Appeasing Pheromones for the Management of Stress and Aggression during Conservation of Wild Canids: Could the Solution Be Right under Our Nose?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Conservation Management of Wild Canids
2.1. Translocation of Wild Animals
2.2. Artificial Pack Formation
3. Stress and Aggression during Conservation Management
3.1. Impact on Reproduction
3.2. Impact on Immune Function
4. Management of Stress and Aggression
4.1. Pharmaceuticals
4.2. Pheromones
5. Pheromones for Conservation
5.1. Appeasing Pheromones
5.2. Dog Appeasing Pheromone
5.3. Application of Dog Appeasing Pheromones to Wild Canids
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sillero-Zubiri, C.; Hoffmann, M.; Macdonald, D.W. Canids: Foxes, Wolves, Jackals, and Dogs: Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 2004. [Google Scholar]
- Farris, Z.J.; Christopher, D.G.; Sarah, K.; Asia, M.; Dean, S.; Felix, R.; Vonjy, A.; Christopher, M.H.; Marcella, J.K. Hunting, Exotic Carnivores, and Habitat Loss: Anthropogenic Effects on a Native Carnivore Community, Madagascar. PLoS ONE 2015, 10, e0136456. [Google Scholar] [CrossRef] [Green Version]
- Woodroffe, R.; Davies-Mostert, H.; Ginsberg, J.; Graf, J.; Leigh, K.; McCreery, K.; Robbins, R.; Mills, G.; Pole, A.; Rasmussen, G.; et al. Rates and Causes of Mortality in Endangered African Wild Dogs Lycaon pictus: Lessons for Management and Monitoring. Oryx 2007, 41, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Frankham, R. Relationship of Genetic Variation to Population Size in Wildlife. Conserv. Biol. 1996, 10, 1500–1508. [Google Scholar] [CrossRef] [Green Version]
- Liberg, O.; Andrén, H.; Pedersen, H.-C.; Sand, H.; Sejberg, D.; Wabakken, P.; Kesson, M.; Bensch, S. Severe Inbreeding Depression in a Wild Wolf (Canis lupus) Population. Biol. Lett. 2005, 1, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Laikre, L.; Ryman, N. Inbreeding Depression in a Captive Wolf (Canis lupus) Population. Conserv. Biol. 1991, 5, 33–40. [Google Scholar] [CrossRef]
- Fredrickson, R.J.; Siminski, P.; Woolf, M.; Hedrick, P.W. Genetic Rescue and Inbreeding Depression in Mexican Wolves. Proc. R. Soc. B Biol. Sci. 2007, 274, 2365–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsden, C.D.; Mable, B.K.; Woodroffe, R.; Rasmussen, G.S.A.; Cleaveland, S.; McNutt, J.W.; Emmanuel, M.; Thomas, R.; Kennedy, L.J. Highly Endangered African Wild Dogs (Lycaon pictus) Lack Variation at the Major Histocompatibility Complex. J. Hered. 2009, 100, S54–S65. [Google Scholar] [CrossRef] [Green Version]
- Marsden, C.D.; Woodroffe, R.; Mills, M.G.L.; McNutt, J.W.; Creel, S.; Groom, R.; Emmanuel, M.; Cleaveland, S.; Kat, P.; Rasmussen, G.S.A.; et al. Spatial and Temporal Patterns of Neutral and Adaptive Genetic Variation in the Endangered African Wild Dog (Lycaon pictus): Spatial and Temporal Diversity in Wild Dogs. Mol. Ecol. 2012, 21, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Leigh, K.A.; Zenger, K.R.; Tammen, I.; Raadsma, H.W. Loss of Genetic Diversity in an Outbreeding Species: Small Population Effects in the African Wild Dog (Lycaon pictus). Conserv. Genet. 2012, 13, 767–777. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Angles, J.M.; Barnes, A.; Carter, S.D.; Francino, O.; Gerlach, J.A.; Happ, G.M.; Ollier, W.E.R.; Thomson, W.; Wagner, J.L. Nomenclature for Factors of the Dog Major Histocompatibility System (Dla), 2000: Second Report of the Isag Dla Nomenclature Committee. Anim. Genet. 2001, 32, 193–199. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Angles, J.M.; Barnes, A.; Carmichael, L.E.; Radford, A.D.; Ollier, W.E.R.; Happ, G.M. Dla-Drb1, Dqa1, and Dqb1 Alleles and Haplotypes in North American Gray Wolves. J. Hered. 2007, 98, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Ellegren, H. Mhc Class Ii Genes in European Wolves: A Comparison with Dogs. Immunogenetics 2002, 54, 490–500. [Google Scholar] [CrossRef]
- Hedrick, P.W.; Lee, R.N.; Parker, K.M. Major Histocompatibility Complex (Mhc) Variation in the Endangered Mexican Wolf and Related Canids. Heredity 2000, 85, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.J.; Randall, D.A.; Knobel, D.; Brown, J.J.; Fooks, A.R.; Argaw, K.; Shiferaw, F.; Ollier, W.; Sillero-Zubiri, C.; Macdonald, D.W. Major Histocompatibility Complex Diversity in the Endangered Ethiopian Wolf (Canis Simensis). Tissue Antigens 2011, 77, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Woodroffe, R.; Ginsberg, J.; Macdonald, D. The African Wild Dog; IUCN: Gland, Switzerland; Cambridge, UK, 1997. [Google Scholar]
- Armstrong, D.P.; Seddon, P.J. Directions in Reintroduction Biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lindenmayer, D.B. An Assessment of the Published Results of Animal Relocations. Biol. Conserv. 2000, 96, 1–11. [Google Scholar] [CrossRef]
- Wolf, C.M.; Griffith, B.; Reed, C.; Temple, S.A. Avian and Mammalian Translocations: Update and Reanalysis of 1987 Survey Data. Conserv. Biol. 1996, 10, 1142–1154. [Google Scholar] [CrossRef]
- Fritts, S.H.; Bangs, E.E.; Fontaine, J.A.; Johnson, M.R.; Phillips, M.K.; Koch, E.D.; Gunson, J.R. Planning and Implementing a Reintroduction of Wolves to Yellowstone National Park and Central Idaho. Restor. Ecol. 1997, 5, 7–27. [Google Scholar] [CrossRef] [Green Version]
- Marneweck, C.; Becker, P.A.; Beverley, G.; Davies-Mostert, H.T.; Plessis, C.d.; Forssman, K.; Graf, J.; Gusset, M.; Hofmeyr, M.; Kelly, C.; et al. Factors Affecting the Success of Artificial Pack Formation in an Endangered, Social Carnivore: The African Wild Dog. Anim. Conserv. 2019, 22, 493–502. [Google Scholar] [CrossRef]
- Landa, A.; Flagstad, Ø.; Areskoug, V.; Linnell, J.D.C.; Strand, O.; Ulvund, K.R.; Thierry, A.; Rød-Eriksen, L.; Eide, N.E. The Endangered Arctic Fox in Norway—The Failure and Success of Captive Breeding and Reintroduction. Polar Res. 2017, 36, 9. [Google Scholar] [CrossRef] [Green Version]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Van den Berghe, F.; Paris, D.B.B.P.; van Soom, A.; Rijsselaere, T.; van der Weyde, L.; Bertschinger, H.J.; Paris, M.C.J. Reproduction in the Endangered African Wild Dog: Basic Physiology, Reproductive Suppression and Possible Benefits of Artificial Insemination. Anim. Reprod. Sci. 2012, 133, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrekoussis, T.; Kalantaridou, S.N.; Mastorakos, G.; Zoumakis, E.; Makrigiannakis, A.; Syrrou, M.; Lavasidis, L.G.; Relakis, K.; Chrousos, G.P. The Role of Stress in Female Reproduction and Pregnancy: An Update. Ann. N. Y. Acad. Sci. 2010, 1205, 69–75. [Google Scholar] [CrossRef]
- Wells, J.V.; Richmond, M.E. Populations, Metapopulations, and Species Populations: What Are They and Who Should Care? Wildl. Soc. Bull. 1995, 23, 458–462. [Google Scholar]
- Moehrenschlager, A.; Somers, M.J. Canid Reintroductions and Metapopulation Management. In Canids: Foxes, Wolves, Jackals and Dogs. Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 2004; pp. 289–297. [Google Scholar]
- Simonis, J.L.; Harrison, R.B.; Long, S.T.; Rabon, D.R., Jr.; Waddell, W.T.; Faust, L.J. Managed Movement Increases Metapopulation Viability of the Endangered Red Wolf. J. Wildl. Manag. 2018, 82, 573–582. [Google Scholar] [CrossRef]
- Mills, M.G.L.; Ellis, S.; Woodroffe, R.; Maddock, A.; Stander, P.; Rasmussen, G.; Pole, A.; Fletcher, P.; Bruford, M.; Wildt, D.; et al. Population and Habitat Viability Assessment for the African Wild Dog (Lycaon pictus) in Southern Africa; Final Workshop Report; IUCN/SCC Conservation Breeding Specialist Group: Apple Valley, CA, USA, 1998. [Google Scholar]
- IUCN/SSC. Regional Conservation Strategy for the Cheetah and Wild Dog in Southern Africa; IUCN: Gland, Switserland, 2009. [Google Scholar]
- Woodroffe, R.; Sillero-Zubric, C. Lycaon pictus. The Iucn Red List of Threatened Species 2012: E.T12436a16711116. Available online: https://www.iucnredlist.org/species/12436/16711116 (accessed on 24 February 2020).
