piggyBac Transposition and the Expression of Human Cystatin C in Transgenic Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal Care
2.2. Codon Optimization of Human Cystatin C Gene and Construction of Expression Vector into piggyBac Transposon
2.3. Chicken DF1 Cell and PGC Culture for Transfection and Selection
2.4. Transplantation of hCST3-Expressing Chicken PGCs and Testcross Analysis to Screen Transgenic Chicks
2.5. Detection of hCST3-Expressing Transgenic Chickens
2.6. ELISA for Detection of hCST3 Protein
2.7. Western Blotting
2.8. Immunohistochemistry Analysis
2.9. Purification of hCST3 from Egg White and Muscle
2.10. Antimicrobial Activity Test
3. Results
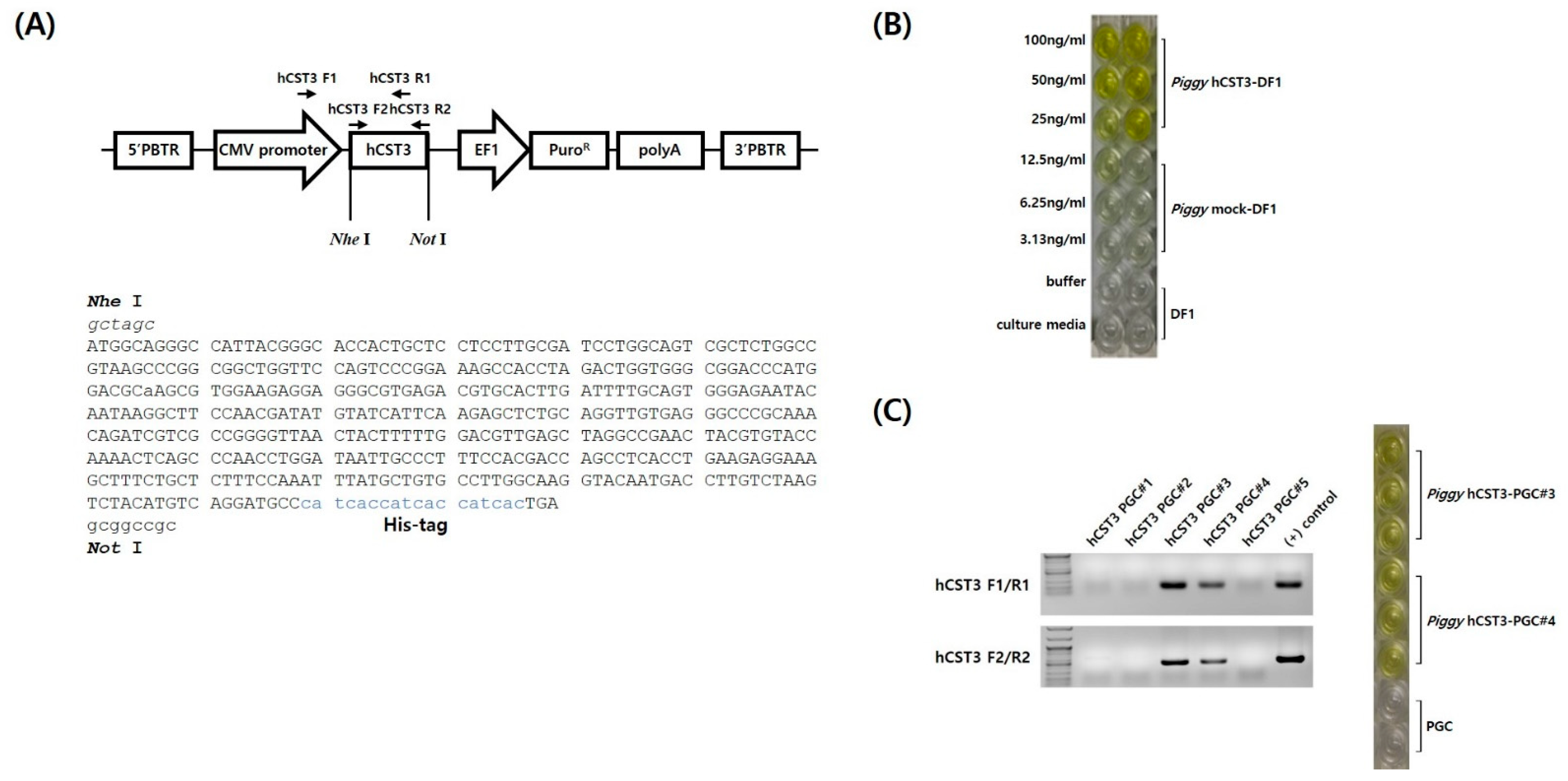
3.1. Codon-Optimization and Construction of hCST3 Expression Vector in piggyBac Transposon
3.2. Detection of hCST3 in DF1 Cells and PGCs after Transfection and Selection
3.3. Production of Transgenic Chickens by Transplanting hCST3-Expressing Chicken PGCs
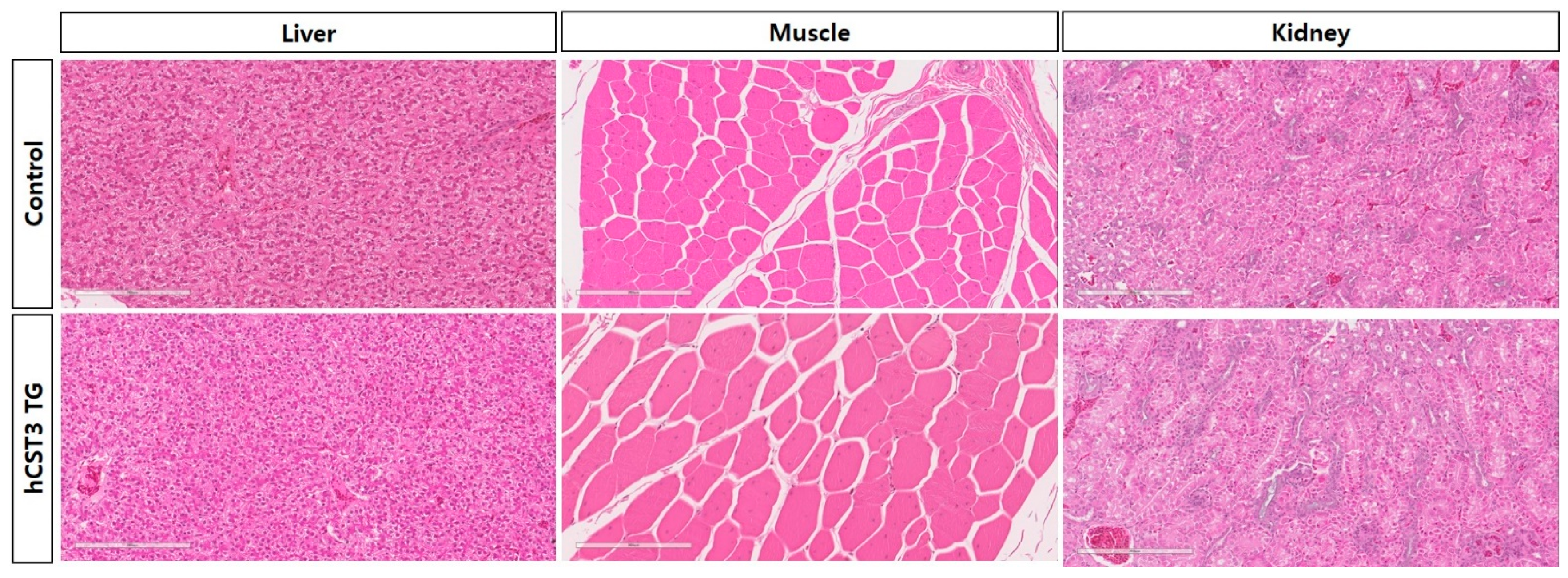
3.4. Immunohistochemistry Analyses of hCST3-Expressing Transgenic Chickens
3.5. Detection of hCST3 in Transgenic Chickens
3.6. Purification of hCST3 and Antimicrobial Activity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kesik-Brodacka, M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Miralles, N.; Domingo-Espín, J.; Corchero, J.L.; Vázquez, E.; Villaverde, A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact 2009, 24, 17. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, N.S. Biopharmaceutical production in transgenic livestock. Trends Biotechnol. 1999, 17, 367–374. [Google Scholar] [CrossRef]
- Houdebine, L.M. Transgenic animal bioreactors. Transgenic Res. 2000, 9, 305–320. [Google Scholar] [CrossRef]
