Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Critical Swimming Test (Ucrit) and Estimation of Metabolic Rates
2.3. Hard-Wire EMG
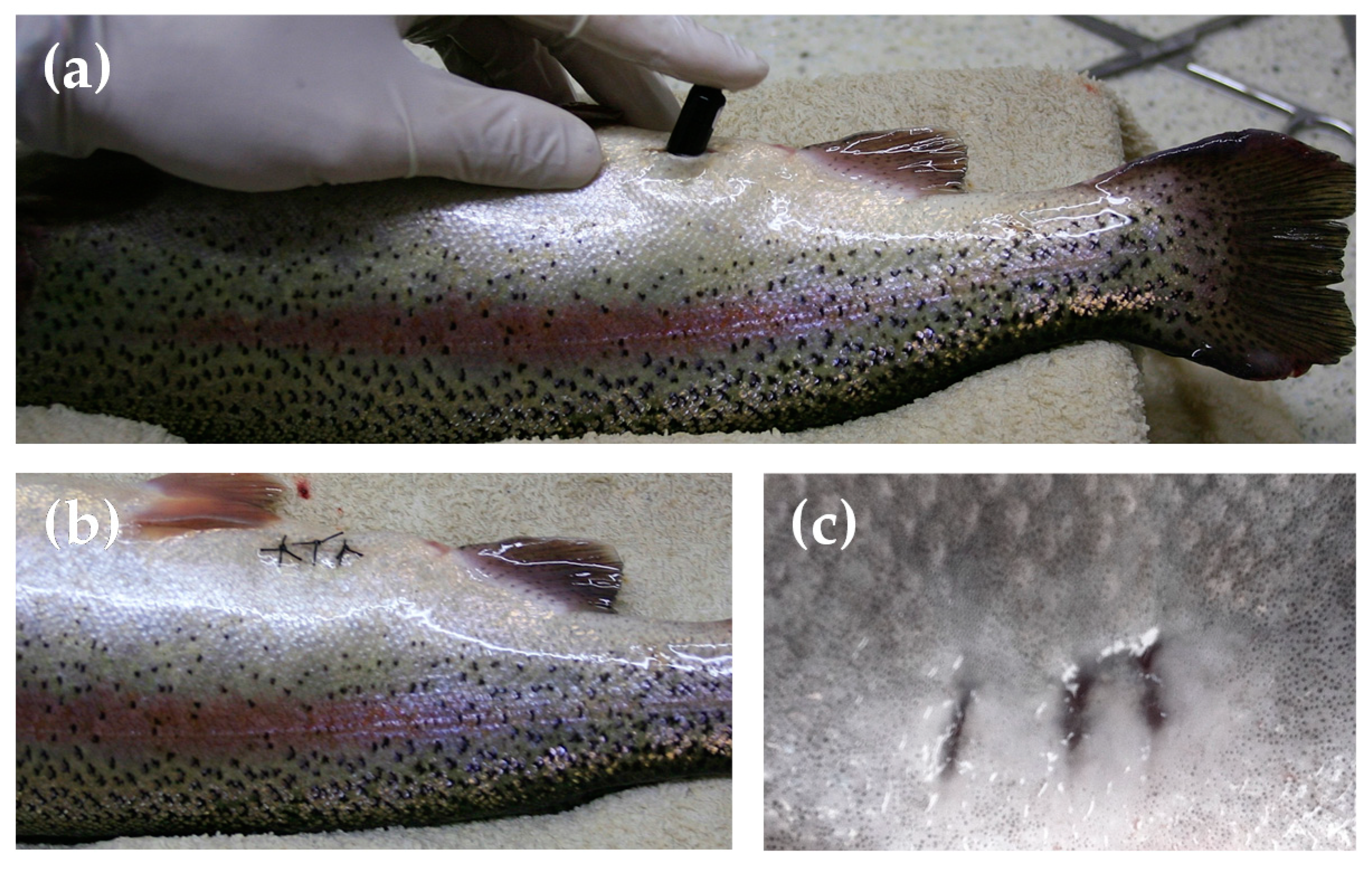
2.4. Calibration of Tailbeat Accelerometer with Oxygen Consumption Rate
2.5. Case Study
2.6. Statistical Analyses
3. Results
3.1. Critical Swimming Speed (Ucrit) and Estimation of Metabolic Rates
3.2. Muscular Activation Pattern
3.3. Calibration of Accelerometer Tag with Oxygen Consumption
3.4. Case Study in Aquaculture Facility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altringham, J.D.; Ellerby, D.J. Fish swimming: Patterns in muscle function. J. Exp. Biol. 1999, 202, 3397–3403. [Google Scholar] [CrossRef]
- Nelson, J.A. Oxygen consumption rate v. rate of energy utilization of fishes: A comparison and brief history of the two measurements. J. Fish. Biol. 2016, 88, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.H.; Gilooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Norin, T.; Clark, T.D. Measurement and relevance of maximum metabolic rate in fishes. J. Fish. Biol. 2016, 88, 122–151. [Google Scholar] [CrossRef] [PubMed]
- Chabot, D.; McKenzie, D.J.; Craig, J.F. Metabolic rate in fishes: Definitions, methods and significance for conservation physiology. J. Fish. Biol. 2016, 88, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish. Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef]
- Brett, J.R. The Respiratory metabolism and swimming performance of young Sockeye salmon. J. Fish. Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Beamish, F.W.H. Swimming capacity. Fish. Physiol. 1978, 7, 101–187. [Google Scholar] [CrossRef]
- Brett, J.R. The metabolic demand for oxygen in fish, particularly salmonids, and a comparison with other vertebrates. Respir. Physiol. 1972, 14, 151–170. [Google Scholar] [CrossRef]
- Norin, T.; Malte, H.; Clark, T.D. Differential plasticity of metabolic rate phenotypes in a tropical fish facing environmental change. Funct. Ecol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, J.D.; Wright, S.; Tudorache, C.; Wilson, R.P. Recent advances in telemetry for estimating the energy metabolism of wild fishes. J. Fish. Biol. 2016, 88, 284–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, S.J.; Hinch, S.G.; Wikelski, M.; Andrews, R.D.; Kuchel, L.J.; Wolcott, T.G.; Butler, P.J. Biotelemetry: A mechanistic approach to ecology. Trends Ecol. Evol. 2004, 19, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Thorstad, E.B.; Hinch, S.G. Activity and energetics of free-swimming fish: Insights from electromyogram telemetry. Fish Fish. 2004, 5, 21–52. [Google Scholar] [CrossRef]
- Wilson, S.M.; Hinch, S.G.; Eliason, E.J.; Farrell, A.P.; Cooke, S.J. Calibrating acoustic acceleration transmitters for estimating energy use by wild adult Pacific salmon. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 491–498. [Google Scholar] [CrossRef]
