Morphometrical Study of the Lumbar Segment of the Internal Vertebral Venous Plexus in Dogs: A Contrast CT-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Anaesthesia Protocol
2.3. CT Venography Protocol
2.4. Morphometric Analysis
- −
- cross sectional area of the vertebral canal (mm2);
- −
- cross sectional area of the dural sac (mm2);
- −
- total area of the IVVP (area of the right and left IVVP component) (mm2);
- −
- area of the epidural space: vertebral canal area minus the area occupied by the dural sac (mm2);
- −
- percentage of the IVVP occupying the vertebral canal;
- −
- percentage of the dural sac occupying the vertebral canal;
- −
- percentage of the IVVP within the epidural space;
2.5. Statistical Analysis
3. Results
3.1. Internal Vertebral Venous Plexus
3.2. Vertebral Canal
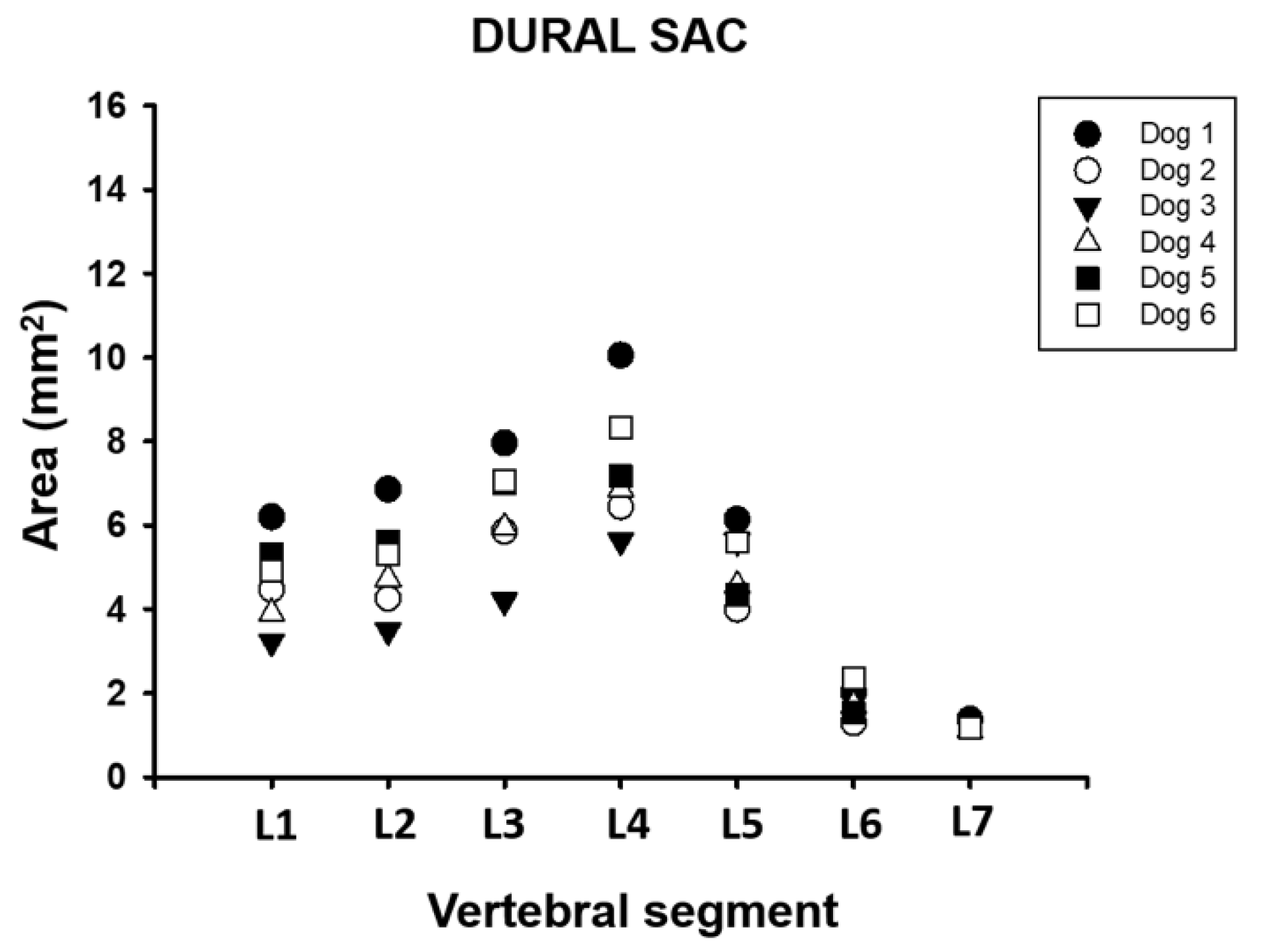
3.3. Dural Sac
3.4. Epidural Space
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Evans, H. Miller’s Anatomy of the Dog, 3rd ed.; WB Saunders Company: Philadelphia, PA, USA, 1993; pp. 713–715. [Google Scholar]
- International Committee on Veterinary Gross Anatomical Nomenclature; World Association of Veterinary Anatomists (W.A.V.A.). Nomina Anatomica Veterinaria (2012); Editorial Committee: Hannover, Germany; Columbia, MO, USA; Ghent, Belgium; Sapporo, Japan, 2012. [Google Scholar]
- Gómez, M.; Freeman, L. Revisión del plexo venosos vertebral en el perro. Int. J. Morphol. 2003, 21, 237–244. [Google Scholar]
- Parke, W. Applied Anatomy of the Spine, 3rd ed.; WB Saunders Company: Philadelphia, PA, USA, 1992; pp. 35–88. [Google Scholar]
- Groen, R.J.; Groenewegen, H.J.; van Alphen, H.A.; Hoogland, P.V. Morphology of the human internal vertebral venous plexus: A cadaver study after intravenous Araldite CY 221 injection. Anat. Rec. 1997, 249, 285–294. [Google Scholar] [CrossRef]
