Dietary Aspergillus oryzae Modulates Serum Biochemical Indices, Immune Responses, Oxidative Stress, and Transcription of HSP70 and Cytokine Genes in Nile Tilapia Exposed to Salinity Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Probiotic Feed Supplement
2.3. Fish Rearing and Management
2.4. Water Quality Parameters
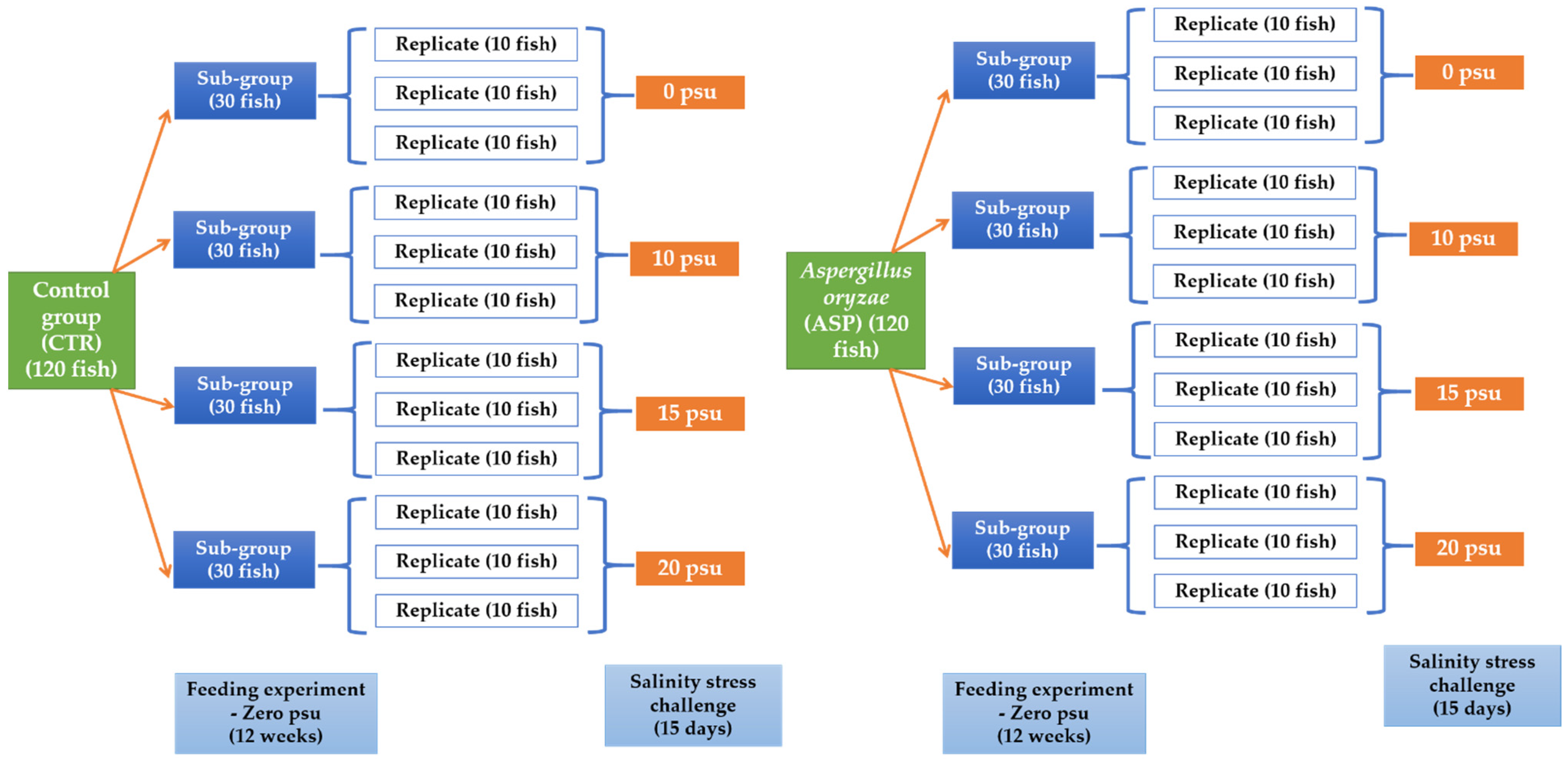
2.5. Experimental Setup
2.5.1. Experiment I: Feeding Trial
2.5.2. Experiment II: Challenge with Salinity Stress
2.6. Sampling
2.6.1. Blood Sampling
2.6.2. Liver Samples
2.7. Serum Biochemical Measurements
2.8. Immunity and Antioxidant Parameters
2.8.1. Immune Parameters
- PA = Total No. of macrophages encompassing yeast cells/total No. of macrophages × 100
- PI = No. of phagocytized yeast cells/No. of phagocytic cells
2.8.2. Oxidative Stress Biomarkers
2.9. Gene Transcription
2.10. Statistical Analysis
3. Results
3.1. Serum Biochemical Measurements
3.2. Non-Specific Immune Parameters
3.3. Antioxidative Capacity Markers
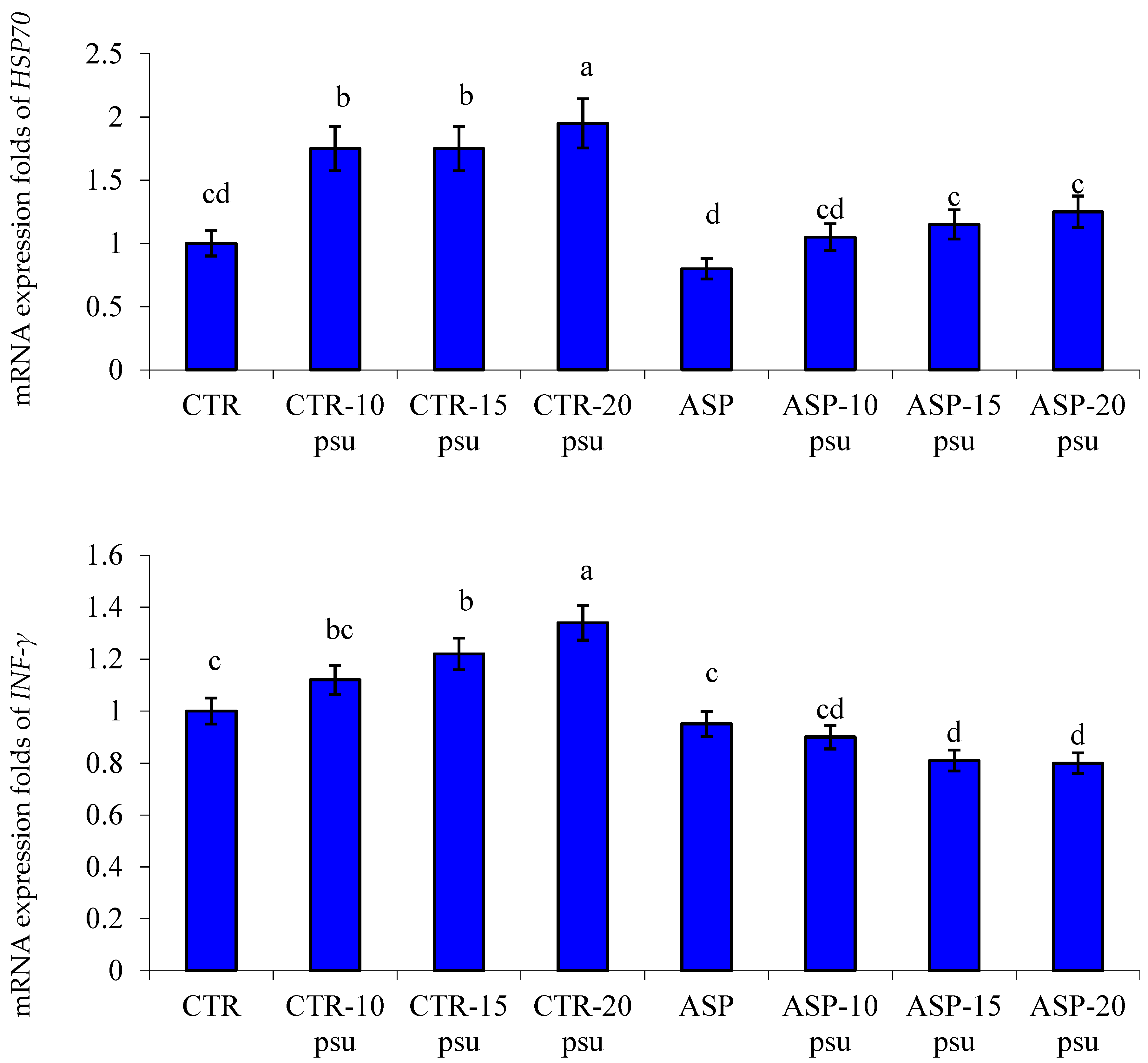
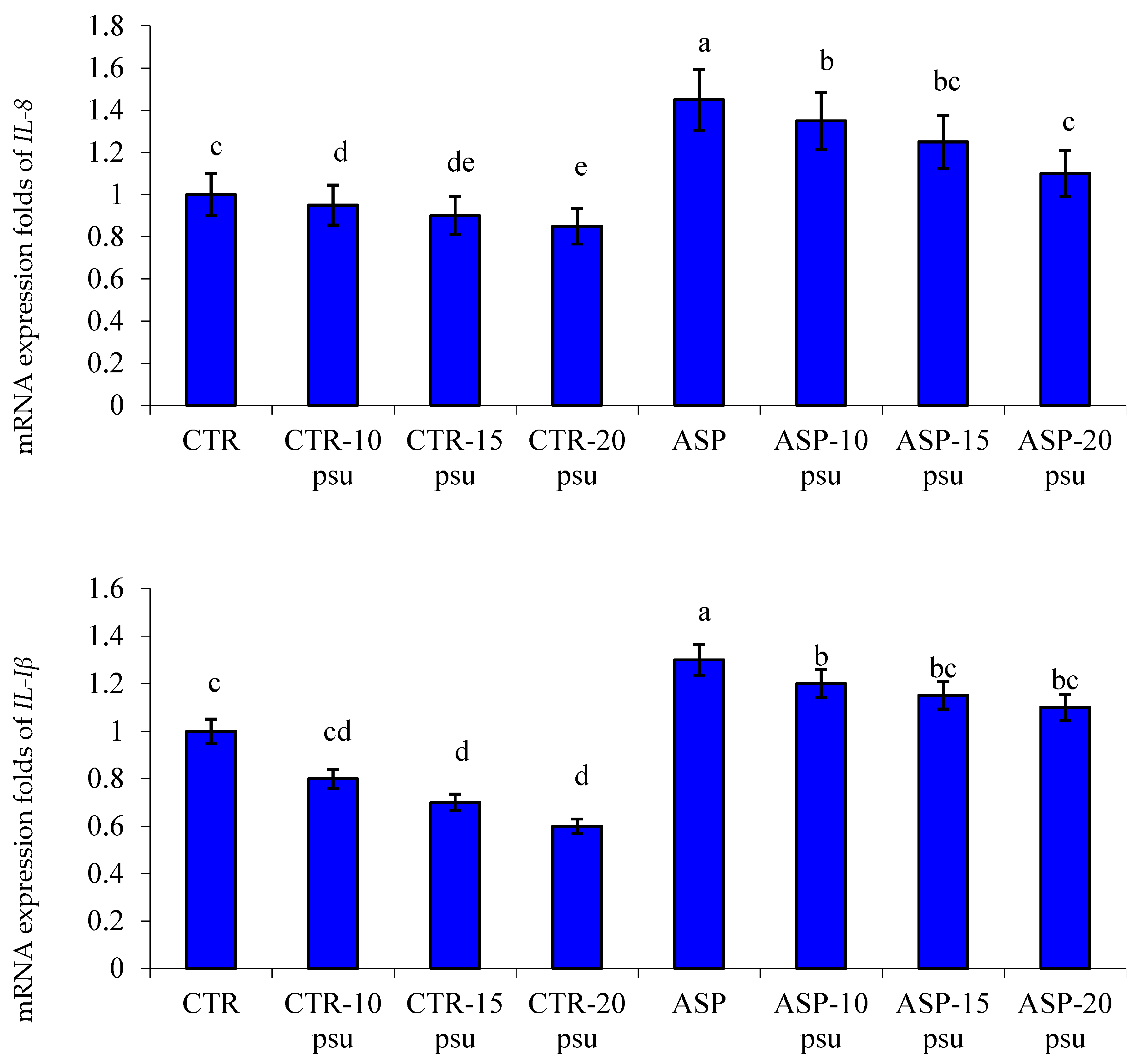
3.4. Transcriptomic Profile
4. Discussion
4.1. Serum Biochemical Indices
4.2. Immune and Antioxidant Measurements
4.3. Gene Transcription
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.-S.; El-Sharawy, M.E.; Atta, A.M.; Elbialy, Z.I.; Abdel-Latif, H.M.; Paray, B.A. The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of Nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Vincent, A.T.; Gauthier, J.; Derome, N.; Charette, S.J. The Rise and Fall of Antibiotics in Aquaculture. In Microbial Communities in Aquaculture Ecosystems; Springer: Cham, Switzerland, 2019; pp. 1–19. [Google Scholar]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Raieni, R.F.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquac. 2020, 10, 1–34. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Gatesoupe, F.J. Chapter 32—Probiotics and Other Microbial Manipulations in Fish Feeds: Prospective Health Benefits. In Bio-active Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Boston, MA, USA, 2010; pp. 541–552. [Google Scholar]
- Gobi, N.; Vaseeharan, B.; Chen, J.-C.; Rekha, R.; Vijayakumar, S.; Anjugam, M.; Iswarya, A. Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol. 2018, 74, 501–508. [Google Scholar] [CrossRef]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.-I.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nat. Cell Biol. 2005, 438, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Lee, S.K.; Lee, B.D. Aspergillus oryzae as probiotic in poultry—A review. Int. J. Poult. Sci. 2006, 5, 1–3. [Google Scholar]