- Davies-Mostert, H.T.; Mills, M.G.L.; Macdonald, D.W. A Critical Assessment of South Africa’s Managed Metapopulation Recovery Strategy for African Wild Dogs; Wiley-Blackwell: Oxford, UK, 2009; pp. 10–42. [Google Scholar]
- Davies-Mostert, H.T.; Mills, M.G.L.; Macdonald, D.W. The Demography and Dynamics of an Expanding, Managed African Wild Dog Metapopulation. Afr. J. Wildl. Res. 2015, 45, 258–273. [Google Scholar] [CrossRef] [Green Version]
- IUCN. Guidelines for Re-Introductions; IUCN/SSC Re-Introduction Specialist Group: Gland, Switxerland; Cambridge, UK, 1998. [Google Scholar]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress: An Inevitable Component of Animal Translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Gosling, L.M.; Sutherland, W.J. Behaviour and Conservation; Cambridge University Press: Cambridge, UK, 2000; 438p. [Google Scholar]
- Letty, J.; Aubineau, J.; Marchandeau, S.; Clobert, J. Effect of Translocation on Survival in Wild Rabbit (Oryctolagus cuniculus). Mamm. Biol. 2003, 68, 250–255. [Google Scholar] [CrossRef]
- Teixeira, C.P.; de Azevedo, C.S.; Mendl, M.; Cipreste, C.F.; Young, R.J. Revisiting Translocation and Reintroduction Programmes: The Importance of Considering Stress. Anim. Behav. 2007, 73, 1–13. [Google Scholar] [CrossRef]
- Weise, T.F. An Experimental Translocation of the Eastern Timber Wolf; National Audubon Society: Washington DC, USA, 1975. [Google Scholar]
- Fritts, S.H.; Paul, W.J.; Mech, L.D. Can Relocated Wolves Survive? Wildl. Soc. Bull. 1985, 13, 459–463. [Google Scholar]
- Phillips, M. Conserving the Red Wolf. Canid News 1995, 3, 13–17. [Google Scholar]
- Carbyn, L.N.; Armbruster, H.J.; Mamo, C. The Swift Fox Reintroduction Program in Canada from 1983 to 1992. In Restoration of Endangered Species: Conceptual Issues, Planning and Implementation; Cambridge University Press: Cambridge, UK, 1994; pp. 247–271. [Google Scholar]
- Woodroffe, R.; Ginsberg, J.R. Edge Effects and the Extinction of Populations inside Protected Areas. Science 1998, 280, 2126–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, P.A.; Davies-Mostert, H.T. South African Action Plan for Conservation of Cheetahs and Wild Dogs. In Report from a National Conservation Action Planning Workshop; Endangered Wildlife Trust: Bela Bela, South Africa, 2009. [Google Scholar]
- Gusset, M.; Stewart, G.B.; Bowler, D.E.; Pullin, A.S. Wild Dog Reintroductions in South Africa: A Systematic Review and Cross-Validation of an Endangered Species Recovery Programme. J. Nat. Conserv. 2010, 18, 230–234. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the Science of Reintroduction Biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Woodroffe, R.; Ginsberg, J.R. Conserving the African Wild Dog Lycaon pictus. Ii. Is There a Role for Reintroduction? Oryx 1999, 33, 143–151. [Google Scholar] [CrossRef]
- Shier, D.M. Effect of Family Support on the Success of Translocated Black-Tailed Prairie Dogs. Conserv. Biol. 2006, 20, 1780–1790. [Google Scholar] [CrossRef]
- McLellan, S.R.; Rabon, D.R., Jr. Translocating Red Wolves Using a Modified Soft-Release Technique. Canid News 2006, 9, 1–10. [Google Scholar]
- Bradley, E.H.; Pletscher, D.H.; Bangs, E.E.; Kunkel, K.E.; Smith, D.W.; Mack, C.M.; Meier, T.J.; Fontaine, J.A.; Niemeyer, C.C.; Jimenez, M.D. Evaluating Wolf Translocation as a Nonlethal Method to Reduce Livestock Conflicts in the Northwestern United States. Conserv. Biol. 2005, 19, 1498–1508. [Google Scholar] [CrossRef]
- Gusset, M.; Slotow, R.; Somers, M.J. Divided We Fail: The Importance of Social Integration for the Re-Introduction of Endangered African Wild Dogs (Lycaon pictus). J. Zool. 2006, 270, 502–511. [Google Scholar] [CrossRef]
- Hayward, M.W.; Adendorff, J.; O’Brien, J.; Sholto-Douglas, A.; Bissett, C.; Moolman, L.C.; Bean, P.; Fogarty, A.; Howarth, D.; Slater, R.; et al. The Reintroduction of Large Carnivores to the Eastern Cape Province, South Africa: An Assessment. Oryx 2007, 41, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Trinkel, M.; Ferguson, N.; Reid, A.; Reid, C.; Somers, M.; Turelli, L.; Graf, J.; Szykman, M.; Cooper, D.; Haverman, P.; et al. Translocating Lions into an Inbred Lion Population in the Hluhluwe-Imfolozi Park, South Africa. Anim. Conserv. 2008, 11, 138–143. [Google Scholar] [CrossRef]
- Buk, K.G.; van der Merwe, V.C.; Marnewick, K.; Funston, P.J. Conservation of Severely Fragmented Populations: Lessons from the Transformation of Uncoordinated Reintroductions of Cheetahs (Acinonyx jubatus) into a Managed Metapopulation with Self-Sustained Growth. Biodivers. Conserv. 2018, 27, 3393–3423. [Google Scholar] [CrossRef]
- McNutt, J.W. Sex-Biased Dispersal in African Wild Dogs, Lycaon pictus. Anim. Behav. 1996, 52, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Creel, S.; Creel, N.M.; Mills, M.G.L.; Monfort, S.L. Rank and Reproduction in Cooperatively Breeding African Wild Dogs: Behavioral and Endocrine Correlates. Behav. Ecol. 1997, 8, 298–306. [Google Scholar] [CrossRef]
- McCreery, E.K. Spatial Relationships as an Indicator of Successful Pack Formation in Free-Ranging African Wild Dogs. Behaviour 2000, 137, 579–590. [Google Scholar] [CrossRef]
- Creel, S.; Creel, N.M. The African Wild Dog: Behaviour, Ecology and Evolution; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Girman, D.J.; Mills, M.G.L.; Geffen, E.; Wayne, R.K. A Molecular Genetic Analysis of Social Structure, Dispersal, and Interpack Relationships of the African Wild Dog (Lycaon pictus). Behav. Ecol. Sociobiol. 1997, 40, 187–198. [Google Scholar] [CrossRef]