- Kreppel, D.A.G.; Seeling, W. Spinal hematoma: A literatura survey with meta-analysis of 613 patients. Neurosurg. Rev. 2003, 26, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Applewhite, A.A.; Wilkens, B.E.; McDonald, D.E.; Radasch, R.M.; Barstad, R.D. Potential central nervous system complications of von Willebrand’s disease. J. Am. Anim. Hosp. Assoc. 1999, 35, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, P.; Toombs, J.; Laverty, P.; Breur, G. Loss of deep pain sensation following thoracolumbar intervertebral disk herniation in dogs: Pathophysiology. Compend. Contin. Educ. Pract. Vet. 2003, 25, 256–264. [Google Scholar]
- King, A.S. Physiological and Clinical Anatomy of the Domestic Mammals; Oxford University Press: Oxford, UK, 1987; pp. 24–30. [Google Scholar]
- Thomas, W.B. Diskospondylitis and other vertebral infections. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 169–182. [Google Scholar] [CrossRef]
- Penwick, R. Fibrocartilaginous embolism and ischemic myelopathy. Compend. Contin. Educ. Pract. Vet. 1989, 11, 287–297. [Google Scholar]
- Spetzler, R.F.; Detwiler, P.W.; Riina, H.A.; Porter, R.W. Modified classification of spinal cord vascular lesions. J. Neurosurg. 2002, 96 (Suppl. 2), 145–156. [Google Scholar] [CrossRef]
- Vicens, L.; Tapia, R.; Bergman, W.; Bergknut, N. Venous Sinus Aneurysm in a Scottish Deerhound. In Proceedings of the 31st Annual Symposium of the ESVN-ECVN, Kopenhagen, Denmark, 20–22 September 2018. [Google Scholar]
- Vernon, J.C.; Durand, A.; Guevar, J.; José-López, R.; Hammond, G.; Stalin, C.; Gutierrez-Quintana, R. Vertebral venous system abnormalities identified with magnetic resonance imaging in sighthounds. Vet. Radiol. Ultrasound 2017, 58, 399–410. [Google Scholar] [CrossRef]
- d’Anjou, M.; Schwarz, T. Hearts and vessels. In Veterinary Computed Tomography, 1st ed.; Schwarz, T., Saunders, J., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 229–242. [Google Scholar]
