Host Genetic Diversity and Infectious Diseases. Focus on Wild Boar, Red Deer and Tuberculosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Impact of Infectious Diseases on Host Populations
3. Ungulates as Hosts
4. Wild Boar, Red Deer, and Tuberculosis
5. Factors Affecting Wild Boar and Red Deer Genetic Diversity. Recommendations to Confront TB
- -
- Isolated wild boar and red deer populations are potentially dangerous populations.
- -
- When translocations are unavoidable, managers should select individuals from populations that occur nearby, in similar habitats, and with low genetic divergence.
- -
- Mainly in red deer, the continuity of vegetation refuges should be maintained to facilitate individual movements between distant areas.
- -
- Mainly in red deer, sex ratios and male age structures should be equilibrated to favor the natural dispersal of males and the action of evolutionary processes related to the mating system and effective population size.
- -
- In wild boar, the decrease in contact between wild boar and domestic pigs might reduce the selective pressures boosting outbreeding avoidance.
6. Disease Transmission and Behavioral Differences between Wild Boar and Red Deer
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohn, M.H.; Murphy, W.J.; Ostrander, E.A.; Wayne, R. Genomics and conservation genetics. Trends Ecol. Evol. 2006, 21, 629–637. [Google Scholar] [CrossRef]
- Frankel, O.H. Genetic conservation: Our evolutionary responsibility. Genetics 1974, 78, 53–65. [Google Scholar] [CrossRef]
- Frankel, O.H. Variation, the essence of life. Proc. Linn. Soc. N. S. W. 1970, 95, 158–169. [Google Scholar]
- Charlesworth, B.; Charlesworth, D. The genetic basis of inbreeding depression. Genet. Res. 1999, 74, 329–340. [Google Scholar] [CrossRef]
- Frankel, O.H.; Soulé, M.E. Conservation and Evolution; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Neaves, L.E.; Eales, J.; Whitlock, R.; Hollingsworth, P.M.; Buerke, T.; Pullin, A.S. The fitness consequences of inbreeding in natural populations and their implications for species conservation—A systematic map. Environ. Evid. 2015, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Frankham, R. Genetic adaptation to captivity in species conservation programs. Mol. Ecol. 2008, 17, 325–333. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Amos, W.; Balmford, A. When does conservation genetics matter? Heredity 2001, 87, 257–265. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, S.J. A role for molecular genetics in biological conservation. Proc. Natl. Scad. Sci. USA 1994, 91, 5748–5755. [Google Scholar] [CrossRef] [Green Version]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Kruse, H.; Kirkemo, A.-M.; Handeland, K. Wildlife as source of zoonotic Infections. Emerg. Infect. Dis. 2004, 10, 2067–2072. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Heymann, D.L.; Chen, L.; Takemi, K.; Fidler, D.P.; Tappero, J.W.; Thomas, M.J.; Kenyon, T.A.; Frieden, T.; Yach, D.; Nishtar, S.; et al. Global health security: The wider lessons from the west African Ebola virus disease epidemic. Lancet 2015, 385, 1884–1901. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Emerging infectious diseases in 2012: 20 years after the institute of medicine report. Mbio 2012, 3, e00494-12. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Stratton, C.W.; Tang, Y. Outbreak of pneumonia of unknown etiology in Wuhan China: The mystery and the miracle. J. Med. Virol. 2020, 93, 401–402. [Google Scholar] [CrossRef] [Green Version]
- McKibbin, W.; Fernando, R. The Global Macroeconomic Impacts of COVID-19: Seven Scenarios. Asian Econ. Pap. 2021, 20, 1–30. [Google Scholar] [CrossRef]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Pike, J.; Bogich, T.L.; Elwood, S.; Finnoff, D.C.; Daszak, P. Economic optimization of a global strategy to reduce the pandemic threat. Proc. Natl. Acad. Sci. USA 2014, 111, 18519–18523. [Google Scholar] [CrossRef] [Green Version]
- Quéméré, E.; Rossi, S.; Petit, E.; Marchard, P.; Merlet, J.; Game, Y.; Galan, M.; Gilot-Fromont, E. Genetic epidemiology of the Alpine ibex reservoir of persistent and virulent brucellosis outbreak. Sci. Rep. 2020, 10, 4400. [Google Scholar] [CrossRef]
- Portanier, E.; Garel, M.; Devillard, S.; Maillard, D.; Poissant, J.; Galan, M.; Benabed, S.; Poirel, M.T.; Duhayer, J.; Itty, C.; et al. Both candidate gene and neutral genetic diversity correlate with parasite resistance in female Mediterranean mouflon. BMC Ecol. 2019, 19, 12. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.; Vitikainen, E.I.K.; Wells, D.A.; Cant, M.A.; Nichols, H.J. Heterozygosity but not inbreeding coefficient predicts parasite burdens in the banded mongoose. J. Zool. 2017, 302, 32–39. [Google Scholar] [CrossRef]
- Benavides, M.V.; Sonstegard, T.S.; Van Tassell, C. Genomic regions associated with sheep resistance to gastrointestinal nematodes. Trends Parasitol. 2016, 32, 470–480. [Google Scholar] [CrossRef]
- Sweeney, T.; Hanrahan, J.P.; Ryan, M.T.; Good, B. Immunogenomics of gastrointestinal nematode infection in ruminants—Breeding for resistance to produce food sustainably and safely. Parasite Immunol. 2016, 38, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.D. Causes and consequences of intra- and inter-host heterogeneity in defense against nematodes. Parasite Immunol. 2013, 35, 362–373. [Google Scholar] [PubMed]
