The Role of the Guanosine Nucleotide-Binding Protein in the Corpus Luteum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Potential Function of RAS in the Ovary
3. The Likelihood of a Novel Role of RAS in the Ovarian Corpus Luteum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Telfer, E.E.; Anderson, R.A. The existence and potential of germline stem cells in the adult mammalian ovary. Climacteric 2019, 22, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Berisha, B.; Schams, D.; Rodler, D.; Pfaffl, M.W. Angiogenesis in the ovary–the most important regulatory event for follicle and corpus luteum development and function in cow—an overview. Anat. Histol. Embryol. 2016, 45, 124–130. [Google Scholar] [CrossRef]
- Richards, J.S. The ovarian cycle. Vitam. Horm. 2018, 107, 1–25. [Google Scholar] [PubMed]
- Csépányi-Kömi, R.; Lévay, M.; Ligeti, E. Small G proteins and their regulators in cellular signalling. Mol. Cell. Endocrinol. 2012, 353, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei-Rad, S.; Haghighi, F.; Nouri, P.; Rezaei Adariani, S.; Lissy, J.; Kazemein Jasemi, N.S.; Ahmadian, M.R. Structural fingerprints, interactions, and signaling networks of RAS family proteins beyond RAS isoforms. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 130–156. [Google Scholar] [CrossRef]
- Serna-Blasco, R.; Sanz-Álvarez, M.; Aguilera, Ó.; García-Foncillas, J. Targeting the RAS-dependent chemoresistance: The Warburg connection. Semin. Cancer Biol. 2019, 54, 80–90. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761. [Google Scholar] [CrossRef] [Green Version]
- Quilliam, L.A. Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier Science: Kidlington, UK, 2013; pp. 12–16. [Google Scholar]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS proteins and their regulators in human disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.S.; Chen, Y.H. Ras family of small GTPases in immunity and inflammation. Curr. Opin. Pharmacol. 2012, 12, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.E.; Rukhlenko, O.S.; Posner, R.G.; Hlavacek, W.S.; Kholodenko, B.N. New insights into RAS biology reinvigorate interest in mathematical modeling of RAS signaling. Semin. Cancer Biol. 2019, 54, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Lambright, D.G. Invited review: Small GTPases and their GAPs. Biopolymers 2016, 105, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, R.O.; Mildorf, T.J.; Hilger, D.; Manglik, A.; Borhani, D.W.; Arlow, D.H.; Hubbell, W.L. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 2015, 348, 1361–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llavero, F.; Arrazola Sastre, A.; Luque Montoro, M.; Martín, M.A.; Arenas, J.; Lucia, A.; Zugaza, J.L. Small GTPases of the Ras superfamily and glycogen phosphorylase regulation in T cells. Small GTPases 2019, 12, 106–113. [Google Scholar] [CrossRef]
