ab | 21.2 a | 41.0 b |
| Neomycin | 33.4 a | 29.1 b | 37.5 c |
| Amoxicillin/clavulanic acid | 33.4 a | 11.3 b | 55.3 a |
| Oxytetracycline | 32.8 a | 30.6 b | 36.7 a |
| Ticarcillin | 32.0 a | 14.8 a | 53.2 a |
| Ampicillin | 22.4 a | 15.7 a | 61.9 b |
| Apramycin | 18.3 a | 16.9 b | 64.8 c |
| Penicillin | 17.2 a | 7.6 a | 75.2 b |
| Cephaloridine | 16.9 a | 4.9 b | 78.2 c |
| Doxycycline | 14.5 a | 32.0 b | 53.5 c |
| E. coli | Staphylococcus spp. | Streptococcus spp. | P. aeruginosa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | IM | R | S | IM | R | S | IM | R | S | IM | R | |
| GM | 53.3 a | 20.0 ab | 26.7 b | 40.7 a | 18.5 a | 40.7 a | 44.7 a | 12.8 a | 42.6 a | 56.5 a | 21.7 a | 21.7 a |
| AK | 63.3 a | 28.3 a | 8.3 b | 48.1 a | 24.1 ab | 27.8 b | 46.8 a | 25.5 a | 27.7 a | 78.3 a | 17.4 ab | 4.3 ab |
| AMP | 16.7 a | 20.0 ab | 63.3 b | 38.9 a | 13.0 a | 48.1 a | 25.5 a | 19.1 a | 55.3 a | 17.4 a | 13.0 a | 69.6 a |
| P | 13.3 a | 8.3 a | 78.3 b | 27.8 ab | 7.4 b | 64.8 a | 38.3 ab | 6.4 b | 55.3 a | 13.0 a | 4.3 a | 82.6 b |
| D | 5.0 a | 33.3 b | 61.7 b | 18.5 a | 24.1 a | 57.4 a | 17.0 a | 36.2 b | 46.8 ab | 0.0 a | 39.1 b | 60.9 b |
| K | 41.7 a | 23.3 ab | 35.0 b | 27.8 a | 20.4 a | 51.9 a | 38.3 a | 23.4 a | 38.3 a | 56.5 a | 13.0 ab | 30.4 b |
| N | 33.3 a | 23.3 a | 43.3 a | 44.4 a | 24.1 a | 31.5 a | 31.9 ab | 36.2 b | 31.9 a | 21.7 a | 52.2 b | 26.1 a |
| APR | 18.3 a | 20.0 a | 61.7 a | 24.1 a | 5.6 a | 70.4 b | 17.0 a | 19.1 ab | 63.8 b | 17.4 a | 17.4 a | 65.2 a |
| TIC | 28.3 a | 11.7 a | 60.0 a | 37.0 a | 18.5 a | 44.4 a | 44.7 a | 21.3 a | 34.0 a | 34.8 a | 13.0 a | 52.2 a |
| AMC | 23.3 a | 10.0 a | 66.7 a | 50.0 a | 7.4 ab | 42.6 b | 42.6 a | 23.4 a | 34.0 a | 21.7 a | 13.0 a | 65.2 a |
| CR | 15.0 a | 8.3 a | 76.7 b | 22.2 a | 3.7 a | 74.1 b | 14.9 a | 8.5 a | 76.6 b | 13.0 a | 0.0 a | 87.0 b |
| SXT | 36.1 a | 25.0 a | 38.9 a | 38.2 a | 20.6 a | 41.2 a | 34.3 a | 11.4 a | 54.3 a | 71.4 a | 7.1 ab | 21.4 b |
| FOX | 55.6 a | 19.4 ab | 25.0 b | 32.4 a | 38.2 b | 29.4 a | 41.4 a | 25.7 ab | 22.9 b | 64.3 a | 21.4 ab | 14.3 b |
| OT | 33.3 a | 22.2 a | 44.4 a | 36.4 ab | 39.4 b | 24.2 a | 50.0 a | 17.6 a | 32.4 a | 15.4 ab | 53.8 b | 30.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Bertrana, M.L.; Deleuze, S.; Pitti Rios, L.; Yeste, M.; Morales Fariña, I.; Rivera del Alamo, M.M. Microbial Prevalence and Antimicrobial Sensitivity in Equine Endometritis in Field Conditions. Animals 2021, 11, 1476. https://doi.org/10.3390/ani11051476
Díaz-Bertrana ML, Deleuze S, Pitti Rios L, Yeste M, Morales Fariña I, Rivera del Alamo MM. Microbial Prevalence and Antimicrobial Sensitivity in Equine Endometritis in Field Conditions. Animals. 2021; 11(5):1476. https://doi.org/10.3390/ani11051476
Chicago/Turabian StyleDíaz-Bertrana, María Luisa, Stefan Deleuze, Lidia Pitti Rios, Marc Yeste, Inmaculada Morales Fariña, and Maria Montserrat Rivera del Alamo. 2021. "Microbial Prevalence and Antimicrobial Sensitivity in Equine Endometritis in Field Conditions" Animals 11, no. 5: 1476. https://doi.org/10.3390/ani11051476
APA StyleDíaz-Bertrana, M. L., Deleuze, S., Pitti Rios, L., Yeste, M., Morales Fariña, I., & Rivera del Alamo, M. M. (2021). Microbial Prevalence and Antimicrobial Sensitivity in Equine Endometritis in Field Conditions. Animals, 11(5), 1476. https://doi.org/10.3390/ani11051476