- Ruiz-López, M.J.; Monello, R.J.; Gompper, M.E.; Eggert, L.S. The effect and relative importance of neutral genetic diversity for predicting parasitism varies across parasite taxa. PLoS ONE 2012, 9, e45404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saddiqi, H.A.; Jabbar, A.; Sarwar, M.; Iqbal, Z.; Muhammad, G.; Nisa, M.; Shahzad, A. Small ruminant resistance against gastrointestinal nematodes: A case of Haemonchus contortus. Parasitol. Res. 2011, 109, 1483–1500. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Whitehouse, K.; Gulland, F.; Greig, D.; Amos, W. Disease susceptibility in California sea lions. Nature 2003, 422, 35. [Google Scholar] [CrossRef] [PubMed]
- Cassinello, J.; Gomendio, M.; Roldan, E.R.S. Relationship between coefficient of inbreeding and parasite burden in endangered gazelles. Conserv. Biol. 2001, 15, 1171–1174. [Google Scholar] [CrossRef]
- Coltman, D.W.; Pilkington, J.G.; Smith, J.A.; Pemberton, J.M. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution 1999, 53, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.; Westerberg, L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002, 11, 2467–2474. [Google Scholar] [CrossRef]
- Bateson, Z.W.; Hammerly, S.C.; Johnson, J.A.; Morrow, M.E.; Whittingham, L.A.; Dunn, P.O. Specific alleles at immune genes, rather than genome-wide heterozygosity, are related to immunity and survival in the critically endangered Attwater’s prairie chicken. Mol. Ecol. 2016, 25, 4730–4744. [Google Scholar] [CrossRef]
- Brambilla, A.; Biebach, I.; Bassano, B.; Bogliani, G.; von Hardenberg, A. Direct and indirect causal effects of heterozygosity on fitness-related traits in Alpine ibex. Proc. R. Soc. B Biol. Sci. 2015, 82, 20141873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. The major histocompatibility complex and its functions. In The Immune System in Health and Disease, 5th ed.; Janeway, C., Ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Aguilar, A.; Roemer, G.; Debenham, S.; Binns, M.; Garcelon, D.; Waine, R.K. High MHC diversity maintained by balancing selection in an otherwise genetically monomorphic mammal. Proc. Natl. Scad. Sci. USA 2004, 101, 3490–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, A.L.; Hughes, M.K.; Howell, C.Y.; Nei, M. Natural selection at the class II major histocompatibility complex loci of mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 359–367. [Google Scholar]
- Hedrick, P.W.; Thomson, G. Evidence for balancing selection at HLA. Genetics 1983, 104, 449–456. [Google Scholar] [CrossRef]
- Spurgin, L.G.; Richardson, D.S. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc. R. Soc. B Biol. Sci. 2010, 277, 979–988. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Whitehouse, K.; Cunningham, A.A. Is MHC enough for understanding wildlife immunogenetics? Trends Ecol. Evol. 2006, 21, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, L.; Landry, C. MHC studies in nonmodel vertebrates: What have we learned about natural selection in 15 years? J. Evol. Biol. 2003, 16, 363–377. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.K.; Begon, M.; Jackson, J.A.; Paterson, S. Evidence for selection at cytokine loci in a natural population of field voles (Microtus agrestis). Mol. Ecol. 2012, 7, 1632–1646. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Samuel, M.D.; Johnson, C.J.; Adams, M.; McKenzie, D.I. Emerging prion disease drives host selection in a wildlife population. Ecol. Appl. 2012, 22, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.; Johnson, J.; Vanderloo, J.P.; Keane, D.; Aiken, J.M.; McKenzie, D. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J. Gen. Virol. 2006, 87, 2109–2114. [Google Scholar] [CrossRef]
- White, P.S.; Choi, A.; Pandey, R.; Menezes, A.; Penley, M.; Gibson, A.K.; de Roode, J.; Morran, L. Host heterogeneity mitigates virulence evolution. Biol. Lett. 2020, 16, 20100744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, D.; Broniewski, J.M.; Westra, E.R.; Buckling, A.; van Houte, S. Host diversity limits the evolution of parasite local adaptation. Mol. Ecol. 2017, 26, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Altizer, S.; Harvell, D.; Friedle, E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol. Evol. 2003, 18, 589–596. [Google Scholar] [CrossRef]
- Regoes, R.R.; Nowak, M.A.; Bonhoeffer, S. Evolution of virulence in a heterogeneous host population. Evolution 2000, 54, 64–71. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Anderson, R.M.; May, R.M. The invasion, persistence and spread of infectious diseases within animal and plant communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 533–570. [Google Scholar]
- Ekroth, A.K.E.; Rafaluk-Mohr, C.; King, K.C. Host genetic diversity limits parasite success beyond agricultural systems: A meta-analysis. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191811. [Google Scholar] [CrossRef] [PubMed]
- van Houte, S.; Ekroth, A.K.; Broniewski, J.M.; Chabas, H.; Ashby, B.; Bondy-Denomy, J.; Gandon, S.; Boots, M.; Paterson, S.; Buckling, A.; et al. The diversity-generating benefits of a prokaryotic adaptive immune system. Nature 2016, 532, 385–388. [Google Scholar] [CrossRef] [Green Version]
- King, K.C.; Lively, C.M. Does genetic diversity limit disease spread in natural host populations? Heredity 2012, 109, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.; Noble, L.R.; Rollinson, D.; Southgate, V.R.; Webster, J.P.; Jones, C.S. Low genetic diversity in a snail intermediate host (Biomphalaria pfeifferi Krass, 1848) and schistosomiasis transmission in the Senegal River Basin. Mol. Ecol. 2010, 19, 241–256. [Google Scholar] [CrossRef]
- Bani, L.; Orioli, V.; Pisa, G.; Dondina, O.; Fagiani, S.; Fabbri, E.; Randi, E.; Mortelliti, A.; Sozio, G. Landscape determinants of genetic differentiation, inbreeding and genetic drift in the hazel dormouse (Muscardinus avellanarius). Conserv. Genet. 2017, 19, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Gubili, C.; Mariani, S.; Weckworth, B.V.; Galpern, P.; McDevitt, A.D.; Hebblewhite, M.; Nickel, B.; Musiani, M. Environmental and anthropogenic drivers of connectivity patterns: A basis for prioritizing conservation efforts for threatened populations. Evol. Appl. 2017, 10, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlesworth, B. Effective population size and patterns of molecular evolution and variation. Nat. Rev. Genet. 2009, 10, 195–205. [Google Scholar] [CrossRef]