- Hancock, J.F. Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003, 4, 373. [Google Scholar] [CrossRef]
- Chavan, T.S.; Muratcioglu, S.; Marszalek, R.; Jang, H.; Keskin, O.; Gursoy, A.; Nussinov, R.; Gaponenko, V. Plasma membrane regulates Ras signaling networks. Cell. Logist. 2016, 5, e1136374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, E.J. Hormone and Receptor Structure and Function, 2nd ed.; CABI Publishing: Wallingford, UK, 2010; pp. 1–46. [Google Scholar]
- Norris, D.O.; Lopez, K.H. The Endocrinology of the Mammalian Ovary; Academic Press: London, UK, 2011; pp. 59–72. [Google Scholar]
- Krsmanovic, L.Z.; Hu, L.; Leung, P.K.; Feng, H.; Catt, K.J. The hypothalamic GnRH pulse generator: Multiple regulatory mechanisms. Trends Endocrinol. Metab. 2009, 20, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, I.R.; Kaiser, U.B. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol. Cell. Endocrinol. 2014, 385, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Invest. 2010, 120, 963–972. [Google Scholar] [CrossRef]
- Jiang, X.; Dias, J.A.; He, X. Structural biology of glycoprotein hormones and their receptors: Insights to signaling. Mol. Cell. Endocrinol. 2014, 382, 424–451. [Google Scholar] [CrossRef]
- Howles, C.M. Role of LH and FSH in ovarian function. Mol. Cell. Endocrinol. 2000, 161, 25–30. [Google Scholar] [CrossRef]
- Gromoll, J.; Simoni, M. Genetic complexity of FSH receptor function. Trends Endocrinol. Metab. 2005, 16, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Aguirre, A.; Reiter, E.; Crepieux, P. FSH receptor signaling: Complexity of interactions and signal diversity. Endocrinology 2018, 159, 3020–3035. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Lei, Z.M. Consequences of targeted inactivation of LH receptors. Mol. Cell. Endocrinol. 2002, 187, 57–67. [Google Scholar] [CrossRef]
- Lalioti, M.D. Impact of follicle stimulating hormone receptor variants in fertility. Curr. Opin. Obstet. Gynecol. 2011, 23, 158–167. [Google Scholar] [CrossRef]
- Bramble, M.S.; Goldstein, E.H.; Lipson, A.; Ngun, T.; Eskin, A.; Gosschalk, J.E.; Arboleda, V.A. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: A fertility application of whole exome sequencing. Hum. Reprod. 2016, 31, 905–914. [Google Scholar] [CrossRef]