- Crossey, B.; Chimimba, C.; Plessis, C.d.; Hall, G.; Ganswindt, A. Using Faecal Glucocorticoid Metabolite Analyses to Elucidate Stressors of African Wild Dogs Lycaon pictus from South Africa. Wildl. Biol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Marneweck, C.J.; Marchal, A.F.J.; Marneweck, D.G.; Beverley, G.; Davies-Mostert, H.T.; Parker, D.M. A Novel Technique for Artificial Pack Formation in African Wild Dogs Using Odour Familiarity. Afr. J. Wildl. Res. 2019, 49, 116–120. [Google Scholar] [CrossRef]
- Johnston, S.D.; Ward, D.; Lemon, J.; Gunn, I.; MacCallum, C.A.; Keeley, T.; Blyde, D. Studies of Male Reproduction in Captive African Wild Dogs ( Lycaon pictus). Anim. Reprod. Sci. 2007, 100, 338–355. [Google Scholar] [CrossRef]
- Vlamings, B.H.A.C. Dog Appeasing Pheromone®: A Useful Tool to Minimize Stress and Aggression of African Wild Dogs (Lycaon Picuts)? Master’s Thesis, University of Utrecht, Utrecht, The Netherlands, 2011. [Google Scholar]
- Scheepers, J.L.; Venzke, K.A.E. Attempts to Re-Introduce African Wild Dogs Lycaon pictus into Entosha National Park, Namibia. S. Afr. J. Wildl. Res. 1995, 25, 138–140. [Google Scholar]
- Creel, S.; Creel, N.M.; Munson, L.; Sanderlin, D.; Appel, M.J. Serosurvey for Selected Viral Diseases and Demography of African Wild Dogs in Tanzania. J. Wildl. Dis. 1997, 33, 823–832. [Google Scholar] [CrossRef] [Green Version]
- von Engelhard, N.; Kappeler, P.M.; Heistermann, M. Androgen Levels and Female Social Dominance in Lemur Catta. Proc. R. Soc. London Ser. B Biol. Sci. 2000, 267, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Potgieter, K.R.; O’Riain, M.J.; Davies-Mostert, H.T. Behavioural Cues Can Be Used to Predict the Outcome of Artificial Pack Formation in African Wild Dogs (Lycaon pictus). Afr. J. Wildl. Res. 2015, 45, 215–222. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary Cortisol in Psychoneuroendocrine Research: Recent Developments and Applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Acute Stress Enhances While Chronic Stress Suppresses Skin Immunity: The Role of Stress Hormones and Leukocyte Trafficking. Ann. N. Y. Acad. Sci. 2000, 917, 876–893. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M. Physiological Stress in Ecology: Lessons from Biomedical Research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed] [Green Version]
- Sapolsky, R.M. Stress, the Aging Brain, and the Mechanisms of Neuron Death, Stress, the Aging Brain, and the Mechanisms of Neuron Death; The MIT Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Goymann, W.; East, M.L.; Hofer, H. Androgens and the Role of Female “Hyperaggressiveness” in Spotted Hyenas (Crocuta crocuta). Horm. Behav. 2001, 39, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Dloniak, S.M.; French, J.A.; Holekamp, K.E. Rank-Related Maternal Effects of Androgens on Behaviour in Wild Spotted Hyaenas. Nature 2006, 440, 1190–1193. [Google Scholar] [CrossRef]
- Van den Berghe, F.; Paris, M.C.J.; Sarnyai, Z.; Briggs, M.B.; Millar, R.P.; Ganswindt, A.; Paris, D.B.B.P. Social Rank Does Not Affect Sperm Quality in Male African Wild Dogs (Lycaon pictus). Reprod. Fertil. Dev. 2019, 31, 875. [Google Scholar] [CrossRef]
- Sundqvist, C.; Lukola, A.; Valtonen, M. Relationship between Serum Testosterone Concentrations and Fertility in Male Mink (Mustela vison). Reproduction 1984, 70, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Kalantaridou, S.N.; Zoumakis, E.; Makrigiannakis, A.; Lavasidis, L.G.; Vrekoussis, T.; Chrousos, G.P. Corticotropin-Releasing Hormone, Stress and Human Reproduction: An Update. J. Reprod. Immunol. 2010, 85, 33–39. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.M.; Reichard, D.G.; Pavilis, K.; Ketterson, E.D. Experimentally-Elevated Testosterone, Female Parental Care, and Reproductive Success in a Songbird, the Dark-Eyed Junco (Junco hyemalis). Horm. Behav. 2008, 54, 571–578. [Google Scholar] [CrossRef]
- Torres-Calleja, J.; Gonzalez-Unzaga, M.; DeCelis-Carrillo, R.; Calzada-Sanchez, L.; Pedron, N. Effect of Androgenic Anabolic Steroids on Sperm Quality and Serum Hormone Levels in Adult Male Bodybuilders. Life Sci. 2001, 68, 1769–1774. [Google Scholar] [CrossRef]
- Batson, W.G.; Gordon, I.J.; Fletcher, D.B.; Manning, A.D. The Effect of Pre-Release Captivity on Post-Release Performance in Reintroduced Eastern Bettongs Bettongia Gaimardi. Oryx 2016, 50, 664–673. [Google Scholar] [CrossRef]
- Waters, S.S. Swift Fox Vulpes Velox Reintroductions: A Review of Release Protocols. Int. Zoo Yearb. 2010, 44, 173–182. [Google Scholar] [CrossRef]
- Van der Weyde, L.K.; Martin, G.B.; Paris, M.C.J. Monitoring Stress in Captive and Free-Ranging African Wild Dogs (Lycaon pictus ) Using Faecal Glucocorticoid Metabolites. Gen. Comp. Endocrinol. 2015, 226, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creel, S. Dominance, Aggression, and Glucorticoid Levels in Social Carnivores. J. Mammal. 2005, 86, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Monfort, S.L.; Wasser, S.K.; Mashburn, K.L.; Burke, M.; Brewer, B.A.; Creel, S.R. Steroid Metabolism and Validation of Noninvasive Endocrine Monitoring in the African Wild Dog (Lycaon pictus). Zoo Biol. 1997, 16, 533–548. [Google Scholar] [CrossRef]
- Newell-Fugate, A.E.; Nöthling, J.O.; Bertschinger, H.J. Seasonal Changes in Steroid Hormone Profiles, Body Weight, Semen Quality, and the Reproductive Tract in Captive African Wild Dogs (Lycaon pictus) in South Africa. Gen. Comp. Endocrinol. 2012, 178, 272–281. [Google Scholar] [CrossRef]
- Creel, S. Social Dominance and Stress Hormones. Trends Ecol. Evol. 2001, 16, 491–497. [Google Scholar] [CrossRef]
- Franklin, A.D.; Waddell, W.T.; Behrns, S.; Goodrowe, K.L. Estrous Cyclicity and Reproductive Success Are Unaffected by Translocation for the Formation of New Reproductive Pairs in Captive Red Wolves (Canis rufus). Zoo Biol. 2020, 39, 230–238. [Google Scholar] [CrossRef]