- Epps, C.W.; Wehausen, J.D.; Bleich, V.C.; Torres, S.G.; Brashares, J.S. Optimizing dispersal and corridor models using landscape genetics. J. Appl. Ecol. 2007, 44, 714–724. [Google Scholar] [CrossRef]
- Lawson Handley, J.L.; Perrin, N. Advances in our understanding of mammalian sex-biased dispersal. Mol. Ecol. 2007, 16, 1559–1578. [Google Scholar] [CrossRef]
- Briton, J.; Nurthen, R.K.; Briscoe, D.A.; Frankham, R. Modelling problems in conservation genetics using Drosophila: Consequences of harem. Biol. Conserv. 1994, 69, 267–275. [Google Scholar] [CrossRef]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Scad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [Green Version]
- Wright, S. Size of population and breeding structure in relation to evolution. Science 1938, 87, 1417–1429. [Google Scholar]
- Arauco-Shapiro, G.; Schmacher, K.I.; Boersma, D.; Bouzat, J.L. The role of demographic history and selection in shaping genetic diversity of the Galápagos penguin (Spheniscus mendiculus). PLoS ONE 2020, 15, e0226439. [Google Scholar] [CrossRef] [Green Version]
- Bouzat, J.L. Conservation genetics of population bottlenecks: The role of change, selection, and history. Conserv. Genet. 2010, 11, 463–478. [Google Scholar] [CrossRef]
- Hedrick, P.W. Genetic of Populations; Jones & Bartlett Publishers: Sudbury, MA, USA, 2005. [Google Scholar]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Hedrick, P.W.; García-Dorado, A. Understanding inbreeding depression, purging, and genetic rescue. Trends Ecol. Evol. 2016, 31, 940–952. [Google Scholar] [CrossRef]
- Groombridge, J.J.; Jones, C.G.; Bruford, M.W.; Nichols, R.A. ‘Ghost’ alleles of the Mauritius kestrel. Nature 2000, 403, 616. [Google Scholar] [CrossRef] [PubMed]
- Bonnell, M.L.; Selander, R.L. Elephant seals: Genetic variation and near extinction. Science 1974, 134, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D.; Axelrod, R.; Tanese, R. Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl. Acad. Sci. USA 1990, 87, 3566–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Råberg, L.; Sim, D.; Read, A.F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007, 318, 812–814. [Google Scholar] [CrossRef] [Green Version]
- Bishop, S.C.; Stear, M.J. Modeling of host genetics and resistance to infectious diseases: Understanding and controlling nematode infections. Vet. Parasitol. 2003, 115, 147–166. [Google Scholar] [CrossRef]
- Roy, B.; Kirchner, J. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 2000, 54, 51–63. [Google Scholar] [CrossRef]
- Blanchong, J.A.; Robinson, S.J.; Samuel, M.D.; Foster, J.T. Application of genetics and genomics to wildlife epidemiology. J. Wildl. Manage. 2016, 80, 593–608. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Padilla, J.; Vergara, P.; Mougeot, F.; Redpath, S.M. Parasitized mates increase infection risk for partners. Am. Nat. 2012, 179, 811–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, H.; Cruz, S.; Deak, T. From models to mechanisms: Odorant communication as a key determinant of social behavior in rodents during illness-associated states. Neurosci. Biobehav. Rev. 2011, 35, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Hillgarth, N. Ectoparasite transfer during matins in ring-necked pheasants Phasianus colchicus. J. Avian Biol. 1996, 27, 260–262. [Google Scholar] [CrossRef]
- Hamilton, W.D.; Zuk, M. Heritable true fitness and bright birds: A role for parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Clutton-Brock, T.H. The Evolution of Parental Care; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Trivers, R.L. Parental investment and sexual selection. In Sexual Selection and the Descent of Man: 1871–1971; Campbell, B., Ed.; Aldine Press: Chicago, IL, USA, 1972; pp. 136–179. [Google Scholar]
- Setchell, J.M.; Charpentier, M.J.E.; Abbot, K.M.; Wickings, E.J.; Knapp, L.A. Opposites attract: MHC-associated mate choice in a polygynous primate. J. Evol. Biol. 2010, 23, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Penn, D.J. The scent of genetic compatibility: Sexual selection and the major histocompatibility complex. Ethology 2002, 108, 1–21. [Google Scholar] [CrossRef]
- Penn, D.J.; Potts, W.K. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 1999, 153, 145–164. [Google Scholar] [CrossRef]
- Campbell, L.J.; Head, M.L.; Wilfert, L.; Griffiths, A.G.F. An ecological role for assortative mating under infection? Conserv. Genet. 2017, 18, 983–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teacher, A.G.F.; Garner, T.W.J.; Nichols, R.A. Population genetic patterns suggest a behavioural change in wild common frogs (Rana temporaria) following disease outbreaks (Ranavirus). Mol. Ecol. 2009, 18, 3163–3172. [Google Scholar] [CrossRef]
- Bruniche-Olsen, A.; Burridge, C.P.; Austin, J.J.; Jones, M.E. Disease induced changes in gene flow patterns among Tasmanian devil populations. Biol. Conserv. 2013, 165, 69–78. [Google Scholar] [CrossRef]
- Lachish, S.; McCallum, H.; Jones, M. Demography, disease and the devil: Life-history changes in a disease-affected population of Tasmanian devils (Sarcophilus harrisii). J. Anim. Ecol. 2009, 78, 427–436. [Google Scholar] [CrossRef]
- Serieys, L.E.K.; Lea, A.; Pollinger, J.P.; Riley, S.P.D.; Wayne, R.K. Disease and freeways drive genetic change in urban bobcat populations. Evol. Appl. 2015, 8, 75–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Valen, L. A new evolutionary law. Evol. Theory 1973, 1, 1–30. [Google Scholar]