- Wright, S.; Metcalfe, J.D.; Hetherington, S.; Wilson, R. Estimating activity-specific energy expenditure in a teleost fish, using accelerometer loggers. Mar. Ecol. Prog. Ser. 2014, 496, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Carbonara, P.; Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Pretto, T.; Spedicato, M.T.; Lembo, G. Behavioral and physiological responses to stocking density in sea bream (Sparus aurata): Do coping styles matter? Physiol. Behav. 2019, 212, 112698. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Kiessling, A.; Berg, C.; Gräns, A. Remote physiological monitoring provides unique insights on the cardiovascular performance and stress responses of freely swimming rainbow trout in aquaculture. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Bone, Q. On the function of the two types of myotomal muscle fibre in elasmobranch fish. J. Mar. Biol. Assoc. U. K. 1966, 46, 321–349. [Google Scholar] [CrossRef]
- Noda, T.; Kawabata, Y.; Arai, N.; Mitamura, H.; Watanabe, S. Monitoring escape and feeding behaviours of cruiser fish by inertial and magnetic sensors. PLoS ONE 2013, 8, e79392. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, Y.; Noda, T.; Nakashima, Y.; Nanami, A.; Sato, T.; Takebe, T.; Mitamura, H.; Arai, N.; Yamaguchi, T.; Soyano, K. Use of a gyroscope/accelerometer data logger to identify alternative feeding behaviours in fish. J. Exp. Biol. 2014, 217, 3204–3208. [Google Scholar] [CrossRef] [Green Version]
- Cooke, S.J.; Chandroo, K.P.; Beddow, T.A.; Moccia, R.D.; McKinley, R.S. Swimming activity and energetic expenditure of captive rainbow trout Oncorhynchus mykiss (Walbaum) estimated by electromyogram telemetry. Aquac. Res. 2000, 31, 495–505. [Google Scholar] [CrossRef]
- Gesto, M.; Zupa, W.; Alfonso, S.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using acoustic telemetry to assess behavioral responses to acute hypoxia and ammonia exposure in farmed rainbow trout of different competitive ability. Appl. Anim. Behav. Sci. 2020, 230, 105084. [Google Scholar] [CrossRef]
- Carbonara, P.; Alfonso, S.; Gai, F.; Gasco, L.; Palmegiano, G.; Spedicato, M.T.; Zupa, W.; Lembo, G. Moderate stocking density does not influence the behavioural and physiological responses of rainbow trout (Oncorhynchus mykiss) in organic aquaculture. Aquac. Res. 2020, 51, 3007–3016. [Google Scholar] [CrossRef] [Green Version]
- Huntingford, F.A.; Kadry, S. Welfare and fish. Fish Welf. 2008, 1, 19–32. [Google Scholar]
- Saraiva, J.L.; Arechavala-Lopez, P. Welfare of fish—no longer the elephant in the room. Fishes 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Conte, F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223. [Google Scholar] [CrossRef]