- Thompson, M.; Graham, J.; Mariani, C. Diagnosis of a porto-azygous shunt using helical computed tomograpy angiography. Vet. Radiol Ultrasound 2003, 44, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Freeman, L.; Jones, J.; Lanz, O.; Arnold, P. Computed tomographic anatomy of the canine cervical vertebral venous system. Vet. Radiol. Ultrasound 2004, 45, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Jones, J.C.; Broadstone, R.V.; Inzana, K.D.; Freeman, L.E. Evaluation of the internal vertebral venous plexus, vertebral canal, dural sac, and vertebral body via nonselective computed tomographic venography in the cervical vertebral column in healthy dogs. Am. J. Vet. Res. 2005, 66, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.; Zwingenberger, A.; Hardam, E.; Lucena, J.; Schwarz, T. Helical computed tomography of the normal canine páncreas. Vet. Radiol. Ultasound 2006, 47, 270–278. [Google Scholar] [CrossRef]
- Jung, J.; Chang, J.; Sunkyoung, O.; Junghee, Y.; Mincheol, C. Computed tomography for evaluation of pulmonary embolism in a experimental model and heartworm infested dogs. Vet. Radiol. Ultrasound 2010, 51, 288–293. [Google Scholar] [CrossRef] [PubMed]
- <named-content content-type="background:white">Oui, H.; Kim, J.; Bae, Y.; Oh, J.; Park, S.; Lee, G.; Jeon, S.; Choi, J. Computed tomography angiography of situs inversus, portosystemic shunt and multiple vena cava anomalies in a dog. J. Vet. Med. Sci. 2013, 75, 1525–1528. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; Pisani, A.; Sabbatini, M.; Michael, A. Acute kidney injury by radiographic contrast media: Pathogenesis and prevention. Biomed. Res. Int. 2014, 2014, 362725. [Google Scholar] [CrossRef]
- Pollard, R.; Puchalski, S. CT contrast media an applications. In Veterinary Computed Tomography; Schwarz, T., Saunders, J., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 57–66. [Google Scholar]