- Gómez, P.; Ashby, B.; Buckling, A. Population mixing promotes arms race host-parasite coevolution. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornhill, R.; Fincher, C.L. The parasite-driven-wedge model of parapatric speciation. J. Zool. 2013, 291, 23–33. [Google Scholar] [CrossRef]
- Fincher, C.L.; Thornhill, R. A parasite-driven wedge: Infectious diseases may explain language and other biodiversity. Oikos 2008, 117, 1289–1297. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef]
- Cleaveland, S.; Laurenson, M.K.; Taylor, L.H. Diseases of humans and their domestic mammals: Pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 991–999. [Google Scholar] [CrossRef]
- Han, B.A.; Kramer, A.M.; Drake, J.M. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016, 32, 565–577. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; George, D.; Pepin, K.M.; Pitzer, V.E.; Pulliam, J.R.C.; Dobson, A.P.; Hudson, P.J.; Grenfell, B.T. Epidemic dynamics at the human-animal interface. Science 2009, 326, 1362–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Wiethoelter, A.K.; Beltrán-Alcrudo, D.; Kock, R.; Mor, S.M. Global trends in infectious diseases at the wildlife–livestock interface. Proc. Natl. Acad. Sci. USA 2015, 112, 9662–9667. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Olson, S.H.; Uejio, C.K.; Gibbs, H.K. Disease emergence from global climate and land use change. Med. Clin. North. Am. 2008, 92, 1473–1491. [Google Scholar] [CrossRef]
- Cleaveland, S.; Haydon, D.T.; Taylor, L. Overviews of pathogen emergence: Which pathogens emerge, when and why? Curr. Top. Microbiol. Inmunol. 2007, 315, 85–111. [Google Scholar]
- Dehove, A.; Commault, J.; Petitclerc, M.; Teissier, M.; Macé, J. Economic analysis and costing of animal health: A literature review of methods and importance. Rev. Sci. Tech. 2012, 31, 605–617. [Google Scholar] [CrossRef]
- Williams, E.S.; Yuill, T.; Artois, M.; Fischer, J.; Haigh, S.A. Emerging infectious diseases in wildlife. Rev. Sci. Tech. 2002, 21, 39–157. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and Human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Ripple, W.J. Collapse of the world’s largest herbivores. Sci. Adv. 2015, 1, e1400103. [Google Scholar] [CrossRef] [Green Version]
- Linnell, J.D.C.; Zachos, F.E. Status and distribution patterns of European ungulates: Genetics, population history and conservation. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 12–53. [Google Scholar]
- Frantz, A.C.; Bertouille, S.; Eloy, M.C.; Licoppe, A.; Chaumont, F.; Flamand, M.C. Comparative landscape genetic analyses show a Belgian motorway to be a gene flow barrier for red deer (Cervus elaphus), but not wild boars (Sus scrofa). Mol. Ecol. 2012, 21, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, R.; Hindenlang, K.E.; Holzgang, O.; Senn, J.; Stoeckle, B.; Sperisen, C. Genetic effect of transportation infrastructure on roe deer populations (Capreolus capreolus). J. Hered. 2007, 98, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epps, C.W.; Palsbøll, P.J.; Wehausen, J.D.; Roderick, G.K.; Ramey, R.R., II; McCullough, D.R. Highways block gene flow and cause a rapid decline in genetic diversity of desert bighorn sheep. Ecol. Lett. 2005, 8, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Sæther, B.-E.; Engen, S.; Solberg, E.J. Effective size of harvested ungulate populations. Anim. Conserv. 2009, 12, 488–495. [Google Scholar] [CrossRef]
- Coltman, D.W. Molecular ecological approaches to studying the evolutionary impact of selective harvesting in wildlife. Mol. Ecol. 2008, 17, 221–235. [Google Scholar] [CrossRef]
- Queirós, J.; Vicente, J.; Alves, P.C.; de la Fuente, J.; Gortazar, C. Tuberculosis, genetic diversity and fitness in the red deer, Cervus elaphus. Infect. Genet. Evol. 2016, 43, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kotzé, A.; Smith, R.M.; Moodley, Y.; Luikart, G.; Birss, C.; Van Wyk, A.M.; Dalton, D.L. Lessons for conservation management: Monitoring temporal changes in genetic diversity of cape mountain zebra (Equus zebra zebra). PLoS ONE 2019, 14, e0220331. [Google Scholar] [CrossRef] [Green Version]
- Frantz, A.C.; Zachos, F.E.; Bertouille, S.; Eloy, M.C.; Colyn, M.; Flamand, M.C. Using genetic tolos to estimate the prevalence of non-native red deer (Cervus elaphus) in a western European population. Ecol. Evol. 2017, 7, 7650–7660. [Google Scholar] [CrossRef]
- Strucken, E.M.; Lee, S.H.; Jang, G.W.; Porto-Neto, L.R.; Gondro, C. Towards breed formation by island model divergence in Korean cattle. BMC Evol. Biol. 2015, 15, 284. [Google Scholar] [CrossRef] [Green Version]
- Queirós, J.; Vicente, J.; Boadella, M.; Gortázar, C.; Alves, P.C. The impact of management practices and past demographic history on the genetic diversity of red deer (Cervus elaphus): An assessment of population and individual fitness. Biol. J. Linn. Soc. 2014, 111, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Smitz, N.; Corneli, D.; Chardonnet, P.; Caron, A.; de Garine-Wichatitsky, M.; Jori, F.; Mouton, A.; Latinne, A.; Pigneur, L.M.; Melletti, M. Genetic structure of fragmented southern populations of African Cape buffalo (Syncerus caffer caffer). BMC Evol. Biol. 2014, 14, 203. [Google Scholar] [CrossRef] [Green Version]
- Wan, Q.H.; Zhang, P.; Ni, X.W.; Wu, X.W.; Chen, Y.Y.; Kuang, Y.Y.; Ge, Y.F.; Fang, S.G. A novel HURRAH protocol reveals high numbers of monomorphic MHC class II loci and tow asymmetric multi-locus haplotypes in the Pere David’s deer. PLoS ONE 2011, 6, e14518. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Mera, I.G.; Vicente, J.; Pérez de la Lastra, J.M.; Mangold, A.J.; Naranjo, V.; Fierro, Y.; de la Fuente, J.; Gortázar, C. Reduced major histocompatibility complex class II polymorphism in a hunter-managed isolated Iberian red deer population. J. Zool. 2009, 277, 157–170. [Google Scholar] [CrossRef]