- Casarini, L.; Crepieux, P. Molecular mechanisms of action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef]
- Wei, S.; Shen, X.; Lai, L.; Liang, H.; Deng, Y.; Gong, Z.; Che, T. FSH receptor binding inhibitor impacts K-Ras and c-Myc of ovarian cancer and signal pathway. Oncotarget 2018, 9, 22498. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gong, Z.; Shen, X.; Bai, S.; Bai, X.; Wei, S. FSH receptor binding inhibitor depresses carcinogenesis of ovarian cancer via decreasing levels of K-Ras, c-Myc and FSHR. Anim. Biotechnol. 2021, 32, 84–91. [Google Scholar] [CrossRef]
- Hunzicker-Dunn, M.E.; Lopez-Biladeau, B.; Law, N.C.; Fiedler, S.E.; Carr, D.W.; Maizels, E.T. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2979–E2988. [Google Scholar] [CrossRef] [Green Version]
- Duggavathi, R.; Murphy, B.D. Ovulation signals. Science 2009, 324, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Sugiura, K.; Li, Q.; Wigglesworth, K.; Matzuk, M.M.; Eppig, J.J. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol. Endocrinol. 2010, 24, 1230–1239. [Google Scholar] [CrossRef]
- Woods, D.C.; Johnson, A.L. Protein kinase C activity mediates LH-induced ErbB/Erk signaling in differentiated hen granulosa cells. Reproduction 2007, 133, 733–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.Y.; Liu, Z.; Mullany, L.K.; Richards, J.S. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol. Cell. Endocrinol. 2012, 356, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.S.; Ascoli, M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol. Metab. 2018, 29, 313–325. [Google Scholar] [CrossRef]
- Fan, H.Y.; Richards, J.S. Minireview: Physiological and pathological actions of RAS in the ovary. Mol. Endocrinol. 2010, 24, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.S.; LaVoie, H.A. The Ovary, 3rd ed.; Academic Press: London, UK, 2019; pp. 237–253. [Google Scholar]
- Devoto, L.; Henríquez, S.; Kohen, P.; Strauss, J.F. The significance of estradiol metabolites in human corpus luteum physiology. Steroids 2017, 123, 50–54. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Maalouf, S.W.; Smith, C.L.; Pate, J.L. Changes in microRNA expression during maturation of the bovine corpus luteum: Regulation of luteal cell proliferation and function by microRNA-34a. Biol. Reprod. 2016, 94, 71. [Google Scholar] [CrossRef]