- Iwashita, M.K.P.; Nakandakare, I.B.; Terhune, J.S.; Wood, T.; Ranzani-Paiva, M.J.T. Dietary supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae enhance immunity and disease resistance against Aeromonas hydrophila and Streptococcus iniae infection in juvenile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2015, 43, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Effects of feeding regimen of dietary Aspergillus oryzae on the growth performance, intestinal morphometry and blood profile of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2019, 25, 1063–1072. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Synbiotic Effects of Aspergillus oryzae and β-Glucan on Growth and Oxidative and Immune Responses of Nile Tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 2019, 12, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Eweedah, N.M.; Moustafa, E.M.; Farahat, E.M. Probiotic effects of Aspergillus oryzae on the oxidative status, heat shock protein, and immune related gene expression of Nile tilapia (Oreochromis niloticus) under hypoxia challenge. Aquaculture 2020, 520, 734669. [Google Scholar] [CrossRef]
- Hedayati, S.A.; Bagheri, T.; Hoseinifar, S.H.; Van Doan, H. Growth performances and hemato-immunological responses of common carp (Cyprinus carpio Linnaeus, 1758) to fermented Aspergillus oryzae. Iran. J. Fish. Sci. 2020, 19, 1749–1756. [Google Scholar]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, P.L.; Ballantyne, J.S. Metabolic responses to salinity acclimation in juvenile shortnose sturgeon Acipenser brevirostrum. Aquaculture 2003, 219, 891–909. [Google Scholar] [CrossRef]
- Zarejabad, A.M.; Jalali, M.A.; Sudagar, M.; Pouralimotlagh, S. Hematology of great sturgeon (Huso huso Linnaeus, 1758) juvenile exposed to brackish water environment. Fish Physiol. Biochem. 2010, 36, 655–659. [Google Scholar] [CrossRef]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.; Xu, D. Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Watanabe, W.O.; Kuo, C.-M. Observations on the reproductive performance of Nile tilapia (Oreochromis niloticus) in laboratory aquaria at various salinities. Aquaculture 1985, 49, 315–323. [Google Scholar] [CrossRef]