- Wojcik, J.M.; Kawalko, A.; Tokarska, M.; Jaarola, M.; Vallenback, P.; Pertodi, C. Post-bottleneck mtDNA diversity in a free-living population of European bison: Implications for conservation. J. Zool. 2009, 277, 81–87. [Google Scholar] [CrossRef]
- Wilson, G.A.; Nishi, J.S.; Elkin, B.T.; Strobeck, C. Effects of a recent founding event and intrinsic population dynamics on genetic diversity in an ungulate population. Conserv. Genet. 2005, 6, 905–916. [Google Scholar] [CrossRef]
- Hedrick, P.W.; Parker, K.M.; Gutiérrez-Espeleta, G.A.; Rattink, A.; Lievers, K. Major histocompatibility complex variation in the Arabian oryx. Evolution 2000, 54, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Devadasan, M.J.; Surya, T.; Vineeth, M.R.; Choudhary, A.; Sivalingam, J.; Kataria, R.S.; Niranjan, S.K.; Tantia, M.S.; Verma, A. Genomic diversity and selection sweeps identified in Indian swamp buffaloes reveals it’s uniqueness with riverine buffaloes. Genomics 2020, 112, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.B.; Hobson, E.A. Edge weight variance: Population genetic metrics for social network analysis. Anim. Behav. 2018, 136, 239–250. [Google Scholar] [CrossRef]
- Janova, E.; Futas, J.; Klumplerova, M.; Putnova, L.; Vrtkva, I.; Vyskocil, M.; Frolkova, P.; Horin, P. Genetic diversity and conservation in a small endangered horse population. J. Appl. Genet. 2013, 54, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Larison, B.; Kaelin, C.B.; Harrigan, R.; Henegar, C.; Rubenstein, D.I.; Kamath, P.; Aschenborn, O.; Smith, T.B.; Barsh, G.S. Population structure, inbreeding and stripe pattern abnormalities in plains zebras. Mol. Ecol. 2021, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, W.G.; Grobler, J.P. Genetic variation among different springbok (Antidorcas marsupialis) colour variants. Mol. Ecol. 2019, 99, 42–53. [Google Scholar] [CrossRef]
- Sasidharan, S.P.; Ludwig, A.; Harper, C.; Moodley, Y.; Bertschinger, H.J.; Guthrie, A.J. Comparative genetics of sarcoid tumour-affected and non-affected mountain zebra (Equus zebra) populations. S. Afr. J. Wildl. Res. 2011, 41, 36–49. [Google Scholar] [CrossRef]
- Pérez-González, J.; Carranza, J.; Torres-Porras, J.; Fernández-García, J.L. Low heterozygosity at microsatellite markers in Iberian red deer with small antlers. J. Hered. 2010, 101, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Marais, H.J.; Nel, P.; Bertschinger, H.J.; Schoeman, J.P.; Zimmerman, D. Prevalence and body distribution of sarcoids in South African Cape mountain zebra (Equus zebra zebra). J. S. Afr. Vet. Assoc. 2007, 78, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachos, F.E.; Althoff, C.; von Steynitz, Y.; Eckert, I.; Hartl, G.B. Genetic analysis of an isolated red deer (Cervus elaphus) population showing signs of inbreeding depression. Eur. J. Wildl. Res. 2007, 53, 61–67. [Google Scholar] [CrossRef]
- Da Silva, A.; Gaillard, J.M.; Yoccoz, N.G.; Hewison, A.J.M.; Galan, M.; Coulson, T.; Allaine, D.; Vial, L.; Delorme, D.; Van Laere, G.; et al. Heterozygosity-fitness correlations revealed by neutral and candidate gene markers in roe deer from a long-term study. Evolution 2009, 63, 403–417. [Google Scholar] [CrossRef]
- Kaeuffer, R.; Reale, D.; Pontier, D.; Chapuis, J.L.; Coltman, D.W. Local effects of inbreeding on embryo number and consequences for genetic diversity in Kerguelen mouflon. Biol. Lett. 2008, 4, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Latch, E.K.; Amann, R.P.; Jacobson, J.P.; Rhodes, O.E. Competing hypotheses for the etiology of cryptorchidism in sitka black-tailed deer: An evaluation of evolutionary alternatives. Anim. Conserv. 2008, 11, 234–246. [Google Scholar] [CrossRef]
- Laikre, L.; Hoban, S.; Bruford, M.W.; Segelbacher, G.; Allendorf, F.W.; Gajardo, G.; Rodríguez, A.G.; Hedrick, P.W.; Heuertz, M.; Hohenlohe, P.A.; et al. Post-2020 goals overlook genetic diversity. Science 2020, 367, 1083–1085. [Google Scholar]
- Ralls, K.; Ballou, J.D.; Dudash, M.R.; Eldridge, M.D.B.; Fenster, C.B.; Lacy, R.C.; Sunnucks, P.; Frankham, R. Call for a Paradigm Shift in the Genetic Management of Fragmented Populations. Conserv. Lett. 2018, 11, e12412. [Google Scholar] [CrossRef]
- Hoban, S.M.; Hauffe, H.C.; Pérez-Espona, S.; Arntzen, J.W.; Bertorelle, G.; Bryja, J.; Frith, K.; Gaggiotti, O.E.; Galbusera, P.; Godoy, J.A.; et al. Bringing genetic diversity to the forefront of conservation policy and management. Conserv. Genet. Res. 2013, 5, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.C.; Reis, A.C.; Ramos, B.; Cunha, M.V. Animal tuberculosis: Impact of disease heterogeneity in transmission, diagnosis and control. Transbound. Emerg. Dis. 2020, 67, 1–19. [Google Scholar] [CrossRef]
- Gortázar, C.; Torres, J.; Vicente, J.; Acevedo, P.; Reglero, M.; de la Fuente, J.; Negro, J.J.; Aznar-Martin, J. Bovine tuberculosis in Doñana Biosphere Reserve: The role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS ONE 2008, 3, e2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Campos, S.; Smith, N.H.; Boniotti, M.B.; Aranaz, A. Overview and phylogeny of Mycobacterium tuberculosis complex organisms: Implications for diagnostics and legislation of bovine tuberculosis. Res. Vet. Sci. 2014, 97, S5–S19. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.A.; Downs, S.H.; Goodchild, A.V.; Broughan, J.M.; Upton, P.A.; Smith, N.H. Bovine TB infection status in cattle in Great Britain in 2012. Vet. Rec. 2014, 174, 600–604. [Google Scholar] [CrossRef]