- Dawkins, M.S. From an animal’s point of view: Motivation, fitness, and animal welfare. Behav. Brain Sci. 1990, 13, 1–61. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Arechavala-Lopez, P.; Castanheira, M.F.; Volstorf, J.; Studer, B.H. A global assessment of welfare in farmed fishes: The fishethobase. Fishes 2019, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Huntingford, F.A.; Kadri, S. Taking account of fish welfare: Lessons from aquaculture. J. Fish. Biol. 2009, 75, 2862–2867. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish. Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Thorstad, E. Effects of telemetry transmitters on swimming performance of adult Atlantic salmon. J. Fish. Biol. 2000, 57, 531–535. [Google Scholar] [CrossRef]
- Quintella, B.R.; Póvoa, I.; Almeida, P.R. Swimming behaviour of upriver migrating sea lamprey assessed by electromyogram telemetry. J. Appl. Ichthyol. 2009. [Google Scholar] [CrossRef]
- Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; Zupa, W.; Mckinley, R.S.; Lembo, G. Muscle activity as a key indicator of welfare in farmed European sea bass (Dicentrarchus labrax L. 1758). Aquac. Res. 2015, 46, 2133–2146. [Google Scholar] [CrossRef]
- Lembo, G.; Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; McKinley, R.S. Use of muscle activity indices as a relative measure of well-being in cultured sea bass Dicentrarchus labrax (Linnaeus, 1758). Hydrobiologia 2007, 582, 271–280. [Google Scholar] [CrossRef]
- Zupa, W.; Carbonara, P.; Spedicato, M.T.; Lembo, G. Modelling swimming activities and energetic costs in European sea bass (Dicentrarchus labrax L., 1758) during critical swimming tests. Mar. Freshw. Behav. Physiol. 2015. [Google Scholar] [CrossRef]
- Blazka, P.; Volf, M.; Cepela, M. A new type of respirometer for the determination of the metabolism of fish in an active state. Physiol. Bohemoslov. 1960, 9, 553–558. [Google Scholar]
- Smit, H.; Amelink-Koutstaal, J.M.; Vijverberg, J.; Von Vaupel-Klein, J.C. Oxygen consumption and efficiency of swimming goldfish. Comp. Biochem. Physiol. Part A Physiol. 1971, 39, 1–28. [Google Scholar] [CrossRef]
- Halsey, L.G.; Killen, S.S.; Clark, T.D.; Norin, T. Exploring key issues of aerobic scope interpretation in ectotherms: Absolute versus factorial. Rev. Fish. Biol. Fish. 2018, 28, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.G.; McKinley, R.S.; Colavecchia, M. The use of clove oil as an anesthetic for rainbow trout and its effects on swimming performance. North. Am. J. Fish. Manag. 1997, 17, 301–307. [Google Scholar] [CrossRef]
- Iversen, M.; Finstad, B.; McKinley, R.S.; Eliassen, R.A. The efficacy of metomidate, clove oil, Aqui-STM and Benzoak® as anaesthetics in Atlantic salmon (Salmo salar L.) smolts, and their potential stress-reducing capacity. Aquaculture 2003, 221, 549–566. [Google Scholar] [CrossRef]
- Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Dioguardi, M.; Dara, M.; Lembo, G.; Carbonara, P.; Cammarata, M. Surgical implantation of electronic tags does not induce medium-term effect: Insights from growth and stress physiological profile in two marine fish species. Anim. Biotelemetry 2020, 8, 21. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Lennox, R.J.; Danylchuk, A.J.; Cooke, S.J. Estimating fish swimming metrics and metabolic rates with accelerometers: The influence of sampling frequency. J. Fish. Biol. 2018, 93, 207–214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Akaike, H. Information theory and an extensión of the maximum likelihood principle. Int. Symp. Inf. Theory 1973, 267–281. [Google Scholar] [CrossRef]