- van Kesteren, F.; Sillero-Zubiri, C.; Millar, R.; Argaw, K.; Macdonald, D.W.; Paris, M. Sex, Stress and Social Status: Patterns in Fecal Testosterone and Glucocorticoid Metabolites in Male Ethiopian Wolves. Gen. Comp. Endocrinol. 2012, 179, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Barja, I.; Silván, G.; Illera, J.C. Relationships between Sex and Stress Hormone Levels in Feces and Marking Behavior in a Wild Population of Iberian Wolves (Canis lupus signatus). J. Chem. Ecol. 2008, 34, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Kreeger, T.J.; Seal, U.S.; Plotka, E.D. Influence of Hypothalamic-Pituitary-Adrenocortical Hormones on Reproductive Hormones in Gray Wolves (Canis lupus). J. Exp. Zool. 1992, 264, 32–41. [Google Scholar] [CrossRef]
- Yordy, J.; Mossotti, R.H. Kinship, Maternal Effects, and Management: Juvenile Mortality and Survival in Captive African Painted Dogs, Lycaon pictus. Zoo Biol. 2016, 35, 367–377. [Google Scholar] [CrossRef]
- Williamson, S.R. North American Regional Studbook: African Wild Dog (Lycaon pictus); Chicago Zoological Society, Brookfield Zoo: Chicago, IL, USA, 2013. [Google Scholar]
- Frantzen, M.A.J.; Ferguson, J.W.H.; de Villiers, M.S. The Conservation Role of Captive African Wild Dogs (Lycaon pictus). Biol. Conserv. 2001, 100, 253–260. [Google Scholar] [CrossRef]
- Wayne, R.K.; Geffen, E.; Girman, D.J.; Koepfli, K.P.; Lau, L.M.; Marshall, C.R. Molecular Systematics of the Canidae. Syst. Biol. 1997, 46, 622–653. [Google Scholar] [CrossRef]
- Troisi, A.; D’Amato, F.R. Mechanisms of Primate Infant Abuse: The Maternal Anxiety Hypothesis. In Infanticide Parent. Care; Routledge: London, UK, 1994; pp. 199–210. [Google Scholar]
- Maestripieri, D.; Carroll, K.A. Behavioral and Environmental Correlates of Infant Abuse in Group-Living Pigtail Macaques. Infant Behav. Dev. 1998, 21, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Maestripieri, D.; Carroll, K.A. Child Abuse and Neglect: Usefulness of the Animal Data. Psychol. Bull. 1998, 123, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, W.; Abbott, D.H. Effects of Elevated Circulating Cortisol Concentrations on Maternal Behavior in Common Marmoset Monkeys (Callithrix jacchus ). Psychoneuroendocrinology 2009, 34, 1222–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovejoy, M.C.; Graczyk, P.A.; O’Hare, E.; Neuman, G. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin. Psychol. Rev. 2000, 20, 561–592. [Google Scholar] [CrossRef]
- Windham, A.M.; Rosenberg, L.; Fuddy, L.; McFarlane, E.; Sia, C.; Duggan, A.K. Risk of Mother-Reported Child Abuse in the First 3 Years of Life. Child Abus. Negl. 2004, 28, 645–667. [Google Scholar] [CrossRef]
- McCanne, T.R.; Hagstrom, A.H. Physiological Hyperreactivity to Stressors in Physical Child Abusers and Individuals at Risk for Being Physically Abusive. Aggress. Violent Behav. 1996, 1, 345–358. [Google Scholar] [CrossRef]
- Bos, P.A.; Hechler, C.; Beijers, R.; Shinohara, K.; Esposito, G.; de Weerth, C. Prenatal and Postnatal Cortisol and Testosterone Are Related to Parental Caregiving Quality in Fathers, but Not in Mothers. Psychoneuroendocrinology 2018, 97, 94–103. [Google Scholar] [CrossRef]
- Ben-Eliyahu, S.; Shakhar, G.; Page, G.G.; Stefanski, V.; Shakhar, K. Suppression of Nk Cell Activity and of Resistance to Metastasis by Stress: A Role for Adrenal Catecholamines and Β-Adrenoceptors. Neuroimmunomodulation 2000, 8, 154–164. [Google Scholar] [CrossRef]
- Quan, N.; Avitsur, R.; Stark, J.L.; He, L.; Shah, M.; Caligiuri, M.; Padgett, D.A.; Marucha, P.T.; Sheridan, J.F. Social Stress Increases the Susceptibility to Endotoxic Shock. J. Neuroimmunol. 2001, 115, 36–45. [Google Scholar] [CrossRef]
- Sergestrom, S.C.; Miller, G.E. Psychological Stress and the Human Immune System: Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Tyrrell, D.A.J.; Smith, A.P. Psychological Stress and Susceptibility to the Common Cold. N. Engl. J. Med. 1991, 325, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Peterson, P.K.; Filice, G.A.; Pomeroy, C.; Sharp, B.M. Effects of Immobilization Stress on the Pathogenesis of Acute Murine Toxoplasmosis. Brain Behav. Immun. 1990, 4, 162–169. [Google Scholar] [CrossRef]
- Marucha, P.T.; Kiecolt-Glaser, J.K.; Favagehi, M. Mucosal Wound Healing Is Impaired by Examination Stress. Psychosom. Med. 1998, 60, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Glaser, R.; Kiecolt-Glaser, J.K.; Bonneau, R.H.; Malarkey, W.; Kennedy, S.; Hughes, J. Stress-Induced Modulation of the Immune Response to Recombinant Hepatitis B Vaccine. Psychosom. Med. 1992, 54, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiecolt-Glaser, J.K.; Glaser, R.; Gravenstein, S.; Malarkey, W.B.; Sheridan, J. Chronic Stress Alters the Immune Response to Influenza Virus Vaccine in Older Adults. Proc. Natl. Acad. Sci. USA 1996, 93, 3043–3047. [Google Scholar] [CrossRef] [Green Version]
- Stammen, R.L.; Cohen, J.K.; Meeker, T.L.; Crane, M.M.; Amara, R.R.; Hicks, S.L.; Meyer, J.S.; Ethun, K.F. Effect of Chronic Social Stress on Prenatal Transfer of Antitetanus Immunity in Captive Breeding Rhesus Macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 357–367. [Google Scholar] [CrossRef]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, J.C.; Ramenofsky, M. Hormones and the Behavioural Ecology of Stress; Baum, P.H.M., Ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Kiecolt-Glaser, J.K.; Loving, T.J.; Stowell, J.R.; Malarkey, W.B.; Lemeshow, S.; Dickinson, S.L.; Glaser, R. Hostile Marital Interactions, Proinflammatory Cytokine Production, and Wound Healing. Arch. Gen. Psychiatry 2005, 62, 1377–1384. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Coussons-Read, M. Elevated Plasma Inflammatory Markers in Individuals with Intermittent Explosive Disorder and Correlation with Aggression in Humans. JAMA Psychiatry 2014, 71, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Deuri, S.K.; Sarmah, A.; Pathak, K.; Baruah, A.; Sengupta, S.; Mehta, S.; Avinash, P.R.; Kalita, K.N.; Hazarika, J. Aggression as an Independent Entity Even in Psychosis-the Role of Inflammatory Cytokines. J. Neuroimmunol. 2016, 292, 45–51. [Google Scholar] [CrossRef]