- Malone, K.M.; Gordon, S.V. Mycobacterium tuberculosis complex members adapted to wild and domestic animals. Adv. Exp. Med. Biol. 2017, 1019, 135–154. [Google Scholar] [PubMed]
- Brites, D.; Gagneux, S. The nature and evolution of genomic diversity in the Mycobacterium tuberculosis complex. Adv. Exp. Med. Biol. 2017, 1019, 1–26. [Google Scholar]
- Galagan, J.E. Genomic insights into tuberculosis. Nat. Rev. Genet. 2014, 15, 307–320. [Google Scholar] [CrossRef]
- Pepperell, C.S.; Casto, A.M.; Kitchen, A.; Granka, J.M.; Conerejo, O.E.; Holmes, E.C.; Birren, B.; Galagan, J.; Feldman, M.W. The role of selection in shaping diversity of natural M. tuberculosis populations. PLoS Pathog. 2013, 9, e1003543. [Google Scholar] [CrossRef]
- Gagneux, S. Host-pathogen coevolution in human tuberculosis. Philos. Trans. R. Soc. B 2012, 367, 850–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019.
- Nebreda, T.; Álvarez-Prida, E.; Blanco, B.; Remacha, M.A.; Samper, S.; Jiménez, M.S. Peritoneal tuberculosis due to Mycobacterium caprae. IDCases 2016, 4, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.; Sánchez, L.P.; Pérez, S.; Herrera, L.; Jiménez, M.S.; Samper, S.; Iglesias, M.J. Human tuberculosis due to Mycobacterium bovis and M. caprae in Spain, 2004–2007. Int. J. Tuberc. Lung. Dis. 2009, 13, 1536–1541. [Google Scholar] [PubMed]
- Pérez-Morote, R.; Pontones-Rosa, C.; Gortázar-Schmidt, C.; Muñoz-Cardona, A.I. Quantifying the economic impact of bovine tuberculosis on livestock farms in south-western Spain. Animals 2020, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Risco, D.; Salguero, F.J.; Cerrato, R.; Gutierrez-Merino, J.; Lanham-New, S.; Barquero-Pérez, O.; Hermoso de Mendoza, J.; Fernández-Llario, P. Association between vitamin D supplementation and severity of tuberculosis in wild boar and red deer. Res. Vet. Sci. 2016, 108, 116–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naranjo, V.; Gortazar, C.; Vicente, J.; de la Fuente, J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Höfle, U.; Garrido, J.M.; Fernández-DeMera, I.G.; Juste, R.; Barral, M.; Gortazar, C. Wild boar and red deer display high prevalence of tuberculosis-like lesions in Spain. Vet. Res. 2006, 37, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Hermoso de Mendoza, J.; Parra, A.; Tato, A.; Alonso, J.M.; Rey, J.M.; Peña, A.; García-Sánchez, A.; Larrasa, J.; Teixido, J.; Manzano, G.; et al. Bovine tuberculosis in wild boar (Sus scrofa), red deer (Cervus elaphus) and cattle (Bos taurus) in a Mediterranean ecosystem (1992–2004). Prev. Vet. Med. 2006, 74, 239–247. [Google Scholar] [CrossRef]
- Gortázar, C.; Vicente, J.; Samper, S.; Garrido, J.M.; Fernández-De-Mera, I.G.; Gavín, P.; Juste, R.A.; Martín, C.; Acevedo, P.; De La Puente, M.; et al. Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in southcentral Spain. Vet. Res. 2005, 36, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artois, M.; Delahay, R.; Guberti, V.; Cheeseman, C. Control of infectious diseases of wildlife in Europe. Vet. J. 2001, 162, 141–152. [Google Scholar] [CrossRef]
- Cross, P.C.; Drewe, J.; Patrek, V.; Pearce, G.; Samuel, M.D.; Delahay, R.J. Wildlife population structure and parasite transmission: Implications for disease management. In Management of Disease in Wild Mammals; Delahay, R.J., Smith, G.C., Hutchings, M.R., Eds.; Springer: Tokyo, Japan, 2009; pp. 9–29. [Google Scholar]
- Vicente, J.; Hofle, U.; Garrido, J.M.; Fernández-de-Mera, I.G.; Acevedo, P.; Juste, R.; Barral, M.; Gortazar, C. Risk factors associated with the prevalence of tuberculosis-like lesions in fenced wild boar and red deer in south central Spain. Vet. Res. 2007, 38, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Queirós, J.; Alves, P.C.; Vicente, J.; Gortázar, C.; de la Fuente, J. Genome-wide associations identify novel candidate loci associated with genetic susceptibility to tuberculosis in wild boar. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Whitehouse, K.; Vicente, J.; Gortazar, C.; Höflem, U.; Fernández-de-Mera, I.; Amos, W. Genetic resistance to bovine tuberculosis in the Iberian wild boar. Mol. Ecol. 2005, 14, 3209–3217. [Google Scholar] [CrossRef]
- Amos, W.; Acevedo-Whitehouse, K. A new test for genotype-fitness associations reveals a single microsatellite allele that strongly predicts the nature of tuberculosis in wild boar. Mol. Ecol. Res. 2009, 9, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Malo, A.F.; Coulson, T. Heterozygosity-fitness correlations and associative overdominance: New detection method and proof of principle in the Iberian wild boar. Mol. Ecol. 2009, 18, 2741–2742. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Gortázar, C.; De La Fuente, J.; Vicente, J. Host Richness Increases Tuberculosis Disease Risk in Game-Managed Areas. Microorganisms 2019, 7, 182. [Google Scholar] [CrossRef] [Green Version]
- Galarza, J.A.; Sánchez-Fernández, B.; Fandos, P.; Soriguer, R. Intensive management and natural genetic variation in red deer (Cervus elaphus). J. Hered. 2017, 108, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Galarza, J.; Sánchez-Fernández, B.; Fandos, P.; Soriguer, R. The genetic landscape of the Iberian red deer (Cervus elaphus hispanicus) after 30 years of big-game hunting in southern Spain. J. Wildl. Manage. Wildl. Monogr. 2015, 79, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Medrano, J.M.; Megens, H.; Groenen, M.A.; Ramis, G.; Bosse, M.; Pérez-Enciso, M.; Crooijmans, R.P.M.A. Conservation genomic analysis of domestic and wild pig populations from the Iberian Peninsula. BMC Genet. 2013, 14, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-González, J.; Frantz, A.C.; Torres-Porras, J.; Castillo, L.; Carranza, J. Population structure, habitat features and genetic structure of managed red deer populations. Eur. J. Wildl. Res. 2012, 58, 933–943. [Google Scholar] [CrossRef]