- Lillico, S.G.; McGrew, M.J.; Sherman, A.; Sang, H.M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today 2005, 10, 191–196. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Harvey, A.J.; Speksnijder, G.; Baugh, L.R.; Morris, J.A.; Ivarie, R. Expression of exogenous protein in the egg white of transgenic chickens. Nat. Biotechnol. 2002, 20, 396–399. [Google Scholar] [CrossRef]
- Kamihira, M.; Ono, K.; Esaka, K.; Nishijima, K.; Kigaku, R.; Komatsu, H.; Yamashita, T.; Kyogoku, K.; Iijima, S. High-level expression of single-chain Fv-Fc fusion protein in serum and egg white of genetically manipulated chickens by using a retroviral vector. J. Virol. 2005, 79, 10864–10874. [Google Scholar] [CrossRef] [Green Version]
- Lillico, S.G.; Sherman, A.; McGrew, M.J.; Robertson, C.D.; Smith, J.; Haslam, C.; Barnard, P.; Radcliffe, P.A.; Mitrophanous, K.A.; Elliot, E.A.; et al. Oviduct-specific expression of two therapeutic proteins in transgenic hens. Proc. Natl. Acad. Sci. USA 2007, 104, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- Herron, L.R.; Pridans, C.; Turnbull, M.L.; Smith, N.; Lillico, S.; Sherman, A.; Gilhooley, H.J.; Wear, M.; Kurian, D.; Papadakos, G.; et al. A chicken bioreactor for efficient production of functional cytokines. BMC Biotechnol. 2018, 18, 82. [Google Scholar] [CrossRef]
- Park, T.S.; Lee, H.G.; Moon, J.K.; Lee, H.J.; Yoon, J.W.; Yun, B.N.R.; Kang, S.; Kim, J.; Kim, H.; Han, J.Y.; et al. Deposition of bioactive human epidermal growth factor in the egg white of transgenic hens using an oviduct-specific minisynthetic promoter. FASEB J. 2015, 29, 2386–2396. [Google Scholar] [CrossRef] [Green Version]
- Kling, J. First US approval for a transgenic animal drug. Nat. Biotechnol. 2009, 27, 302–304. [Google Scholar] [CrossRef]
- Park, T.S.; Han, J.Y. piggyBac transposition into primordial germ cells is an efficient tool for transgenesis in chickens. Proc. Natl. Acad. Sci. USA 2012, 109, 9337–9341. [Google Scholar] [CrossRef] [Green Version]