- Stringer, M.D.; Restieaux, M.; Fisher, A.L.; Crosado, B. The vertebral venous plexuses: The internal veins are muscular and external veins have valves. Clin. Anat. 2012, 25, 609–618. [Google Scholar] [CrossRef]
- Demondion, X.; Delfaut, E.M.; Drizenko, A.; Boutry, N.; Francke, J.P.; Cotten, A. Radio-anatomic demonstration of the vertebral lumbar venous plexuses: An MRI experimental study. Surg. Radiol. Anat. 2000, 22, 151–156. [Google Scholar] [CrossRef]
- Zippel, K.C.; Lillywhite, H.B.; Mladinich, C.R. New vascular system in reptiles: Anatomy and postural hemodynamics of the vertebral venous plexus in snakes. J. Morphol. 2001, 250, 173–184. [Google Scholar] [CrossRef]
- Zippel, K.C.; Lillywhite, H.B.; Mladinich, C.R. Anatomy of the crocodilian spinal vein. J. Morphol. 2003, 258, 327–335. [Google Scholar] [CrossRef]
- Kotzé, S.; Vorster, J.; Hoogland, P. A morphological study of the vertebral venous plexus and its connections in the Cape dune mole-rat, Bathyergus suillus (Bathyergidae). J. Morphol. 2010, 272, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, V.L.; Patel, A.; Pham, M.H.; Strickland, B.A.; Ohiorhenuan, I.; Chen, T. Spine Surgery Complicated by an Engorged Lumbar Epidural Venous Plexus from Cerebrospinal Fluid Overshunting: A Case Report and Review of the Literature. World Neurosurg. 2018, 111, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Rhue, K.E.; Taylor, A.R.; Cole, R.C.; Winter, R.L. Bilateral Vertebral Venous Sinus Thrombosis Causing Cervical Spinal Cord Compression in a Dog. Front. Vet. Sci. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin-Vaquero, P.; da Costa, R.C. Magnetic resonance imaging features of Great Danes with and without clinical signs of cervical spondylomyelopathy. J. Am. Vet. Med. Assoc. 2014, 245, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology 2001, 56, 1746–1748. [Google Scholar] [CrossRef]