- Martínez, J.G.; Carranza, J.; Fernández-García, J.L.; Sánchez-Prieto, C.B. Genetic variation of red deer populations under hunting exploitation in Southwestern Spain. J. Wildl. Manage. 2002, 66, 1273–1282. [Google Scholar] [CrossRef]
- Torres-Porras, J.; Carranza, J.; Pérez-González, J.; Mateos, C.; Alarcos, S. The tragedy of the commons: Unsustainable population structure of Iberian red deer in hunting estates. Eur. J. Wildl. Res. 2014, 60, 351–357. [Google Scholar] [CrossRef]
- Pérez-González, J.; Carranza, J. Female-biased dispersal under conditions of low male mating competition in a polygynous mammal. Mol. Ecol. 2009, 18, 4617–4630. [Google Scholar] [CrossRef]
- Sánchez-Prieto, C.B.; Carranza, J.; Pérez-González, J.; Alarcos, S.; Mateos, C. Effects of small barriers on habitat use by red deer: Implications for conservation practices. J. Nat. Conserv. 2010, 18, 196–201. [Google Scholar] [CrossRef]
- Pérez-González, J.; Costa, V.; Santos, P.; Slate, J.; Carranza, J.; Fernández-Llario, P.; Zsolnai, A.; Monteiro, N.M.; Anton, I.; Buzgó, J.; et al. Males and females contribute unequally to offspring genetic diversity in the polygynandrous mating system of wild boar. PLoS ONE 2014, 9, e115394. [Google Scholar] [CrossRef] [PubMed]
- Carranza, J.; Pérez-González, J.; Mateos, C.; Fernández-García, J.L. Parents’ genetic dissimilarity and offspring sex in a polygynous mammal. Mol. Ecol. 2009, 18, 4964–4973. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, J.; Mateos, C.; Carranza, J. Polygyny can increase rather than decrease genetic diversity contributed by males relative to females: Evidence from red deer. Mol. Ecol. 2009, 18, 1591–1600. [Google Scholar] [CrossRef]
- Iacolina, L.; Corlatti, L.; Buzan, E.; Safner, T.; Šprem, N. Hybridization in European ungulates: A review of the current status, causes and consequences. Mammal. Rev. 2019, 49, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Randi, E. Management of Wild Ungulate Populations in Italy: Captive-Breeding, Hybridisation and Genetic Consequences of Translocations. Vet. Res. Commun. 2005, 29, 71–75. [Google Scholar] [CrossRef]
- Whiteley, A.R.; Fitzpatrick, S.W.; Funk, W.C.; Tallmon, D.A. Genetic rescue to the rescue. Trends Ecol. Evol. 2015, 30, 42–49. [Google Scholar] [CrossRef]
- Bell, D.A.; Robinson, Z.L.; Funk, W.C.; Fitzpatrick, S.W.; Allendorf, F.W.; Talmon, D.A.; Whiteley, A.R. The exciting potential and remaining uncertainties of genetic rescue. Trends Ecol. Evol. 2019, 34, 1070–1079. [Google Scholar] [CrossRef]
- Harris, K.; Zhang, Y.; Nielsen, R. Genetic rescue and the maintenance of native ancestry. Conserv. Genet. 2019, 20, 1–5. [Google Scholar] [CrossRef]
- Carranza, J.; Martínez, J.G.; Sánchez-Prieto, C.; Fernández-García, J.L.; Sánchez-Fernández, B.; Álvarez-Álvarez, R.; Valencia, J.; Alarcos, S. Game species: Extinctions hidden by census numbers. Anim. Biodivers. Conserv. 2003, 26, 81–84. [Google Scholar]
- Northover, A.; Lymbery, A.; Wayne, A.; Godfrey, S.; Thompson, R. The hidden consequences of altering host-parasite relationships during fauna translocations. Biol. Conserv. 2018, 220, 140–148. [Google Scholar] [CrossRef]
- Sainsbury, A.W.; Vaughan-Higgins, R.J. Analysing disease risks associated with translocations. Conserv. Biol. 2012, 26, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Mera, I.G.; Vicente, J.; Naranjo, V.; Fierro, Y.; Garde, J.J.; de la Fuente, J.; Gortázar, C. Impact of major histocompatibility complex class II polymorphisms on Iberian red deer parasitism and life history traits. Infect. Genet. Evol. 2009, 9, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Ballou, J.D.; Eldridge, M.D.; Lacy, R.C.; Ralls, K.; Dudash, M.R.; Fenster, C.B. Predicting the probability of outbreeding depression. Conserv. Biol. 2011, 25, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, P.W.; Fredrickson, R. Genetic rescue guidelines with examples from Mexican wolves and Florida panthers. Conserv. Genet. 2010, 11, 615–626. [Google Scholar] [CrossRef]
- Casas-Díaz, E.; Closa-Sebastia, F.; Peris, A.; Miño, A.; Torrentó, J.; Casanovas, R.; Marco, I.; Lavín, S.; Fernández-Llario, P.; Serrano, E. Recorded dispersal of wild boar (Sus scrofa) in northeast Spain: Implications for disease monitoring programs. Wildl. Biol. Pract. 2013, 9, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Albon, S.D.; Langvatn, R. Plant phenology and the benefits of migration in a temperate ungulate. Oikos 1992, 65, 502–513. [Google Scholar] [CrossRef]
- Pérez-González, J.; Carranza, J. Female aggregation interacts with population structure to influence the degree of polygyny in red deer. Anim. Behav. 2011, 82, 957–970. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. Red Deer: Behaviour and Ecology of Two Sexes; University of Chicago Press: Chicago, IL, USA, 1982. [Google Scholar]
- Greenwood, P.J. Mating systems philopatry and dispersal in birds and mammals. Anim. Behav. 1980, 28, 1140–1162. [Google Scholar] [CrossRef]
- Pérez-Espona, S.; Pérez-Barberia, F.J.; McLeod, J.E.; Jiggins, C.D.; Gordon, I.J.; Pemberton, J.M. Landscape features affect gene flow of Scottish Highland red deer (Cervus elaphus). Mol. Ecol. 2008, 17, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Foerster, K.; Coulson, T.; Sheldon, B.C.; Pemberton, J.M.; Clutton-Brock, T.H.; Kruuk, L.E.B. Sexually antagonistic genetic variation for fitness in red deer. Nature 2007, 447, 1107–1110. [Google Scholar] [CrossRef]
- Meagher, S.; Penn, D.J.; Potts, W.K. Male-male competition magnifies inbreeding depression in wild house mice. Proc. Natl. Scad. Sci. USA 2000, 97, 3324–3329. [Google Scholar] [CrossRef]