- Park, T.S.; Lee, H.J.; Kim, K.H.; Kim, J.; Han, J.Y. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl. Acad. Sci. USA 2014, 111, 12716–12721. [Google Scholar] [CrossRef] [Green Version]
- Park, T.S.; Park, J.; Lee, J.H.; Park, J.; Park, B. Disruption of G 0/G 1 switch gene 2 (G0S2) reduced abdominal fat deposition and altered fatty acid composition in chicken. FASEB J. 2019, 33, 1188–1198. [Google Scholar] [CrossRef]
- Kim, G.; Lee, J.; Song, S.; Kim, S.W.; Han, J.S.; Shin, S.P.; Park, B.; Park, T.S. Generation of myostatin-knockout chickens mediated by D10A-Cas9 nickase. FASEB J. 2020, 34, 5688–5696. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, J.; Taylor, L.; Sherman, A.; Kawakami, K.; Takahashi, Y.; Sang, H.M.; McGrew, M.J. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc. Natl. Acad. Sci. USA 2012, 109, E1466–E1472. [Google Scholar] [CrossRef] [Green Version]
- Zi, M.; Xu, Y. Involvement of cystatin C in immunity and apoptosis. Immunol. Lett. 2018, 196, 80–90. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Bellomo, R. Cystatin C in acute kidney injury. Curr. Opin. Crit. Care 2010, 16, 533–539. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Li, Z.; Chen, Y.; Liu, Y.; Liu, B.; Su, Z. Modulation of dendritic cell function and immune response by cysteine protease inhibitor from murine nematode parasite Heligmosomoides polygyrus. Immunology 2013, 138, 370–381. [Google Scholar] [CrossRef]
- Hernandez, H.M.; Marcet, R.; Sarracent, J. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 2014, 21, 54. [Google Scholar] [CrossRef] [Green Version]
- Luthra, K. Antiviral activity of cystatin-C against HIV. Indian J. Med. Res. 2015, 141, 383–384. [Google Scholar] [CrossRef]
- Wesierska, E.; Saleh, Y.; Trziszka, T.; Kopec, W.; Siewinski, M.; Korzekwa, K. Antimicrobial activity of chicken egg white cystatin. World J. Microbiol. Biotechnol. 2005, 21, 59–64. [Google Scholar] [CrossRef]