- Hammer, C. Fatigue and exercise tests with fish. Comp. Biochem. Physiol. Part A Physiol. 1995, 112, 1–20. [Google Scholar] [CrossRef]
- Farrell, A.P.; Johansen, J.A.; Steffensen, J.F.; Moyes, C.D.; West, T.G.; Suarez, R.K. Effects of exercise training and coronary ablation on swimming performance, heart size, and cardiac enzymes in rainbow trout, Oncorhynchus mykiss. Can. J. Zool. 1990, 68, 1174–1179. [Google Scholar] [CrossRef]
- Alsop, D.H.; Wood, C.M. The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1997, 200, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Chandroo, K.P.; Cooke, S.J.; McKinley, R.S.; Moccia, R.D. Use of electromyogram telemetry to assess the behavioural and energetic responses of rainbow trout, Oncorhynchus mykiss (Walbaum) to transportation stress. Aquac. Res. 2005, 36, 1226–1238. [Google Scholar] [CrossRef]
- Lee, C.G. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 2003, 206, 3239–3251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingles, A.; McKenzie, D.J.; Taylor, E.W.; Moretti, A.; Butler, P.J.; Ceradini, S. Effects of sublethal ammonia exposure on swimming performance in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2001, 204, 2691–2698. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Höglund, E.; Dupont-Prinet, A.; Larsen, B.K.; Skov, P.V.; Pedersen, P.B.; Jokumsen, A. Effects of stocking density and sustained aerobic exercise on growth, energetics and welfare of rainbow trout. Aquaculture 2012, 338–341, 216–222. [Google Scholar] [CrossRef]
- Webb, P.W. Form and function in fish swimming. Sci. Am. 1984, 251, 72–83. [Google Scholar] [CrossRef]
- Farrell, A.P. Cardiorespiratory performance during prolonged swimming tests with salmonids: A perspective on temperature effects and potential analytical pitfalls. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2007, 362, 2017–2030. [Google Scholar] [CrossRef] [Green Version]
- Chabot, D.; Koenker, R.; Farrell, A.P. The measurement of specific dynamic action in fishes. J. Fish. Biol. 2016, 88, 152–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Biol. Sci. 2011, 278, 3465–3473. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.O. Metabolic thermal compensation by rainbow-trout—Effects on standard metabolic-rate and potential usable power. Trans. Am. Fish. Soc. 1990, 119, 585–600. [Google Scholar] [CrossRef]
- Dickson, I.W.; Kramer, R.H. Factors influencing scope for activity and active and standard metabolism of Rainbow trout (Salmo gairdneri). J. Fish. Board Can. 1971, 28. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Pedersen, P.B.; Jokumsen, A. Aspects of respiratory physiology and energetics in rainbow trout (Oncorhynchus mykiss) families with different size-at-age and condition factor. Aquaculture 2007, 263, 280–294. [Google Scholar] [CrossRef]