- Kang’Ombe, J.; Brown, J.A. Effect of Salinity on Growth, Feed Utilization, and Survival of Tilapia rendalli Under Laboratory Conditions. J. Appl. Aquac. 2008, 20, 256–271. [Google Scholar] [CrossRef]
- Gan, L.; Xu, Z.X.; Ma, J.J.; Xu, C.; Wang, X.D.; Chen, K.; Chen, L.Q.; Li, E.C. Effects of salinity on growth, body composition, muscle fatty acid composition, and antioxidant status of juvenile Nile tilapia Oreochromis niloticus (Linnaeus, 1758). J. Appl. Ichthyol. 2016, 32, 372–374. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Suo, Y.; Su, Y.; Lu, M.; Zhao, Q.; Qin, J.G.; Chen, L. Histological and transcriptomic responses of two immune organs, the spleen and head kidney, in Nile tilapia (Oreochromis niloticus) to long-term hypersaline stress. Fish Shellfish Immunol. 2018, 76, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Sun, Y.; Liu, Y.; Qiao, F.; Chen, L.; Liu, W.-T.; Du, Z.; Li, E. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture 2016, 454, 72–80. [Google Scholar] [CrossRef]
- Secombes, C.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Miao, Z.; Ding, L.; Teng, X.; Bao, J. Energy metabolism disorder mediated ammonia gas-induced autophagy via AMPK/mTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol. Environ. Saf. 2021, 217, 112219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Song, H.; Gao, H.; Liu, C.; Zhang, Z.; Fu, J. The Antagonistic Effect of Selenium on Lead-Induced Inflammatory Factors and Heat Shock Protein mRNA Level in Chicken Cartilage Tissue. Biol. Trace Element Res. 2016, 173, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.; Dawood, M.; Mahmoud, S.; Shukry, M.; Noreldin, A.; Ghetas, H.; Khallaf, M. Copper Oxide Nanoparticles Alter Serum Biochemical Indices, Induce Histopathological Alterations, and Modulate Transcription of Cytokines, HSP70, and Oxidative Stress Genes in Oreochromis niloticus. Animals 2021, 11, 652. [Google Scholar] [CrossRef]
- Abdel-Latif, H.; Shukry, M.; El Euony, O.; Soliman, M.M.; Noreldin, A.; Ghetas, H.; Dawood, M.; Khallaf, M. Hazardous Effects of SiO2 Nanoparticles on Liver and Kidney Functions, Histopathology Characteristics, and Transcriptomic Responses in Nile Tilapia (Oreochromis niloticus) Juveniles. Biology 2021, 10, 183. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Khalili, M.; Rostami, H.K.; Esteban, M.