- Mannion, A.F.; Fekete, T.F.; Pacifico, D.; O’Riordan, D.; Nauer, S.; von Büren, M.; Schizas, C. Dural sac cross-sectional area and morphological grade show significant associations with patient-rated outcome of surgery for lumbar central spinal stenosis. Eur. Spine J. 2017, 26, 2552–2564. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, Z.; Zhang, P.; Shan, B.; Zhou, Z.; Zhang, Y.; Deng, Y.; Zhou, X. Correlation Between Dural Sac Size in Dynamic Magnetic Resonance Imaging and Clinical Symptoms in Patients with Lumbar Spinal Stenosis. World Neurosurg. 2020, 134, e866–e873. [Google Scholar] [CrossRef]
- Lim, J.; Yoon, Y.; Hwang, T.; Lee, H.C. Novel vertebral computed tomography indices in normal and spinal disorder dogs. J. Vet. Sci. 2018, 19, 296–300. [Google Scholar] [CrossRef]
- Dabanoglu, I.; Kara, M.E.; Turan, E.; Ocal, M.K. Morphometry of the thoracic spine in German shepherd dog: A computed tomographic study. Anat. Histol. Embryol. 2004, 33, 53–58. [Google Scholar] [CrossRef]
- Sturges, B.K.; Westworth, D.R. Congenital spinal malformations in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 951–981. [Google Scholar]
- Bailey, C.S.; Morgan, J.P. Congenital spinal malformations. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 985–1015. [Google Scholar] [CrossRef]
- Thrall, D. Textbook of Veterinary Diagnostic Radiology, 7th ed.; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
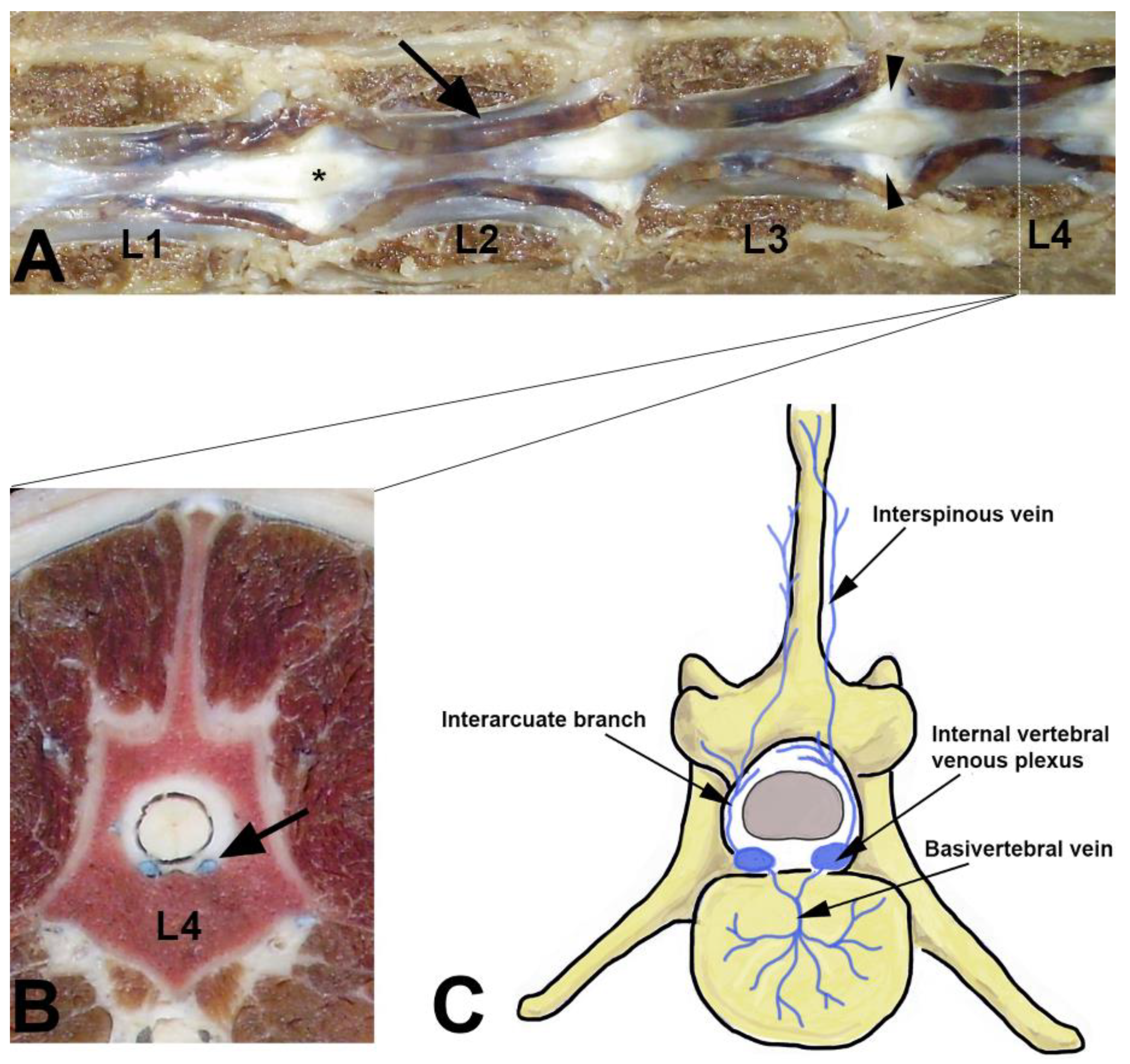
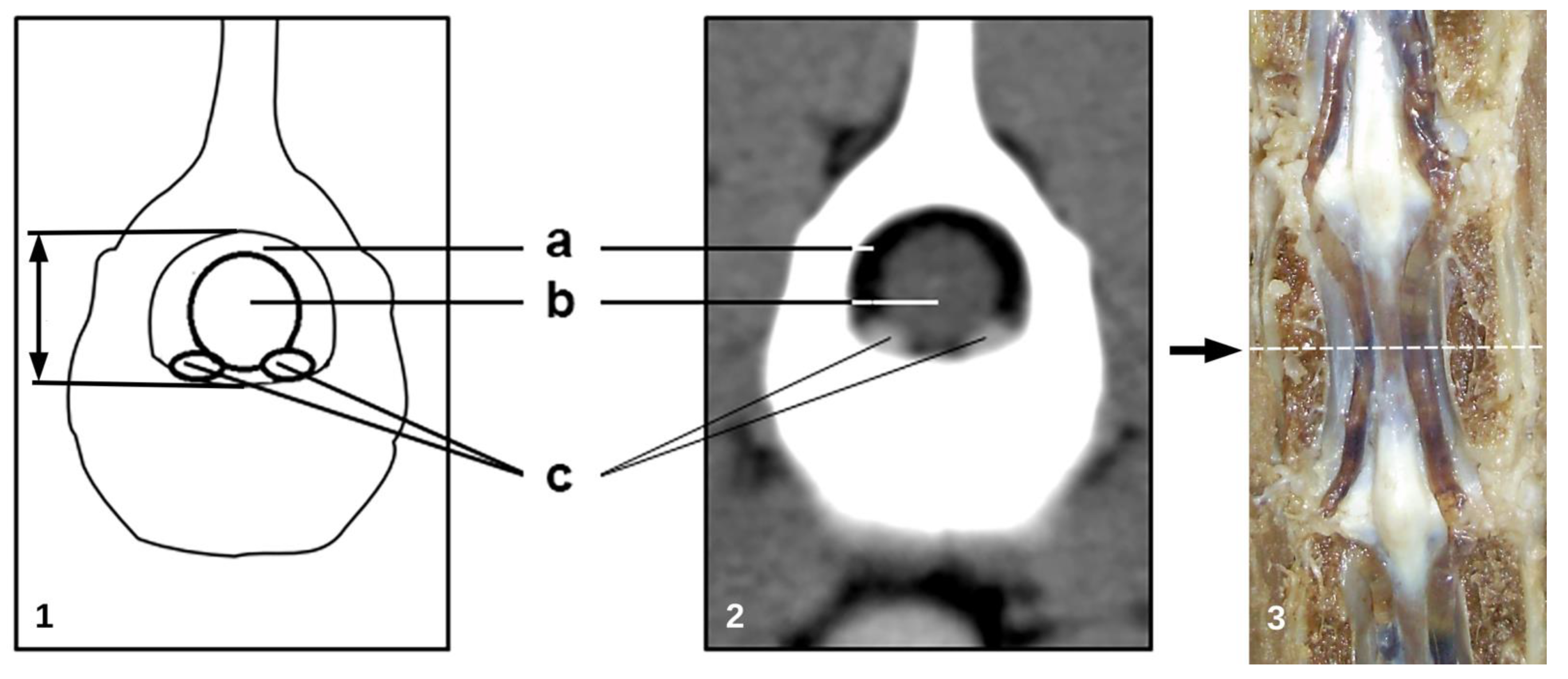





| Morphometric Measures | Vertebral Segment | ||||||
|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | L6 | L7 | |
| Vertebral canal | 7.87 ± 1.3 | 8.24 ± 1.8 | 9.21 ± 1.7 | 10.27 ± 1.9 | 9.77 ± 1.7 | 8.68 ± 1.2 | 8.28 ± 2 |
| Dural sac | 4.67 ± 1.1 | 5.04 ± 1.2 | 6.34 ± 1.3 | 7.42 ± 1.6 | 5.04 ± 0.9 | 1.78 ± 0.4 | 1.22 ± 0.1 |