- Almberg, E.S.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. A Serological Survey of Infectious Disease in Yellowstone National Park’s Canid Community. PLoS ONE 2009, 4, e7042. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.K.; Kat, P.W.; Bulger, J.B.; Maddock, A.H.; Ginsberg, J.R.; Burrows, R.; Mcnutt, J.W.; Mills, M.G.L. Population Dynamics of African Wild Dogs. In Wildlife 2001: Populations; McCullough, D.R., Barrett, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 1125–1139. [Google Scholar]
- Flacke, G.; Becker, P.; Cooper, D.; Gunther, M.S.; Robertson, I.; Holyoake, C.; Donaldson, R.; Warren, K. An Infectious Disease and Mortality Survey in a Population of Free-Ranging African Wild Dogs and Sympatric Domestic Dogs. Int. J. Biodivers. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Goller, K.V.; Fyumagwa, R.D.; Nikolin, V.; East, M.L.; Kilewo, M.; Speck, S.; Müller, T.; Matzke, M.; Wibbelt, G. Fatal Canine Distemper Infection in a Pack of African Wild Dogs in the Serengeti Ecosystem, Tanzania. Vet. Microbiol. 2010, 146, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, K.A.; McNutt, J.W.; Briggs, M.B.; Standers, P.E.; Funston, P.; Hemson, G.; Keet, D.; van Vuuren, M.J. Multi-Host Pathogens and Carnivore Management in Sourthern Africa. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Gordon, C.H.; Banyard, A.C.; Hussein, A.; Laurenson, M.K.; Malcolm, J.R.; Marino, J.; Regassa, F.; Stewart, A.E.; Fooks, A.R.; Sillero-Zubiri, C. Canine Distemper in Endangered Ethiopian Wolves. Emerg. Infect. Dis. 2015, 21, 824. [Google Scholar] [CrossRef] [Green Version]
- Almberg, E.S.; Cross, P.C.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. Infectious Diseases in Yellowstone’s Canid Community. Yelllowstone Sc. 2011, 19, 16–24. [Google Scholar]
- Gasgoyne, S.C.; Laurenson, M.K.; Lelo, S.; Borner, M. Rabies in African Wild Dogs (Lycaon pictus) in the Serengeti Region, Tanzania. J. Wild. Dis. 1993, 29, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Kat, P.W.; Alexander, K.A.; Smith, J.S.; Munson, L. Rabies and African Wild Dogs in Kenya. Proc. R. Soc. B 1995, 262, 229–233. [Google Scholar]
- Hofmeyr, M.; Hofmeyr, D.; Nel, L.; Bingham, J. A Second Outbreak of Rabies in African Wild Dogs (Lycaon pictus) in Madikwe Game Reserve, South Africa, Demonstrating the Efficacy of Vaccination against Natural Rabies Challenge. Anim. Conserv. 2004, 7, 193–198. [Google Scholar] [CrossRef]
- Randall, D.A.; Marino, J.; Haydon, D.T.; Sillero-Zubiri, C.; Knobel, D.L.; Tallents, L.A.; Macdonald, D.W.; Laurenson, M.K. An Integrated Disease Management Strategy for the Control of Rabies in Ethiopian Wolves. Biol. Conserv. 2006, 131, 151–162. [Google Scholar] [CrossRef]
- Randall, D.A.; Williams, S.D.; Kuzmin, I.V.; Rupprecht, C.E.; Tallents, L.A.; Tefera, Z.; Argaw, K.; Shiferaw, F.; Knobel, D.L.; Sillero-Zubiri, C. Rabies in Endangered Ethiopian Wolves. Emerg. Infect. Dis. 2004, 10, 2214. [Google Scholar] [CrossRef]
- Sillero-Zubiric, C.; King, A.A.; Macdonald, D.W. Rabies and Mortality in Ethopian Wolves (Canis simensis). J. Wildl. Dis. 1996, 32, 80–86. [Google Scholar] [CrossRef]
- Haydon, D.T.; Laurenson, M.K.; Sillero-Zubiri, C. Integrating Epidemiology into Population Viability Analysis: Managing the Risk Posed by Rabies and Canine Distemper to the Ethiopian Wolf. Conserv. Biol. 2002, 16, 1372–1385. [Google Scholar] [CrossRef]
- Marino, J.; Sillero-Zubiri, C.; Gottelli, D.; Johnson, P.J.; Macdonald, D.W. The Fall and Rise of E Thiopian Wolves: Lessons for Conservation of Long-Lived, Social Predators. Anim. Conserv. 2013, 16, 621–632. [Google Scholar] [CrossRef]
- Woodroffe, R. Assessing the Risks of Intervention: Immobilization, Radio-Collaring and Vaccination of African Wild Dogs. Oryx 2001, 35, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Burrows, R.; Hofer, H.; East, M.L. Demography, Extinction and Intervention in a Small Population: The Case of the Serengeti Wild Dogs. Proc. R. Soc. London Ser. B Biol. Sci. 1994, 256, 281–292. [Google Scholar]
- Burrows, R. Rabies in Wild Dogs. Nature 1992, 359, 277. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, S.C.; King, A.A.; Laurenson, M.K.; Borner, M.; Schildger, B.; Barrat, J. Aspects of Rabies Infection and Control in the Conservation of the African Wild Dog (Lycaon pictus) in the Serengeti Region, Tanzania. Onderstepoort J. Vet. Res. 1993, 60, 415–420. [Google Scholar]
- D’Onise, K.; Hazel, S.; Caraguel, C. Mandatory Desexing of Dogs: One Step in the Right Direction to Reduce the Risk of Dog Bite? A Systematic Review. Inj. Prev. 2017, 23, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Houpt, K.A.; Scarlett, J.M. Evaluation of Treatments for Separation Anxiety in Dogs. J. Am. Vet. Med. Assoc. 2000, 217, 342–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodman, N.H.; Donnelly, R.; Shuster, L.; Mertens, P.; Rand, W.; Miczek, K. Use of Fluoxetine to Treat Dominance Aggression in Dogs. J. Am. Vet. Med. Assoc. 1996, 209, 1585–1587. [Google Scholar] [PubMed]
- Simpson, B.S.; Papich, M.G. Pharmacologic Management in Veterinary Behavioral Medicine. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 365–404. [Google Scholar] [CrossRef]
- Ciribassi, J.J.; Ballantyne, K. Using Clonidine and Trazodone for Anxiety-Based Behavior Disorders in Dogs. Vet. Med. 2014, 109, 131. [Google Scholar]