Á. Dietary galactooligosaccharide affects intestinal microbiota, stress resistance, and performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immunol. 2013, 35, 1416–1420. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Pal, A.K.; Sahu, N.P.; Ciji, A. Hemato-immunological responses of Labeo rohita juveniles to temperature and salinity stress: Effect of dietary L-tryptophan. Isr. J. Aquac. 2013, 65, 1–8. [Google Scholar]
- Akhtar, M.S.; Pal, A.K.; Sahu, N.P.; Ciji, A.; Gupta, S.K.; Dasgupta, S. Serum Electrolytes, Osmolarity and Selected Enzyme Activities of Labeo rohita Juveniles Exposed to Temperature and Salinity Stress: Effect of Dietary l-Tryptophan; Springer: Berlin/Heidelberg, Germany, 2014; pp. 973–980. [Google Scholar]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; El-Sabagh, M.; Yokoyama, S.; Wang, W.-L.; Yukun, Z.; Olivier, A. Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiol. Biochem. 2017, 43, 179–192. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N. Stimulatory effect of dietary taurine on growth performance, digestive enzymes activity, antioxidant capacity, and tolerance of common carp, Cyprinus carpio L., fry to salinity stress. Fish Physiol. Biochem. 2018, 44, 639–649. [Google Scholar] [CrossRef]
- Yokoyama, S.; Ishikawa, M.; Koshio, S. Dietary bovine lactoferrin enhances defense factors on body surface and anti-parasitic effects against Neobenedenia girellae infection, and mitigates low-salinity stress in amberjack (Seriola dumerili) juveniles. Aquaculture 2019, 504, 52–58. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Fadl, S.; Ahmed, H.A.; El Asely, A.; Abdel-Daim, M.M.; Alkahtani, S. The modulatory effect of mannanoligosaccharide on oxidative status, selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major). Aquac. Rep. 2020, 16, 100278. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Yan, M.; Tang, S.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Inulin alleviates hypersaline-stress induced oxidative stress and dysbiosis of gut microbiota in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 529, 735681. [Google Scholar] [CrossRef]
- Tang, S.; Liu, S.; Zhang, J.; Zhou, L.; Wang, X.; Zhao, Q.; Weng, W.; Qin, J.G.; Chen, L.; Li, E. Relief of hypersaline stress in Nile tilapia Oreochromis niloticus by dietary supplementation of a host-derived Bacillus subtilis strain. Aquaculture 2020, 528, 735542. [Google Scholar] [CrossRef]