- Robinson, R.S.; Woad, K.J.; Hunter, M.G.; Sinclair, K.D.; Laird, M.; Joseph, C.; Mann, G.E. Corpus luteum development and angiogenesis. Bioscientifica Proc. 2019, 8, 327–343. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Meidan, R.; Ochoa, J.; Baez, G.M.; Giordano, J.O.; Ferreira, J.C.P.; Sartori, R. Maintenance or regression of the corpus luteum during multiple decisive periods of bovine pregnancy. Anim. Reprod. 2018, 13, 217–233. [Google Scholar] [CrossRef]
- Woad, K.J.; Robinson, R.S. Luteal angiogenesis and its control. Theriogenology 2016, 86, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.M.; Ferreira-Dias, G.; Skarzynski, D.J. Cytokines and angiogenesis in the corpus luteum. Mediat. Inflamm. 2013, 2013, 420186. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.S. The critical importance of ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaessmeyer, S.; Huenigen, H.; Al Masri, S.; Dieckhoefer, P.; Richardson, K.; Plendl, J. Corpus luteal angiogenesis in a high milk production dairy breed differs from that of cattle with lower milk production levels. Vet. Med. 2016, 61, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef]
- Meidan, R.; Klipper, E.; Zalman, Y.; Yalu, R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 343–350. [Google Scholar] [CrossRef]
- Folkman, J.; Klagsbrun, M. Angiogenic factors. Science 1987, 235, 442–447. [Google Scholar] [CrossRef]
- Tamanini, C.; De Ambrogi, M. Angiogenesis in developing follicle and corpus luteum. Reprod. Domest. Anim. 2004, 39, 206–216. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R.; Parmar, M.S.; Chouhan, V.S.; Rajesh, G.; Yadav, V.P.; Bharti, M.K.; Dangi, S.S. Expression and localization of fibroblast growth factor (FGF) family in corpus luteum during different stages of estrous cycle and synergistic role of FGF2 and vascular endothelial growth factor (VEGF) on steroidogenesis, angiogenesis and survivability of cultured buffalo luteal cells. Agri. Gene 2016, 1, 53–68. [Google Scholar]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Berisha, B. Regulation of corpus luteum function in cattle–an overview. Reprod. Domest. Anim. 2004, 39, 241–251. [Google Scholar] [CrossRef]
- Lu, E.; Li, C.; Wang, J.; Zhang, C. Inflammation and angiogenesis in the corpus luteum. J. Obstet. Gynaecol. Res. 2019, 45, 1967–1974. [Google Scholar] [CrossRef] [Green Version]
- Duncan, W.C.; Myers, M.; Dickinson, R.E.; Van Den Driesche, S.; Fraser, H.M. Paracrine regulation of luteal development and luteolysis in the primate. Anim. Reprod. 2018, 6, 34–46. [Google Scholar]