- Moltesen, M.; Ebbesson, L.; Dabelsteen, T.; Vindas, M.A.; de Lourdes Ruiz-Gomez, M.; Øverli, Ø.; Höglund, E.; Skov, P.V.; Winberg, S. Cognitive appraisal of aversive stimulus differs between individuals with contrasting stress coping styles; evidences from selected rainbow trout (Oncorhynchus mykiss) strains. Behaviour 2016, 153, 1567–1587. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Rogers, S.C.; Patch, J.R. Oxygen consumption of active rainbow trout, Salmo gairdneri Richardson, derived from electromyograms obtained by radiotelemetry. J. Fish. Biol. 1982, 479–489. [Google Scholar] [CrossRef]
- Beddow, T.A.; Mckinley, R.S. Effects of thermal environment on Electromyographical signals obtained from Atlantic salmon (Salmo salar L.) during forced swimming. In Advances in Invertebrates and Fish Telemetry; Springer: Dordrecht, The Netherlands, 1998; pp. 225–232. [Google Scholar]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef] [Green Version]
- McFarlane, W.J.; Cubitt, K.F.; Williams, H.; Rowsell, D.; Moccia, R.; Gosine, R.; McKinley, R.S. Can feeding status and stress level be assessed by analyzing patterns of muscle activity in free swimming rainbow trout (Oncorhynchus mykiss Walbaum)? Aquaculture 2004, 239, 467–484. [Google Scholar] [CrossRef]
- Lembo, G.; Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; Bjørnsen, J.E.; Holand, B.; McKinley, R.S. Introduction of a new physiological sensor: An acoustic EMG transmitter. Fish. Manag. Ecol. 2008, 15, 333–338. [Google Scholar] [CrossRef]
- Jepsen, N.; Davis, L.E.; Schreck, C.B.; Siddens, B. The physiological response of chinook salmon smolts to two methods of radio-tagging. Trans. Am. Fish. Soc. 2011, 130, 495–500. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Rosengren, M.; Sundell, K.; Berg, C.; Axelsson, M.; Gräns, A. Prospects and pitfalls of using heart rate bio-loggers to assess the welfare of rainbow trout (Oncorhynchus mykiss) in aquaculture. Aquaculture 2019, 509, 188–197. [Google Scholar] [CrossRef]
- Sloman, K.A.; Motherwell, G.; O’connor, K.I.; Taylor, A.C. The effect of social stress on the Standard Metabolic Rate (SMR) of brown trout, Salmo trutta. Fish. Physiol. Biochem. 2000, 23, 49–53. [Google Scholar] [CrossRef]
- Barton, B.A.; Schreck, C.B. Metabolic cost of acute physical stress in juvenile Steelhead. Trans. Am. Fish. Soc. 1987, 116, 257–263. [Google Scholar] [CrossRef]
- Svendsen, E.; Føre, M.; Økland, F.; Gräns, A.; Hedger, R.D.; Alfredsen, J.A.; Uglem, I.; Rosten, C.M.; Frank, K.; Erikson, U.; et al. Heart rate and swimming activity as stress indicators for Atlantic salmon (Salmo salar). Aquaculture 2021, 531, 735804. [Google Scholar] [CrossRef]
- Lefrançois, C.; Claireaux, G.; Mercier, C.; Aubin, J. Effect of density on the routine metabolic expenditure of farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 2001, 195, 269–277. [Google Scholar] [CrossRef]