- Cui, W.; Ma, A.; Huang, Z.; Liu, Z.; Yang, K.; Zhang, W. myo-inositol facilitates salinity tolerance by modulating multiple physiological functions in the turbot Scophthalmus maximus. Aquaculture 2020, 527, 735451. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Kluwer Academic Publishers: Boston, MA, USA, 1998. [Google Scholar]
- Henry, R.J. Colorimetric determination of total protein. In Clinical Chemistry; Harper and Row Publishers: New York, NY, USA, 1964. [Google Scholar]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; SOS Publications: Cambridge, UK, 1990; pp. 101–103. [Google Scholar]
- Mörsky, P. Turbidimetric determination of lysozyme with Micrococcus lysodeikticus cells: Reexamination of reaction conditions. Anal. Biochem. 1983, 128, 77–85. [Google Scholar] [CrossRef]
- Kawahara, E.; Ueda, T.; Nomura, S. In Vitro Phagocytic Activity of White-Spotted Char Blood Cells after Injection with Aeromonas salmonicida Extracellular Products. Fish Pathol. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Nayak, S. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- Sakai, M. Current research status of fish immunostimulants. Aquaculture 1999, 172, 63–92. [Google Scholar] [CrossRef]
- Euony, O.I.E.; Elblehi, S.S.; Abdel-Latif, H.M.; Abdel-Daim, M.M.; El-Sayed, Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environ. Sci. Pollut. Res. 2020, 27, 23108–23128. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Bonga, S.E.W. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A. Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 2020, 526, 735432. [Google Scholar] [CrossRef]
- Patriche, T.; Patriche, N.; Tenciu, M. Cyprinids total blood proteins determination. J. Anim. Sci. Biotechnol. 2009, 42, 95–101. [Google Scholar]
- Laiz-Carrión, R.; Sangiao-Alvarellos, S.; Guzmán, J.M.; Del Río, M.P.M.; Míguez, J.M.; Soengas, J.L.; Mancera, J.M. Energy Metabolism in Fish Tissues Related to Osmoregulation and Cortisol Action. Fish Physiol. Biochem. 2002, 27, 179–188. [Google Scholar] [CrossRef]
- Neissi, A.; Rafiee, G.; Nematollahi, M.; Razavi, S.H.; Maniei, F. Influence of supplemented diet with Pediococcus acidilactici on non-specific immunity and stress indicators in green terror (Aequidens rivulatus) during hypoxia. Fish Shellfish Immunol. 2015, 45, 13–18. [Google Scholar] [CrossRef]
- Bruslé, J.; Anadon, G.G. The structure and function of fish liver. Fish Morphol. 1996, 76, 545–551. [Google Scholar]