- Berisha, B.; Schams, D.; Rodler, D.; Sinowatz, F.; Pfaffl, M.W. Expression pattern of HIF 1alpha and vasohibins during follicle maturation and corpus luteum function in the bovine ovary. Reprod. Domest. Anim. 2017, 52, 130–139. [Google Scholar] [CrossRef]
- Watson, E.D.; Fraser, H.M. Angiogenesis and vascular endothelial growth factor expression in the equine corpus luteum. Reproduction 2003, 125, 259–270. [Google Scholar]
- Maia, V.N.; Batista, A.M.; Neto, S.C.; Silva, D.M.; Adrião, M.; Wischral, A. Expression of angiogenic factors and luteinizing hormone receptors in the corpus luteum of mares induced to ovulate with deslorelin acetate. Theriogenology 2016, 85, 461–465. [Google Scholar] [CrossRef]
- Schams, D.; Berisha, B. Angiogenic factors in the bovine corpus luteum. J. Reprod. Dev. 2002, 48, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Boonyaprakob, U.; Gadsby, J.E.; Hedgpeth, V.; Routh, P.A.; Almond, G.W. Expression and localization of hypoxia inducible factor-1alpha mRNA in the porcine ovary. Can. J. Vet. Res. 2005, 69, 215–222. [Google Scholar] [PubMed]
- Wu, L.; Zhang, Z.; Pan, X.; Wang, Z. Expression and contribution of the HIF-1α/VEGF signaling pathway to luteal development and function in pregnant rats. Mol. Med. Rep. 2015, 12, 7153–7159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, J.; Acosta, T.J.; Berisha, B.; Tetsuka, M.; Matsui, M.; Kobayashi, S.; Miyamoto, A. Relative changes in mRNA expression of angiopoietins and receptors tie in bovine corpus luteum during estrous cycle and prostaglandin F2α-induced luteolysis: A possible mechanism for the initiation of luteal regression. J. Reprod. Dev. 2004, 50, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, D.J.; Ferreira-Dias, G.; Okuda, K. Regulation of luteal function and corpus luteum regression in cows: Hormonal control, immune mechanisms and intercellular communication. Reprod. Domest. Anim. 2008, 43, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Meidan, R.; Girsh, E.; Mamluk, R.; Levy, N.; Farberov, S. The Life Cycle of the Corpus Luteum; Springer Cham: Basingstoke, UK, 2017; pp. 159–182. [Google Scholar]
- Hojo, T.; Piotrowska-Tomala, K.K.; Jonczyk, A.W.; Lukasik, K.; Jankowska, K.; Okuda, K.; Skarzynski, D.J. Receptor interacting protein kinases-dependent necroptosis as a new, potent mechanism for elimination of the endothelial cells during luteolysis in cow. Theriogenology 2019, 128, 193–200. [Google Scholar] [CrossRef]
- Miyamoto, A.; Shirasuna, K. Luteolysis in the cow: A novel concept of vasoactive molecules. Anim. Reprod. 2009, 6, 47–59. [Google Scholar]