- Gilbert-Gregory, S.E.; Stull, J.W.; Rice, M.R.; Herron, M.E. Effects of Trazodone on Behavioral Signs of Stress in Hospitalized Dogs. J. Am. Vet. Med. Assoc. 2016, 249, 1281–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, A.C.; Dwinnell, S.P.; Lasharr, T.N.; Jakopak, R.P.; Denryter, K.; Huggler, K.S.; Hayes, M.M.; Aikens, E.O.; Verzuh, T.L.; May, A.B.; et al. Effectiveness of Partial Sedation to Reduce Stress in Captured Mule Deer. J. Wildl. Manag. 2020, 84, 1445–1456. [Google Scholar] [CrossRef]
- Jeong, D.-H.; Yang, J.-J.; Yeon, S.-C. Fluoxetine Therapy to Decrease Stereotypic Behavior in the Asiatic Black Bear (Ursus Thibetanus). J. Zoo Wildl. Med. 2019, 50, 718. [Google Scholar] [CrossRef]
- Yalcin, E.; Aytug, N. Use of Fluoxetine to Treat Stereotypical Pacing Behavior in a Brown Bear (Ursus arctos). J. Vet. Behav. 2007, 2, 73–76. [Google Scholar] [CrossRef]
- Uchida, Y.; Dodman, N.H.; DeGhetto, D. Animal Behavior Case of the Month. A Captive Bear Was Observed to Exhibit Signs of Separation Anxiety, Decreased Fear of Human Beings, and Stereotypical Activity. J. Am. Vet. Med. Assoc. 1998, 212, 354–355. [Google Scholar] [PubMed]
- Poulsen, E.M.B.; Honeyman, V.; Valentine, P.A.; Teskey, G.C. Use of Fluoxetine for the Treatment of Stereotypical Pacing Behavior in a Captive Polar Bear. J. Am. Vet. Med. Assoc. 1996, 209, 1470–1474. [Google Scholar]
- Baker, D.G. Combination Therapy for Footpad Lesions in a Captive Bengal Tiger (Panthera Tigris Tigris). J. Zoo Wildl. Med. 2002, 33, 389–391. [Google Scholar]
- Olds, J.E. Use of Oral Fluoxetine for the Treatment of Abnormal Aggression in Two Red-Necked Wallabies (Macropus Rufogriseus). J. Zoo Wildl. Med. 2017, 48, 922–924. [Google Scholar] [CrossRef]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Effect of Fluoxetine on Blood Concentrations of Serotonin, Cortisol and Dehydroepiandrosterone in Canine Aggression. J. Vet. Pharmacol. Ther. 2011, 34, 430–436. [Google Scholar] [CrossRef]
- Hueletl-Soto, M.E.; Carro-Juárez, M.; Rodríguez-Manzo, G. Fluoxetine Chronic Treatment Inhibits Male Rat Sexual Behavior by Affecting Both Copulatory Behavior and the Genital Motor Pattern of Ejaculation. J. Sex. Med. 2012, 9, 1015–1026. [Google Scholar] [CrossRef]
- Karlson, P.; Lüsche, M. ‘Pheromones’: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R739–R743. [Google Scholar] [CrossRef]
- Liberles, S.D. Mammalian Pheromones. Annu. Rev. Physiol. 2014, 76, 151–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From Pheromones to Behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef]
- Wang, Z.; Sindreu, C.B.; Li, V.; Nudelman, A.; Chan, G.C.K.; Storm, D.R. Pheromone Detection in Male Mice Depends on Signaling through the Type 3 Adenylyl Cyclase in the Main Olfactory Epithelium. J. Neurosci. 2006, 26, 7375–7379. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Kelliher, K.R.; Li, X.; Boehm, T.; Leinders-Zufall, T.; Zufall, F. Essential Role of the Main Olfactory System in Social Recognition of Major Histocompatibility Complex Peptide Ligands. J. Neurosci. 2006, 26, 1961–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisami, E.; Bhatnagar, K.P. Structure and Diversity in Mammalian Accessory Olfactory Bulb. Microsc. Res. Tech. 1998, 43, 476–499. [Google Scholar] [CrossRef]
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an Olfactory Sensory Map. Cell 1996, 87, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Ressler, K.J.; Sullivan, S.L.; Buck, L.B. A Zonal Organization of Odorant Receptor Gene Expression in the Olfactory Epithelium. Cell 1993, 73, 597–609. [Google Scholar] [CrossRef]
- Vassar, R.; Ngai, J.; Axel, R. Spatial Segregation of Odorant Receptor Expression in the Mammalian Olfactory Epithelium. Cell 1993, 74, 309–318. [Google Scholar] [CrossRef]
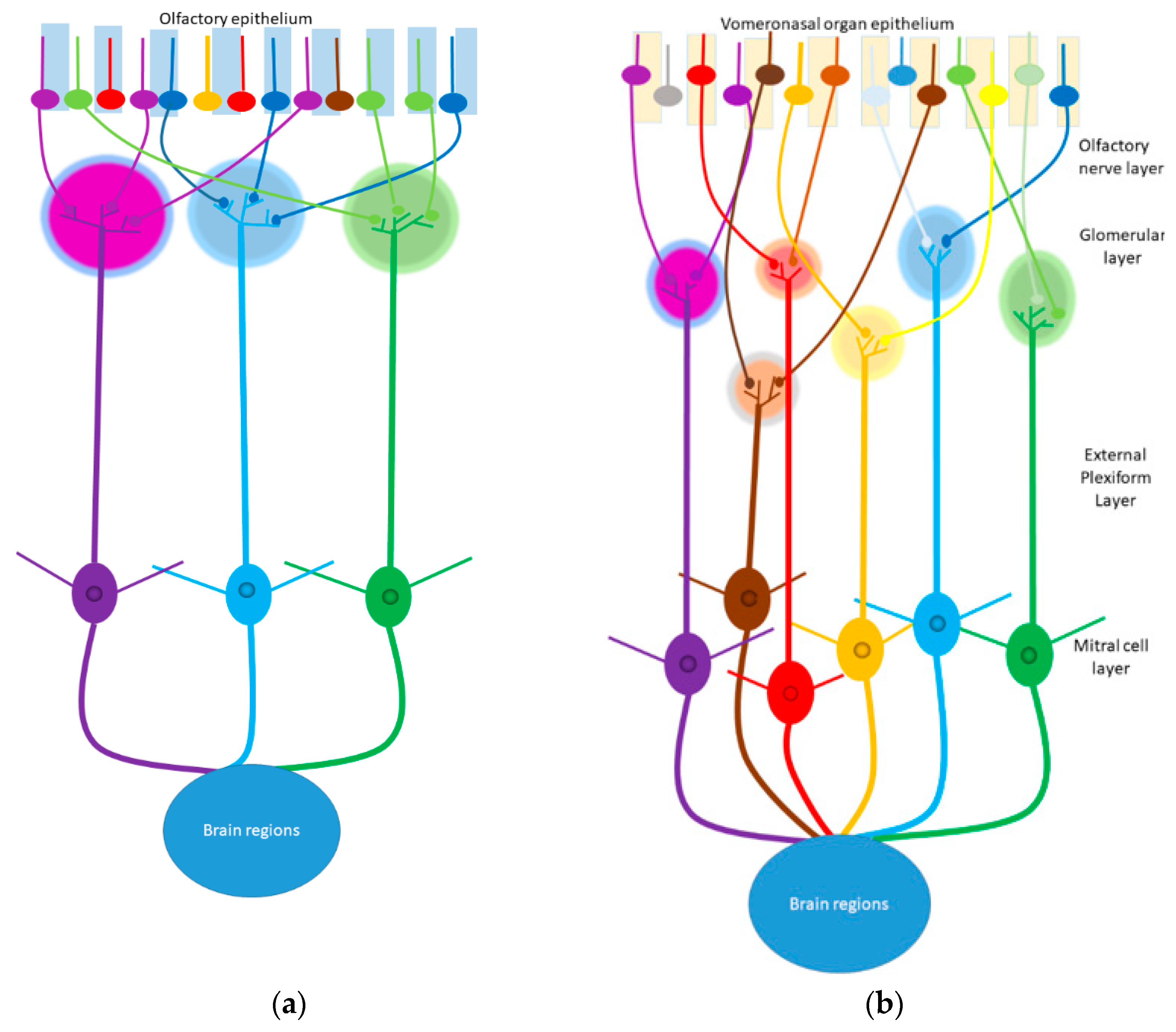
- Ennis, M.; Hamilton, K.A.; Hayar, A. Neurochemistry of the Main Olfactory System; Springer: Boston, MA, USA, 2007; pp. 137–204. [Google Scholar]
- Døving, K.B.; Trotier, D. Structure and Function of the Vomeronasal Organ. J. Exp. Biol. 1998, 201, 2913–2925. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M. Chronic Recording of Vomeronasal Pump Activation in Awake Behaving Hamsters. Physiol. Behav. 1994, 56, 345–354. [Google Scholar] [CrossRef]
- Meredith, M.; Marques, D.M.; O’connell, R.O.; Stern, F.L. Vomeronasal Pump: Significance for Male Hamster Sexual Behavior. Science 1980, 207, 1224–1226. [Google Scholar] [CrossRef]
- Meredith, M.; O’connell, R.J. Efferent Control of Stimulus Access to the Hamster Vomeronasal Organ. J. Physiol. 1979, 286, 301–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, I.; Sánchez-Quinteiro, P.; Alemañ, N.; Prieto, D. Anatomical, Immnunohistochemical and Physiological Characteristics of the Vomeronasal Vessels in Cows and Their Possible Role in Vomeronasal Reception. J. Anat. 2008, 212, 686–696. [Google Scholar] [CrossRef]
- Zancanaro, C.; Caretta, C.M.; Bolner, A.; Sbarbati, A.; Nordera, G.P.; Osculati, F. Biogenic Amines in the Vomeronasal Organ. Chem. Senses 1997, 22, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Spehr, M.; Hatt, H.; Wetzel, C.H. Arachidonic Acid Plays a Role in Rat Vomeronasal Signal Transduction. J. Neurosci. 2002, 22, 8429–8437. [Google Scholar] [CrossRef]
- Taniguchi, M.; Wang, D.; Halpern, M. Chemosensitive Conductance and Inositol 1, 4, 5-Trisphosphate-Induced Conductance in Snake Vomeronasal Receptor Neurons. Chem. Senses 2000, 25, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Von Campenhausen, H.; Mori, K. Convergence of Segregated Pheromonal Pathways from the Accessory Olfactory Bulb to the Cortex in the Mouse. Eur. J. Neurosci. 2000, 12, 33–46. [Google Scholar] [CrossRef]
- Fleischer, J.; Schwarzenbacher, K.; Breer, H. Expression of Trace Amine-Associated Receptors in the Grueneberg Ganglion. Chem. Senses 2007, 32, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Plant, T.M.; Zeleznik, A.J. Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