- Secombes, C.; Fletcher, T. The role of phagocytes in the protective mechanisms of fish. Annu. Rev. Fish Dis. 1992, 2, 53–71. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.-S.; El-Sharawy, M.E.; Ghozlan, A.M.; Abdel-Latif, H.M.; Van Doan, H.; Ali, M.A. The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 2020, 525, 735320. [Google Scholar] [CrossRef]
- Ferguson, R.; Merrifield, D.; Harper, G.; Rawling, M.; Mustafa, S.; Picchietti, S.; Balcázar, J.L.; Davies, S. The effect of Pediococcus acidilactici on the gut microbiota and immune status of on-growing red tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2010, 109, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nakhro, K.; Chowdhury, S.; Kamilya, D. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol. 2013, 35, 1547–1553. [Google Scholar] [CrossRef]
- Le Bras, M.; Clément, M.-V.; Pervaiz, S.; Brenner, C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol. Histopathol. 2005, 20, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free. Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Li, J.; Liu, Y.; Gu, X.; Teng, X. Cadmium-induced Oxidative Stress and Immunosuppression Mediated Mitochondrial Apoptosis via JNK-FoxO3a-PUMA pathway in Common Carp (Cyprinus carpio L.) Gills. Aquat. Toxicol. 2021, 233, 105775. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, J.; Sun, Q.; Xu, Y.; Teng, X. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotoxicol. Environ. Saf. 2020, 203, 110974. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Yang, K.; An, Y.; Teng, X.; Teng, X. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ. Sci. Pollut. Res. 2017, 24, 7555–7564. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.W.A.; Chen, D.; Zhang, J.; Liu, Y.; Ishfaq, M.; Tang, Y.; Teng, X. The effect of ammonia exposure on energy metabolism and mitochondrial dynamic proteins in chicken thymus: Through oxidative stress, apoptosis, and autophagy. Ecotoxicol. Environ. Saf. 2020, 206, 111413. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free. Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Eissa, I.A.; Abdeen, A.; Abdel-Latif, H.M.; Ismail, M.M.; Dawood, M.A.; Hassan, A.M. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2019, 69, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Cordero, H.; Martínez-Tomé, M.; Jiménez-Monreal, A.; Bakhrouf, A.; Mahdhi, A. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2014, 39, 532–540. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol. 2013, 35, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of interleukin-1β. Cytokine Growth Factor Rev. 2002, 13, 483–502. [Google Scholar] [CrossRef]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Iwama, G.K.; Vijayan, M.M.; Forsyth, R.B.; Ackerman, P.A. Heat Shock Proteins and Physiological Stress in Fish. Am. Zool. 1999, 39, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Molina, A.; Biemar, F.; Müller, F.; Iyengar, A.; Prunet, P.; MacLean, N.; Martial, J.A.; Muller, M. Cloning and expression analysis of an inducibleHSP70gene from tilapia fish. FEBS Lett. 2000, 474, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Lu, M.; Chen, G.; Cao, J.; Gao, F.; Wang, M.; Liu, Z.; Zhang, D.; Zhu, H.; Yi, M. Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2018, 76, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Q.; Jiang, C.; Ling, F.; Wang, G.-X. Effects of dietary supplementation of intestinal autochthonous bacteria on the innate immunity and disease resistance of grass carp (Ctenopharyngodon idellus). Aquaculture 2015, 438, 105–114. [Google Scholar] [CrossRef]
| Treatment | Serum Biochemical Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Diets | Salinity Levels | GLU (mg/dL) | CORT (ng/mL) | GLO (g/dL) | ALB (g/dL) | TP (g/dL) | AST (U/L) | ALT (U/L) |
| CTR | 0.0 psu | 10.25 | 54.28 | 1.46 | 1.26 | 2.72 | 24.11 | 30.01 |
| 10 psu | 10.92 | 65.73 | 1.42 | 1.25 | 2.67 | 24.95 | 35.42 | |
| 15 psu | 11.15 | 69.94 | 1.40 | 1.24 | 2.63 | 25.62 | 36.69 | |
| 20 psu | 11.50 | 70.71 | 1.37 | 1.20 | 2.57 | 26.15 | 38.04 | |
| ASP | 0.0 psu | 10.13 | 41.46 | 1.62 | 1.41 | 3.03 | 23.97 | 29.11 |
| 10 psu | 10.76 | 52.18 | 1.53 | 1.38 | 2.91 | 24.66 | 30.33 | |
| 15 psu | 10.88 | 53.16 | 1.58 | 1.29 | 2.87 | 24.89 | 32.70 | |
| 20 psu | 10.97 | 50.90 | 1.58 | 1.21 | 2.78 | 24.99 | 34.22 | |
| SEM | 0.05 | 0.65 | 0.02 | 0.01 | 0.01 | 0.04 | 0.11 | |
| Two-way ANOVA (p values) | ||||||||
| ASP | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | |
| Salinity | ˂0.001 | 0.031 | 0.266 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | |
| ASP × Salinity | 0.034 | 0.012 | 0.001 | ˂0.001 | 0.097 | ˂0.001 | 0.001 | |
| Treatments | Measurements | |||
|---|---|---|---|---|
| Diets | Salinity Levels | LYZ (Units/mL) | PI | PA (%) |
| CTR | 0.0 psu | 8.80 | 0.77 | 9.12 |