- Neuvians, T.P.; Berisha, B.; Schams, D. Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) expression during induced luteolysis in the bovine corpus luteum. Mol. Reprod. Dev. 2004, 67, 389–395. [Google Scholar] [CrossRef]
- Pan, X.Y.; Zhang, Z.H.; Wu, L.X.; Wang, Z.C. Effect of HIF-1a/VEGF signaling pathway on plasma progesterone and ovarian prostaglandin F. Genet. Mol. Res. 2015, 14, 8796–8809. [Google Scholar] [CrossRef]
- Vonnahme, K.A.; Redmer, D.A.; Borowczyk, E.; Bilski, J.J.; Luther, J.S.; Johnson, M.L.; Grazul-Bilska, A.T. Vascular composition, apoptosis, and expression of angiogenic factors in the corpus luteum during prostaglandin F2α-induced regression in sheep. Reproduction 2006, 131, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Neuvians, T.P.; Pfaffl, M.W.; Berisha, B.; Schams, D. The mRNA expression of the members of the IGF-system in bovine corpus luteum during induced luteolysis. Domest. Anim. Endocrinol. 2003, 25, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Berisha, B.; Meyer, H.H.; Schams, D. Effect of prostaglandin F2 alpha on local luteotropic and angiogenic factors during induced functional luteolysis in the bovine corpus luteum. Biol. Reprod. 2010, 82, 940–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Liang, R.R.; Zhou, C.; Wu, M.Y.; Lian, L.; Yuan, G.F.; Chen, K. The association between expressions of Ras and CD68 in the angiogenesis of breast cancers. Cancer Cell Int. 2015, 15, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Liu, Y.; Bellail, A.C.; Olson, J.J.; Sun, S.Y.; Lu, G.; Hao, C. K-Ras mutation-mediated IGF-1-induced feedback ERK activation contributes to the rapalog resistance in pancreatic ductal adenocarcinomas. Cancer Lett. 2012, 322, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, M.; Nakagama, H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef]
- Hardy, K.M.; Yatskievych, T.A.; Konieczka, J.H.; Bobbs, A.S.; Antin, P.B. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol. 2011, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, M.M.; Cavaco, B.M.; Leite, V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr. Relat. Cancer 2015, 22, R235–R252. [Google Scholar] [CrossRef]
- Kranenburg, O.; Gebbink, M.F.; Voest, E.E. Stimulation of angiogenesis by Ras proteins. Biochim. Biophys. Acta-Rev. Cancer 2004, 1654, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, Y.; Takano, O.; Kato, I.; Takahashi, Y.; Shima, F.; Kataoka, T. Ras inhibitors display an anti-metastatic effect by downregulation of lysyl oxidase through inhibition of the Ras-PI3K-Akt-HIF-1α pathway. Cancer let. 2017, 410, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Larcher, F.; Franco, M.; Bolontrade, M.; Rodriguez-Puebla, M.; Casanova, L.; Navarro, M.; Conti, C.J. Modulation of the angiogenesis response through Ha-ras control, placenta growth factor, and angiopoietin expression in mouse skin carcinogenesis. Mol. Carcinogen. 