| IVVP | 0.7 ± 0.2 | 0.66 ± 0.1 | 0.64 ± 0.1 | 0.63 ± 0.1 | 0.74 ± 0.2 | 0.66 ± 0.2 | 0.61 ± 0.1 |
| Epidural space | 3.2 ± 0.6 | 3.2 ± 0.9 | 2.87 ± 0.7 | 2.85 ± 0.8 | 4.72 ± 1.6 | 6.9 ± 0.8 | 7.78 ± 1.6 |
| Morphometric Measures | Vertebral Segment | ||||||
|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | L6 | L7 | |
| Dural sac | 59 (47.7–67,4) | 61.3 (49.1–69.4) | 68.8 (58.8–76.6) | 72.2 (62.6–77.8) | 52.6 (46.8–75.3) | 20.4 (17–21.2) | 13.8 (11.3–15.7) |
| IVVP | 8.9 (6.4–11.8) | 8.3 (5.5–10.3) | 7.1 (4.8–8.7) | 6.3 (4.8–8.8) | 7.4 (5.3–10.8) | 7.7 (4.5–9.5) | 7.9 (4.5–11.2) |
| Epidural space | 41 (32.6–52.3) | 38.7 (30.6–50.9) | 31.2 (23.4–41.2) | 27.8 (20.8–37.4) | 47.4 (24.7–58.9) | 79.6 (75.6–83) | 86.2 (84.3–88.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariete, V.; Barnert, N.; Gómez, M.; Mieres, M.; Pérez, B.; Gutierrez, J.C. Morphometrical Study of the Lumbar Segment of the Internal Vertebral Venous Plexus in Dogs: A Contrast CT-Based Study. Animals 2021, 11, 1502. https://doi.org/10.3390/ani11061502
Ariete V, Barnert N, Gómez M, Mieres M, Pérez B, Gutierrez JC. Morphometrical Study of the Lumbar Segment of the Internal Vertebral Venous Plexus in Dogs: A Contrast CT-Based Study. Animals. 2021; 11(6):1502. https://doi.org/10.3390/ani11061502
Chicago/Turabian StyleAriete, Valeria, Natalia Barnert, Marcelo Gómez, Marcelo Mieres, Bárbara Pérez, and Juan Claudio Gutierrez. 2021. "Morphometrical Study of the Lumbar Segment of the Internal Vertebral Venous Plexus in Dogs: A Contrast CT-Based Study" Animals 11, no. 6: 1502. https://doi.org/10.3390/ani11061502
APA StyleAriete, V., Barnert, N., Gómez, M., Mieres, M., Pérez, B., & Gutierrez, J. C. (2021). Morphometrical Study of the Lumbar Segment of the Internal Vertebral Venous Plexus in Dogs: A Contrast CT-Based Study. Animals, 11(6), 1502. https://doi.org/10.3390/ani11061502