| 10 psu | 8.84 | 0.83 | 9.16 | |
| 15 psu | 8.84 | 0.88 | 9.30 | |
| 20 psu | 8.64 | 0.81 | 9.19 | |
| ASP | 0.0 psu | 9.38 | 1.02 | 10.20 |
| 10 psu | 9.70 | 1.10 | 10.16 | |
| 15 psu | 9.74 | 1.15 | 10.21 | |
| 20 psu | 9.55 | 1.01 | 10.05 | |
| SEM | 0.02 | 0.01 | 0.03 | |
| Two- way ANOVA (p values) | ||||
| ASP | ˂0.001 | ˂0.001 | ˂0.001 | |
| Salinity | ˂0.001 | ˂0.001 | 0.001 | |
| ASP × Salinity | 0.323 | 0.001 | 0.081 | |
| Treatment | Serum Parameters | ||||
|---|---|---|---|---|---|
| Diet | Salinity | MDA (nmol/mL) | GPX (IU/L) | CAT (IU/L) | SOD (IU/L) |
| CTR | 0.0 psu | 10.71 | 8.28 | 7.57 | 6.90 |
| 10 psu | 11.16 | 8.78 | 7.73 | 6.94 | |
| 15 psu | 11.17 | 8.77 | 7.70 | 7.03 | |
| 20 psu | 11.34 | 8.70 | 7.67 | 6.84 | |
| ASP | 0.0 psu | 8.63 | 9.62 | 8.05 | 7.14 |
| 10 psu | 8.82 | 10.11 | 8.37 | 8.20 | |
| 15 psu | 8.97 | 10.27 | 8.48 | 8.13 | |
| 20 psu | 9.01 | 10.28 | 8.46 | 8.29 | |
| SEM | 0.03 | 0.01 | 0.02 | 0.04 | |
| Two-way ANOVA (p values) | |||||
| ASP | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | |
| Salinity | ˂0.001 | 0.001 | 0.022 | 0.937 | |
| ASP × Salinity | 0.015 | ˂0.001 | ˂0.001 | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukry, M.; Abd El-Kader, M.F.; Hendam, B.M.; Dawood, M.A.O.; Farrag, F.A.; Aboelenin, S.M.; Soliman, M.M.; Abdel-Latif, H.M.R. Dietary Aspergillus oryzae Modulates Serum Biochemical Indices, Immune Responses, Oxidative Stress, and Transcription of HSP70 and Cytokine Genes in Nile Tilapia Exposed to Salinity Stress. Animals 2021, 11, 1621. https://doi.org/10.3390/ani11061621
Shukry M, Abd El-Kader MF, Hendam BM, Dawood MAO, Farrag FA, Aboelenin SM, Soliman MM, Abdel-Latif HMR. Dietary Aspergillus oryzae Modulates Serum Biochemical Indices, Immune Responses, Oxidative Stress, and Transcription of HSP70 and Cytokine Genes in Nile Tilapia Exposed to Salinity Stress. Animals. 2021; 11(6):1621. https://doi.org/10.3390/ani11061621
Chicago/Turabian StyleShukry, Mustafa, Marwa F. Abd El-Kader, Basma M. Hendam, Mahmoud A. O. Dawood, Foad A. Farrag, Salama Mostafa Aboelenin, Mohamed Mohamed Soliman, and Hany M. R. Abdel-Latif. 2021. "Dietary Aspergillus oryzae Modulates Serum Biochemical Indices, Immune Responses, Oxidative Stress, and Transcription of HSP70 and Cytokine Genes in Nile Tilapia Exposed to Salinity Stress" Animals 11, no. 6: 1621. https://doi.org/10.3390/ani11061621