2003, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Urakami, T.; Li, F.; Urakami, A.; Zhu, W.; Fukuda, M.; Komatsu, M. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell 2012, 22, 235–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ii, M.; Li, H.; Adachi, Y.; Yamamoto, H.; Ohashi, H.; Taniguchi, H.; Shinomura, Y. The efficacy of IGF-I receptor monoclonal antibody against human gastrointestinal carcinomas is independent of k-ras mutation status. Clin. Cancer Res. 2011, 17, 5048–5059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Lee, S. Change of Ras and its guanosine triphosphatases during development and regression in bovine corpus luteum. Theriogenology 2020, 144, 16–26. [Google Scholar] [CrossRef] [PubMed]
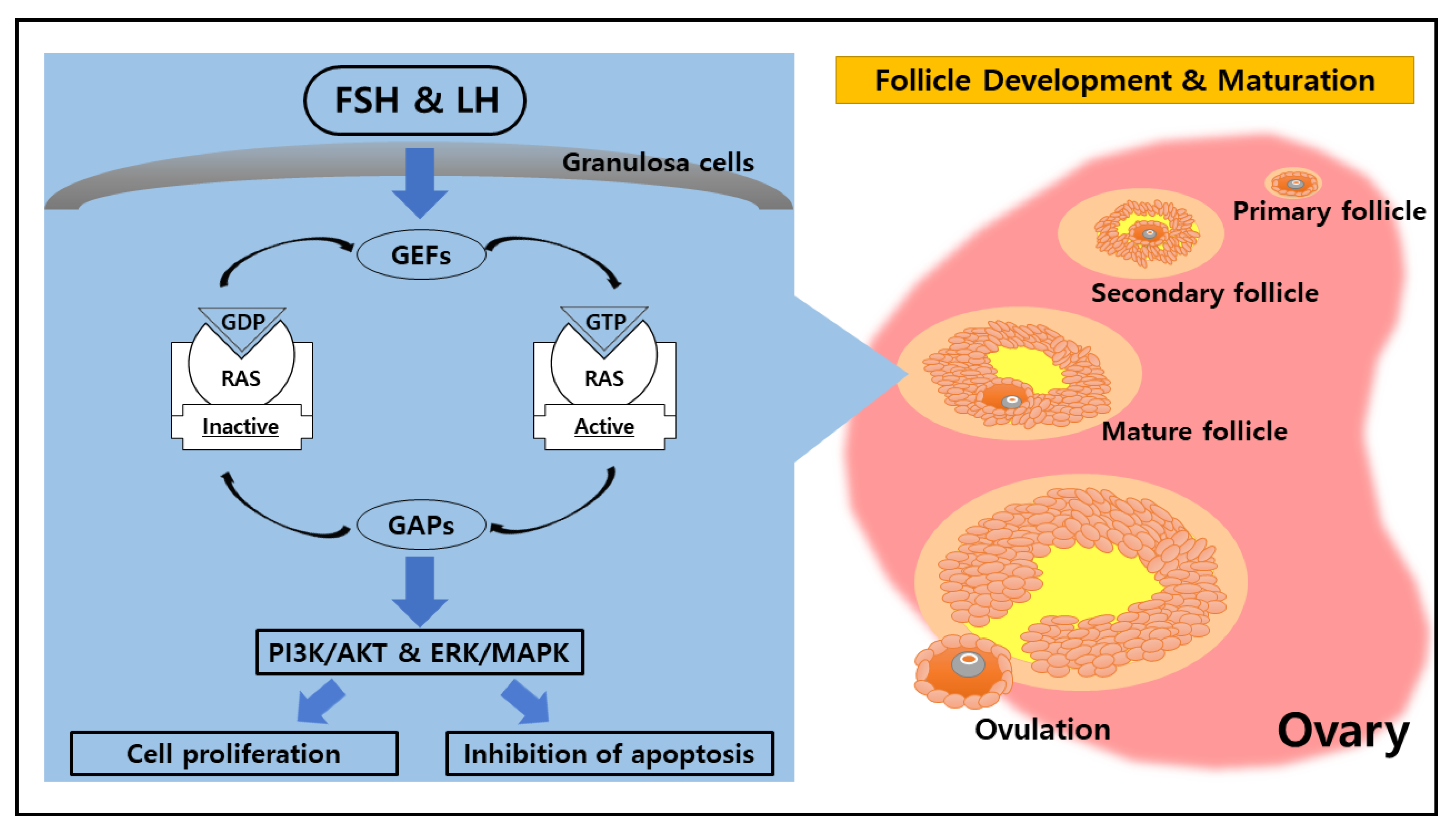
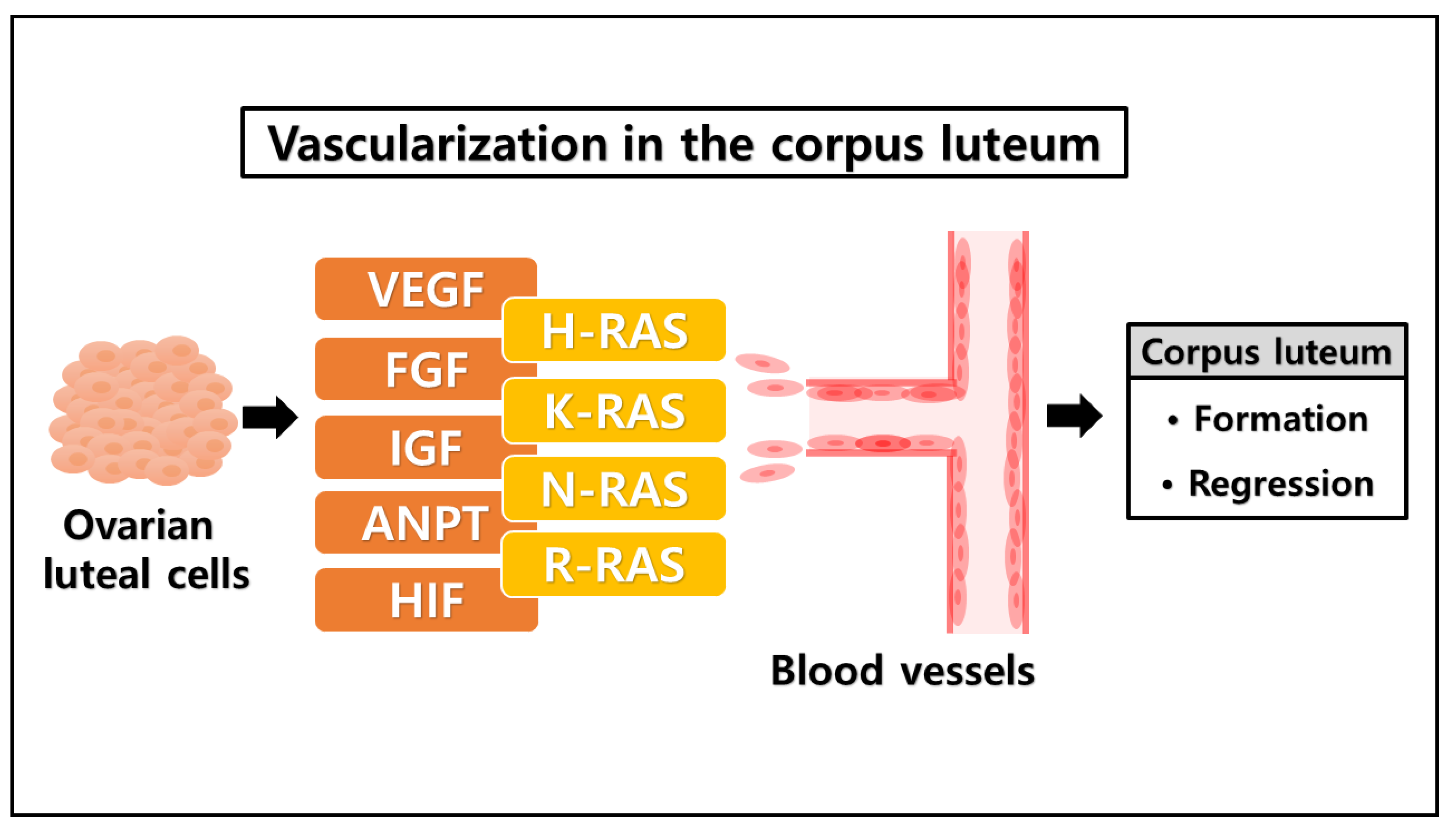
| Conditions | Factors | Functions |
|---|---|---|
| Formation of corpus luteum | Vascular endothelial growth factor (VEGF) | Vasculogenesis [57] |
| Insulin-like growth factor (IGF) | Angiogenesis [58] | |
| Fibroblast growth factor (FGF) | Angiogenic factor [59] | |
| Angiopoietins (ANPT) | Cellular growth factor [60] | |
| Hypoxia-inducible factor (HIF) | Transcription [61] | |
| Regression of corpus luteum | Prostaglandin F2α (PGF2α) | Luteolysis [70] Apoptosis [71] |
| RAS family | H-RAS | Cellular signal transduction [78] |
| K-RAS | ERK pathway [79] | |
| N-RAS | PI3K-AKT pathway [80] | |
| R-RAS | RAS-MAPK pathway [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billhaq, D.H.; Lee, S. The Role of the Guanosine Nucleotide-Binding Protein in the Corpus Luteum. Animals 2021, 11, 1524. https://doi.org/10.3390/ani11061524
Billhaq DH, Lee S. The Role of the Guanosine Nucleotide-Binding Protein in the Corpus Luteum. Animals. 2021; 11(6):1524. https://doi.org/10.3390/ani11061524
Chicago/Turabian StyleBillhaq, Dody Houston, and Seunghyung Lee. 2021. "The Role of the Guanosine Nucleotide-Binding Protein in the Corpus Luteum" Animals 11, no. 6: 1524. https://doi.org/10.3390/ani11061524
APA StyleBillhaq, D. H., & Lee, S. (2021). The Role of the Guanosine Nucleotide-Binding Protein in the Corpus Luteum. Animals, 11(6), 1524. https://doi.org/10.3390/ani11061524