- Bégout Anras, M.L.; Lagardère, J.P. Measuring cultured fish swimming behaviour: First results on rainbow trout using acoustic telemetry in tanks. Aquaculture 2004, 240, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Huyben, D.; Broström, R.; Kiessling, A.; Berg, C.; Gräns, A. The final countdown: Continuous physiological welfare evaluation of farmed fish during common aquaculture practices before and during harvest. Aquaculture 2018, 495, 903–911. [Google Scholar] [CrossRef]
- Carbonara, P.; Dioguardi, M.; Cammarata, M.; Zupa, W.; Vazzana, M.; Spedicato, M.T.; Lembo, G. Basic knowledge of social hierarchies and physiological profile of reared sea bass Dicentrarchus labrax (L.). PLoS ONE 2019, 14, e0208688. [Google Scholar] [CrossRef]








| U Ucrit | |||||
| Variable | Parameters | Estimate | Standard Error | t Value | p Value |
| TL | (intercept) | −0.79 | 0.40 | −1.96 | 0.07 |
| x | 0.06 | 0.01 | 4.93 | <0.001 | |
| BW | (intercept) | 0.42 | 0.13 | 3.23 | 0.005 |
| x | 0.002 | 0.0003 | 6.13 | <0.001 | |
| BL Ucrit | |||||
| Variable | Parameters | Estimate | Standard Error | t Value | p Value |
| TL | (intercept) | 1.06 | 1.22 | 0.86 | 0.40 |
| x | 0.08 | 0.04 | 2.06 | 0.054 | |
| BW | (intercept) | 2.31 | 0.40 | 5.83 | <0.001 |
| x | 0.003 | 0.0008 | 3.27 | 0.004 |
| Parameters | Estimate | St. Error | t Value | p Value | |
|---|---|---|---|---|---|
| Ucrit–oxygen consumption rate | |||||
| Asym | 668.69 | 134.4 | 5.12 | <0.001 | |
| xmid | 0.48 | 0.13 | 3.7 | <0.001 | |
| scal | 0.28 | 0.07 | 3.84 | <0.001 | |
| EMG–red muscle | |||||
| intercept | 0.02 | 0.10 | 0.22 | 0.82 | |
| x | 1.27 | 0.19 | 6.72 | <0.001 | |
| EM–white muscle | |||||
| alpha | 0.11 | 0.01 | 8.87 | <0.001 | |
| beta | 1.50 | 0.17 | 9.03 | <0.001 | |
| Calibration–swimming activity | |||||
| alpha | 18.89 | 2.41 | 7.87 | <0.001 | |
| beta | 1.78 | 0.16 | 11.43 | <0.001 | |
| Calibration–oxygen consumption rate | |||||
| Asym | 675.45 | 54.26 | 12.45 | <0.001 | |
| xmid | 43.98 | 4.23 | 10.39 | <0.001 | |
| scal | 20.79 | 3.11 | 6.23 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupa, W.; Alfonso, S.; Gai, F.; Gasco, L.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study. Animals 2021, 11, 1496. https://doi.org/10.3390/ani11061496
Zupa W, Alfonso S, Gai F, Gasco L, Spedicato MT, Lembo G, Carbonara P. Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study. Animals. 2021; 11(6):1496. https://doi.org/10.3390/ani11061496
Chicago/Turabian StyleZupa, Walter, Sébastien Alfonso, Francesco Gai, Laura Gasco, Maria Teresa Spedicato, Giuseppe Lembo, and Pierluigi Carbonara. 2021. "Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study" Animals 11, no. 6: 1496. https://doi.org/10.3390/ani11061496
APA StyleZupa, W., Alfonso, S., Gai, F., Gasco, L., Spedicato, M. T., Lembo, G., & Carbonara, P. (2021). Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study. Animals, 11(6), 1496. https://doi.org/10.3390/ani11061496