- Stern, C.D. The chick; a great model system becomes even greater. Dev. Cell 2015, 8, 9–17. [Google Scholar]
- Raju, T.S.; Briggs, J.B.; Borge, S.M.; Jones, A.J.S. Species-specific variation in glycosylation of IgG: Evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 2010, 10, 477–486. [Google Scholar] [CrossRef]
- Oishi, I.; Yoshii, K.; Miyahara, D.; Tagami, T. Efficient production of human interferon beta in the white of eggs from ovalbumin gene-targeted hens. Sci. Rep. 2018, 8, 10203. [Google Scholar] [CrossRef]
- Nakhjavan-Shahraki, B.; Yousefifard, M.; Ataei, N.; Baikpour, M.; Ataei, F.; Bazargani, B.; Abbasi, A.; Ghelichkhani, P.; Javidilarijani, F.; Hosseini, M. Accuracy of cystatin-C in prediction of acute kidney injury in children; serum or urine levels: Which one works better? A systematic review and meta-analysis. BMC Nephrol. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, N.; Zadkarami, M.; Chobdar, F.; Hoseini, R.; Khalesi, N.; Panahi, P.; Karimi, A. The Value of Urinary Cystatin-C Level to Predict Neonatal Kidney Injury. Curr. Pharm. Des. 2018, 24, 3002–3004. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.W.; Lee, J.H.; Han, J.S.; Shin, S.P.; Park, T.S. piggyBac Transposition and the Expression of Human Cystatin C in Transgenic Chickens. Animals 2021, 11, 1554. https://doi.org/10.3390/ani11061554
Kim SW, Lee JH, Han JS, Shin SP, Park TS. piggyBac Transposition and the Expression of Human Cystatin C in Transgenic Chickens. Animals. 2021; 11(6):1554. https://doi.org/10.3390/ani11061554
Chicago/Turabian StyleKim, Seo Woo, Jeong Hyo Lee, Ji Seon Han, Seung Pyo Shin, and Tae Sub Park. 2021. "piggyBac Transposition and the Expression of Human Cystatin C in Transgenic Chickens" Animals 11, no. 6: 1554. https://doi.org/10.3390/ani11061554