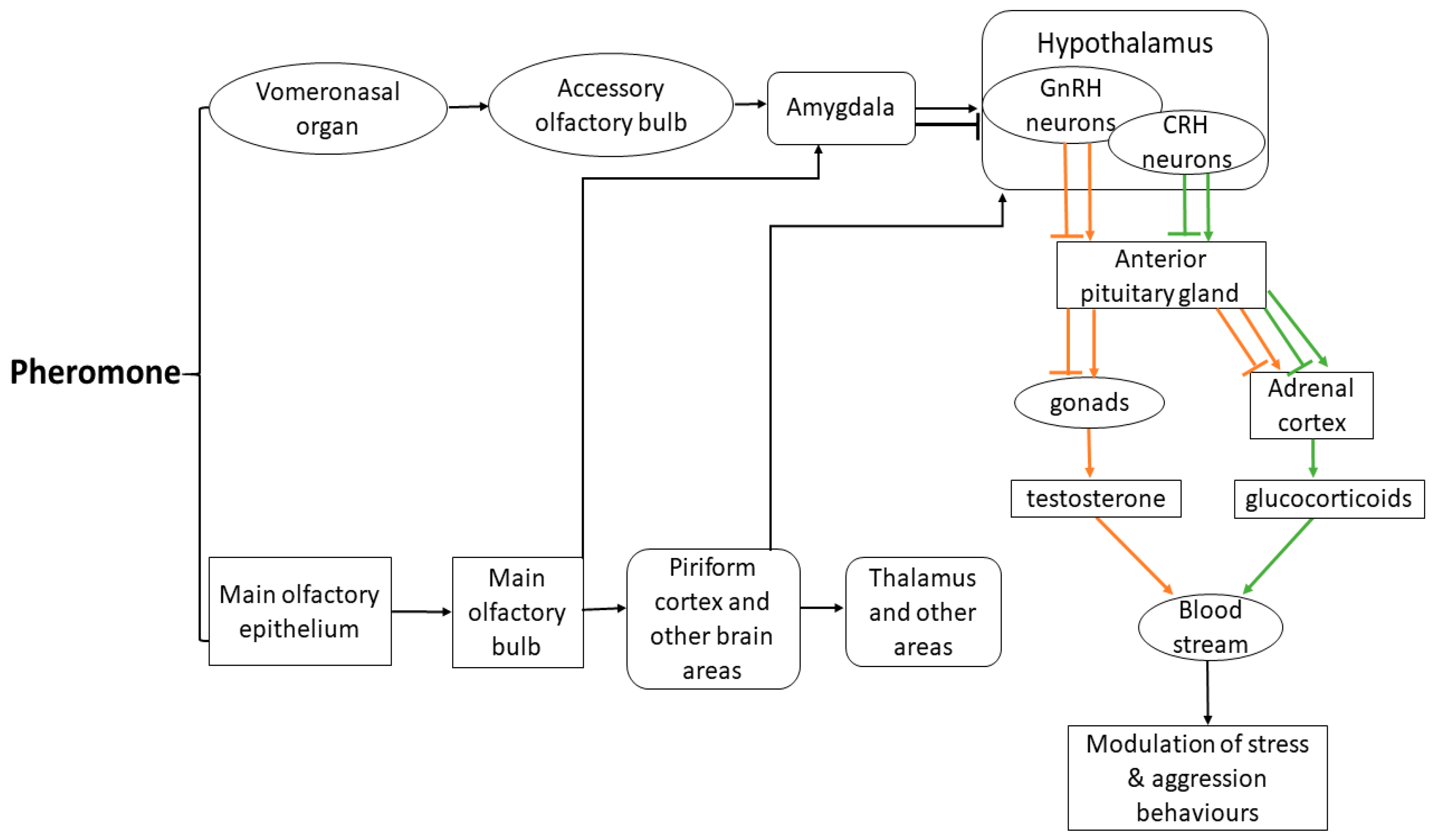
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef]
- Meredith, M. Vomeronasal Function. Chem. Senses 1998, 23, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.J. The Gonadotropin-Releasing Hormone (Gnrh), Neuronal Systems: Immunocytochemistry and in situ hybridization. In The Physiology of Reproduction; Raven: New York, NY, USA, 1988; pp. 1683–1709. [Google Scholar]
- Boehm, U.; Zou, Z.; Buck, L.B. Feedback Loops Link Odor and Pheromone Signaling with Reproduction. Cell 2005, 123, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Enquist, L.W.; Dulac, C. Olfactory Inputs to Hypothalamic Neurons Controlling Reproduction and Fertility. Cell 2005, 123, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Weiss, I.; Hofferberth, J.; Ruther, J.; Stökl, J. Varying Importance of Cuticular Hydrocarbons and Iridoids in the Species-Specific Mate Recognition Pheromones of Three Closely Related Leptopilina Species. Front. Ecol. Evol. 2015, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Buchinger, T.J.; Li, W. The Evolution of (Non) Species-Specific Pheromones. Evol. Ecol. 2020, 34, 455–468. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Baker, C. Species-Specific Pheromones and Their Roles in Shoaling, Migration, and Reproduction: A Critical Review and Synthesis. Fish Pheromones Relat. Cues 2015, 2015, 11–32. [Google Scholar]
- Shine, R.; Reed, R.N.; Shetty, S.; Lemaster, M.; Mason, R.T. Reproductive Isolating Mechanisms between Two Sympatric Sibling Species of Sea Snakes. Evolution 2002, 56, 1655–1662. [Google Scholar] [CrossRef]
- Apps, P.J.; Weldon, P.J.; Kramer, M. Chemical Signals in Terrestrial Vertebrates: Search for Design Features. Nat. Prod. Rep. 2015, 32, 1131–1153. [Google Scholar] [CrossRef]
- Durairaj, R.; Bienboire-Frosini, C.; Cozzi, A.; Pageat, P. Sequence Analysis and Multi-Template Threading of Cat Vomeronasal Type-1 Receptor with an Evaluation of Feline Semiochemical Interactions Using Virtual Screening. In Proceedings of the European Chemoreception Research Organization, Trieste, Italy, 11–14 September 2019. [Google Scholar]
- Grus, W.E.; Zhang, J. Distinct Evolutionary Patterns between Chemoreceptors of 2 Vertebrate Olfactory Systems and the Differential Tuning Hypothesis. Mol. Biol. Evol. 2008, 25, 1593–1601. [Google Scholar] [CrossRef] [Green Version]
- Niimura, Y.; Matsui, A.; Touhara, K. Extreme Expansion of the Olfactory Receptor Gene Repertoire in African Elephants and Evolutionary Dynamics of Orthologous Gene Groups in 13 Placental Mammals. Genome Res. 2014, 24, 1485–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isogai, Y.; Si, S.; Pont-Lezica, L.; Tan, T.; Kapoor, V.; Murthy, V.N.; Dulac, C. Molecular Organization of Vomeronasal Chemoreception. Nature 2011, 478, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmachary, R.L.; Poddar-Sarkar, M. Fifty Years of Tiger Pheromone Research. Curr. Sci. 2015, 108, 2178–2185. [Google Scholar]
- Apps, P.J. Are Mammal Olfactory Signals Hiding Right under Our Noses? Naturwissenschaften 2013, 100, 487–506. [Google Scholar] [CrossRef]
- Apps, P.; Mmualefe, L.; McNutt, J.W. Identification of Volatiles from the Secretions and Excretions of African Wild Dogs (Lycaon pictus). J. Chem. Ecol. 2012, 38, 1450–1461. [Google Scholar] [CrossRef]
- Jackson, C.R.; McNutt, J.W.; Apps, P.J. Managing the Ranging Behaviour of African Wild Dogs (Lycaon pictus) Using Translocated Scent Marks. Wildl. Res. 2012, 39, 31–34. [Google Scholar] [CrossRef]
- Asa, C.S.; Valdespino, C. Canid Reporductive Biology: An Integration of Proximate Mechanisims and Ultimate Causes. Am. Zool. 1998, 38, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Van den Berghe, F.; Paris, M.C.J.; Sarnyai, Z.; Vlamings, B.; Millar, R.P.; Ganswindt, A.; Cozzi, A.; Pageat, P.; Paris, D.B.B.P. Dog Appeasing Pheromone Prevents the Androgen Surge and May Reduce Contact Dominance and Active Submission after Stressful Interventions in African Wild Dogs (Lycaon pictus). PLoS ONE 2019, 14, e0212551. [Google Scholar] [CrossRef] [PubMed]
- Pageat, P.; Mengoli, M.; Cozzi, A. Using Feline Pheromones: From Pet Cats to Wild Felids. In Proceedings of the International Society of Chemical Ecology Annual Congress, Urbana Champaign, IL, USA, 8–12 July 2014. [Google Scholar]
- Mills, D.S.; Ramos, D.; Estelles, M.G.; Hargrave, C. A Triple Blind Placebo-Controlled Investigation into the Assessment of the Effect of Dog Appeasing Pheromone (Dap) on Anxiety Related Behaviour of Problem Dogs in the Veterinary Clinic. Appl. Anim. Behav. Sci. 2006, 98, 114–126. [Google Scholar] [CrossRef]
- Cozzi, A.; Monneret, P.; Lafont-Lecuelle, C.; Bougrat, L.; Gaultier, E.; Pageat, P. The Maternal Cat Appeasing Pheromone: Exploratory Study of the Effects on Aggressive and Affiliative Interactions in Cats. J. Vet. Behav. 2010, 5, 37–38. [Google Scholar] [CrossRef]
- DePorter, T.L.; Bledsoe, D.L.; Beck, A.; Ollivier, E. Evaluation of the Efficacy of an Appeasing Pheromone Diffuser Product Vs Placebo for Management of Feline Aggression in Multi-Cat Households: A Pilot Study. J. Feline Med. Surg. 2019, 21, 293–305. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.J.; Anderson, D.L. Synthetic Maternal Pheromone Stimulates Feeding Behavior and Weight Gain in Weaned Pigs. J. Anim. Sci. 2002, 80, 3179–3183. [Google Scholar] [CrossRef] [Green Version]
- Falewee, C.; Gaultier, E.; Lafont, C.; Bougrat, L.; Pageat, P. Effect of a Synthetic Equine Maternal Pheromone During a Controlled Fear-Eliciting Situation. Appl. Anim. Behav. Sci. 2006, 101, 144–153. [Google Scholar] [CrossRef]
- Pageat, P. Animal Appeasing Pheromones. U.S. Patent 6384252, 7 May 2002. [Google Scholar]