- Pusey, A.; Wolf, M. Inbreeding avoidance in animals. Trends Ecol. Evol. 1996, 11, 201–206. [Google Scholar] [CrossRef]
- Pearse, D.E.; Anderson, E.C. Multiple paternity increases effective population size. Mol. Ecol. 2009, 18, 3124–3127. [Google Scholar] [CrossRef]
- Sugg, D.W.; Chesser, R.K. Effective population sizes with multiple paternity. Genetics 1994, 137, 1147–1155. [Google Scholar] [CrossRef]
- Pérez-González, J.; Costa, V.; Santos, P.; Carranza, J.; Zsolnai, A.; Fernández-Llario, P.; Monteiro, N.; Anton, I.; Beja-Pereira, A. Heterozygosity decrease in wild boar mating system—A case of outbreeding avoidance? J. Zool. 2017, 302, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Scandura, M.; Iacolina, L.; Apollonio, M. Genetic diversity in the European wild boar Sus scrofa: Phylogeography, population structure and wild x domestic hybridization. Mammal. Rev. 2011, 41, 125–137. [Google Scholar] [CrossRef]
- Edmands, S. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution 1999, 53, 1757–1768. [Google Scholar] [CrossRef]
- Barasona, J.A.; Acevedo, P.; Diez-Delgado, I.; Queiros, J.; Carrasco-García, R.; Gortazar, C.; Vicente, J. Tuberculosis-associated death among adult wild boars, Spain, 2009–2014. Emerg. Infect. Dis. 2016, 22, 2178–2180. [Google Scholar] [CrossRef] [Green Version]
- Castillo, L.; Fernández-Llario, P.; Mateos, C.; Carranza, J.; Benítez-Medina, J.M.; García-Jiménez, W.; Bermejo-Martín, F.; Hermoso de Mendoza, J. Management practices and their association with Mycobacterium tuberculosis complex prevalence in red deer populations in Southwestern Spain. Prev. Vet. Med. 2011, 98, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruiz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Beltran-Beck, B.; González-Barrio, D.; Queirós, J.; Montoro, V.; et al. Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transbound. Emerg. Dis. 2013, 60, 92–103. [Google Scholar] [CrossRef]
- Parra, A.; García, A.; Inglis, N.F.; Tato, A.; Alonso, J.M.; Hermoso de Mendoza, M.; Hermoso de Mendoza, J.; Larrasa, J. An epidemiological evaluation of Mycobacterium bovis infections in wild game animals of the Spanish Mediterranean ecosystem. Res. Vet. Sci. 2006, 80, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, A.; de Juan, L.; Montero, N.; Sánchez, C.; Galka, M.; Delso, C.; Álvarez, J.; Romero, B.; Bezos, J.; Vela, A.I.; et al. Bovine tuberculosis (Mycobacterium bovis) in Wildlife in Spain. J. Clin. Microbiol. 2004, 42, 2602–2608. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. Forest. Sci. 2001, 47, 214–218. [Google Scholar]
- Olea, L.; López-Bellido, R.J.; Poblaciones, M.J. Europe types of silvopastoral systems in the Mediterranean area: Dehesa. In Silvopastoralism and Sustainable Land Management; Mosquera, M.R., Rigueiro, A., McAdam, J., Eds.; CABI: Wallingford, Oxfordshire, UK, 2005; pp. 30–35. [Google Scholar]
- Fernández-Llario, P. The sexual function of wallowing in male wild boar (Sus scrofa). J. Ethol. 2005, 23, 9–14. [Google Scholar] [CrossRef]
- Cousins, D.V. Mycobacterium bovis infection and control in domestic livestock. Rev. Sci. Tech. Off. Int. Epizoot. 2001, 20, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, J.; Barbosa, A.M.; Carranza, J.; Torres-Porras, J. Relative effect of food supplementation and natural resources on hind aggregation in a Mediterranean ecosystem. J. Wildl. Manag. 2010, 74, 1701–1708. [Google Scholar] [CrossRef]
- Carranza, J.; Fernandez-Llario, P.; Gomendio, M. Correlates of territoriality in rutting red deer. Ethology 1996, 102, 793–805. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Pérez de Val, B.; Arenas-Montes, A.; Paniagua, J.; Boadella, M.; Gortázar, C.; Arenas, A. Seroprevalence and risk factors associated to Mycobacterium bovis in wild artiodactyl species from Southern Spain, 2006–2010. PLoS ONE 2012, 7, e34908. [Google Scholar] [CrossRef] [Green Version]
- Zanella, G.; Duvauchelle, A.; Hars, J.; Moutou, F.; Boschiroli, M.L.; Durand, B. Patterns of lesions of bovine tuberculosis in wild red deer and wild boar. Vet. Rec. 2008, 163, 43–47. [Google Scholar] [CrossRef] [PubMed]


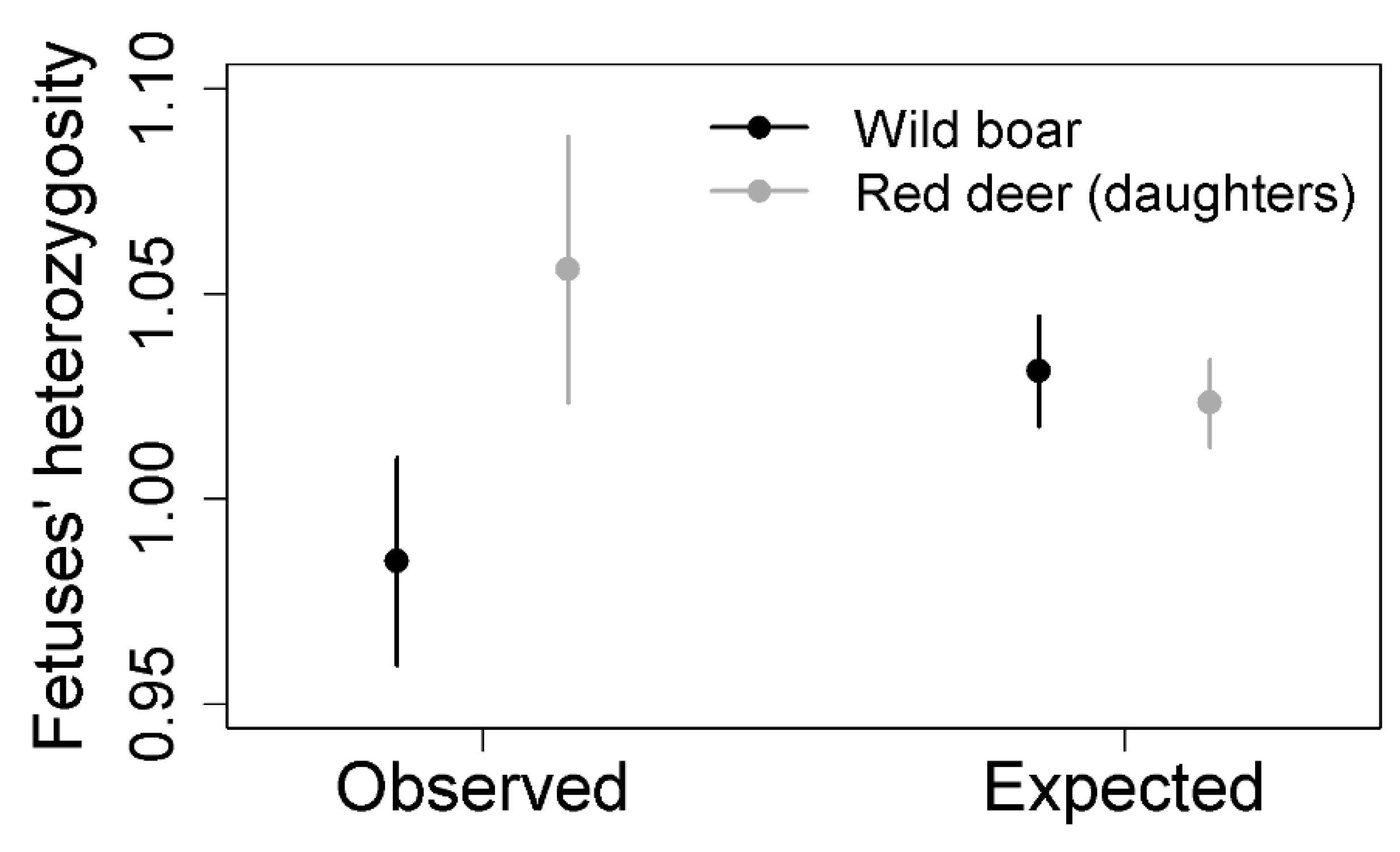
| Species | Total | Selected | Journals | Genetic Diversity | Percentage |
|---|---|---|---|---|---|
| Wild boar | 299 | 217 | 74 | 4 | 1.8 |
| Red deer | 282 | 215 | 69 | 3 | 1.4 |
| Value | SE | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| Intercept | 1.033 | 0.021 | 300 | 49.648 | <0.001 |
| Mating type | −0.047 | 0.022 | 250 | −2.133 | 0.034 |
| Species | −0.010 | 0.026 | 21 | −0.376 | 0.710 |
| Mating type × Species | 0.079 | 0.030 | 250 | 2.639 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-González, J.; Carranza, J.; Martínez, R.; Benítez-Medina, J.M. Host Genetic Diversity and Infectious Diseases. Focus on Wild Boar, Red Deer and Tuberculosis. Animals 2021, 11, 1630. https://doi.org/10.3390/ani11061630
Pérez-González J, Carranza J, Martínez R, Benítez-Medina JM. Host Genetic Diversity and Infectious Diseases. Focus on Wild Boar, Red Deer and Tuberculosis. Animals. 2021; 11(6):1630. https://doi.org/10.3390/ani11061630
Chicago/Turabian StylePérez-González, Javier, Juan Carranza, Remigio Martínez, and José Manuel Benítez-Medina. 2021. "Host Genetic Diversity and Infectious Diseases. Focus on Wild Boar, Red Deer and Tuberculosis" Animals 11, no. 6: 1630. https://doi.org/10.3390/ani11061630
APA StylePérez-González, J., Carranza, J., Martínez, R., & Benítez-Medina, J. M. (2021). Host Genetic Diversity and Infectious Diseases. Focus on Wild Boar, Red Deer and Tuberculosis. Animals, 11(6), 1630. https://doi.org/10.3390/ani11061630