- Taylor, K.; Mills, D.S. A Placebo-Controlled Study to Investigate the Effect of Dog Appeasing Pheromone and Other Environmental and Management Factors on the Reports of Disturbance and House Soiling During the Night in Recently Adopted Puppies (Canis familiaris). Appl. Anim. Behav. Sci. 2007, 105, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Gandia, E.M.; Mills, D.S. Signs of Travel-Related Problems in Dogs and Their Response to Treatment with Dog Appeasing Pheromone. Vet. Rec. 2006, 159, 143–148. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Beck, A.; Lopez, A.; Deniaud, M.; Araujo, J.A.; Milgram, N.W. Dog-Appeasing Pheromone Collars Reduce Sound-Induced Fear and Anxiety in Beagle Dogs: A Placebo-Controlled Study. Vet. Rec. 2015, 177, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermiston, C.; Montrose, V.T.; Taylor, S. The Effects of Dog-Appeasing Pheromone Spray Upon Canine Vocalizations and Stress-Related Behaviors in a Rescue Shelter. J. Vet. Behav. 2018, 26, 11–16. [Google Scholar] [CrossRef]
- Gaultier, E.; Bonnafous, L.; Bougrat, L.; Lafont, C.; Pageat, P. Comparison of the Efficacy of a Synthetic Dog-Appeasing Pheromone with Clomipramine for the Treatment of Separation-Related Disorders in Dogs. Vet. Rec. 2005, 156, 533–538. [Google Scholar] [CrossRef]
- Santos, N.R.; Beck, A.; Blondel, T.; Maenhoudt, C.; Fontbonne, A. Influence of Dog-Appeasing Pheromone on Canine Maternal Behaviour During the Peripartum and Neonatal Periods. Vet. Rec. 2020, 186, 449. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.S.; Hargrave, C. Dog Appeasing Pheromone Reduces the Anxiety of Aggressive Dogs in the Veterinary Practice. Am. Vet. Soc. Anim. Behav. Proc. Phila. 2004, 6–7. [Google Scholar]
- Koolhaas, J.M.; Korte, S.M.; de Boer, S.F.; van der Vegt, B.J.; van Reenen, C.G.; Hopster, H.; de Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping Styles in Animals: Current Status in Behavior and Stress-Physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Carlo, S.; Manteca, X.; Cuenca, R.; Alcalá, M.D.; Alba, A.; Lavín, S.; Pastor, J. Effect of a Synthetic Appeasing Pheromone on Behavioral, Neuroendocrine, Immune, and Acute-Phase Perioperative Stress Responses in Dogs. J. Am. Vet. Med. Assoc. 2010, 237, 673–681. [Google Scholar]
- Tomohiro, Y.; Koori, M.; Kikusui, T.; Mori, Y. Appeasing Pheromone Inhibits Cortisol Augmentation and Agonistic Behaviors During Social Stress in Adult Miniature Pigs. Zool. Sci. 2009, 26, 739–744. [Google Scholar]
- Aviles-Rosa, E.O.; Surowiec, K.; McGlone, J. Identification of Faecal Maternal Semiochemicals in Swine (Sus scrofa) and Their Effects on Weaned Piglets. Sci. Rep. 2020, 10, 5349. [Google Scholar] [CrossRef] [Green Version]
- Dugovich, B.S.; Peel, M.J.; Palmer, A.L.; Zielke, R.A.; Sikora, A.E.; Beechler, B.R.; Jolles, A.E.; Epps, C.W.; Dolan, B.P. Detection of Bacterial-Reactive Natural Igm Antibodies in Desert Bighorn Sheep Populations. PLoS ONE 2017, 12, e0180415. [Google Scholar] [CrossRef] [PubMed]
- Laikre, L.; Olsson, F.; Jansson, E.; Hössjer, O.; Ryman, N. Metapopulation Effective Size and Conservation Genetic Goals for the Fennoscandian Wolf (Canis lupus) Population. Heredity 2016, 117, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Appleby, R.; Mackie, J.; Smith, B.; Bernede, L.; Jones, D. Human–Dingo Interactions on Fraser Island: An Analysis of Serious Incident Reports. Aust. Mammal. 2018, 40, 146–156. [Google Scholar] [CrossRef]
| Study Species | DLA-DQA1 | DLA-DQB1 |
|---|---|---|
| Domestic Dog: European purebred dogs n = > 8000 | 18 | 47 |
| African wild dog: Eastern and Southern Africa n = 368 | 1 | 2 |
| Grey wolf: Canada and Alaska n = 194 | 12 | 15 |
| Grey Wolf: Northern Europe n = 163 | 9 | 10 |
| Grey wolf: Total n = 407 | 18 | 21 |
| Mexican wolf: Captive American Population n < 7 | 5 | 3 |
| Ethiopian wolf: Bale Mountains Ethiopia n = 99 | 2 | 5 |
| Component | Canine % (w/w) | Porcine % (w/w) | Caprine % (w/w) | Bovine % (w/w) | Ovine % (w/w) | Equine % (w/w) |
|---|---|---|---|---|---|---|
| Oleic acid | 21.5–27.8 | 24.7–36.8 | 20.1–22.3 | 24.9–28.6 | 32.8–38.8 | 35.2–40.3 |
| Palmitic acid | 20.8–24.9 | 15.5–26.8 | 22.3–26.8 | 19.2–23.1 | 21.6–25.9 | 22.8–26.7 |
| Linoleic acid | 20.5–25.4 | 29.5–40.6 | 20.2–22.5 | 20.5–24.3 | 21.2–25.7 | 22.1–27.1 |
| Myristic acid | 2.2–3.9 | 3.9–9.6 | 8.5–10.1 | 3.2–5.6 | 3.4–5.9 | 2.0–2.8 |
| Lauric acid | 0.4–1.8 | 2.8–8.7 | 11.4–14.8 | 1.9–4.2 | 2.6–4.4 | 2.3–3.7 |
| Pentadecanoic acid | 1.8–3.1 | |||||
| Cholesterol | 10.2–18.6 | |||||
| Capric acid | 0.5–3.5 | |||||
| Squalene | 9.5–11.2 | |||||
| 1-docosanol 2,2-dimethyl 1,3-dioxolane 4-methanol | 18.4–22.8 | 7.4–9.7 | 4.4–6.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riddell, P.; Paris, M.C.J.; Joonè, C.J.; Pageat, P.; Paris, D.B.B.P. Appeasing Pheromones for the Management of Stress and Aggression during Conservation of Wild Canids: Could the Solution Be Right under Our Nose? Animals 2021, 11, 1574. https://doi.org/10.3390/ani11061574
Riddell P, Paris MCJ, Joonè CJ, Pageat P, Paris DBBP. Appeasing Pheromones for the Management of Stress and Aggression during Conservation of Wild Canids: Could the Solution Be Right under Our Nose? Animals. 2021; 11(6):1574. https://doi.org/10.3390/ani11061574
Chicago/Turabian StyleRiddell, Pia, Monique C. J. Paris, Carolynne J. Joonè, Patrick Pageat, and Damien B. B. P. Paris. 2021. "Appeasing Pheromones for the Management of Stress and Aggression during Conservation of Wild Canids: Could the Solution Be Right under Our Nose?" Animals 11, no. 6: 1574. https://doi.org/10.3390/ani11061574
APA StyleRiddell, P., Paris, M. C. J., Joonè, C. J., Pageat, P., & Paris, D. B. B. P. (2021). Appeasing Pheromones for the Management of Stress and Aggression during Conservation of Wild Canids: Could the Solution Be Right under Our Nose? Animals, 11(6), 1574. https://doi.org/10.3390/ani11061574







