Genetic Aspects of Somatic Cell Count in Holstein Dairy Cows in Iran
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Model
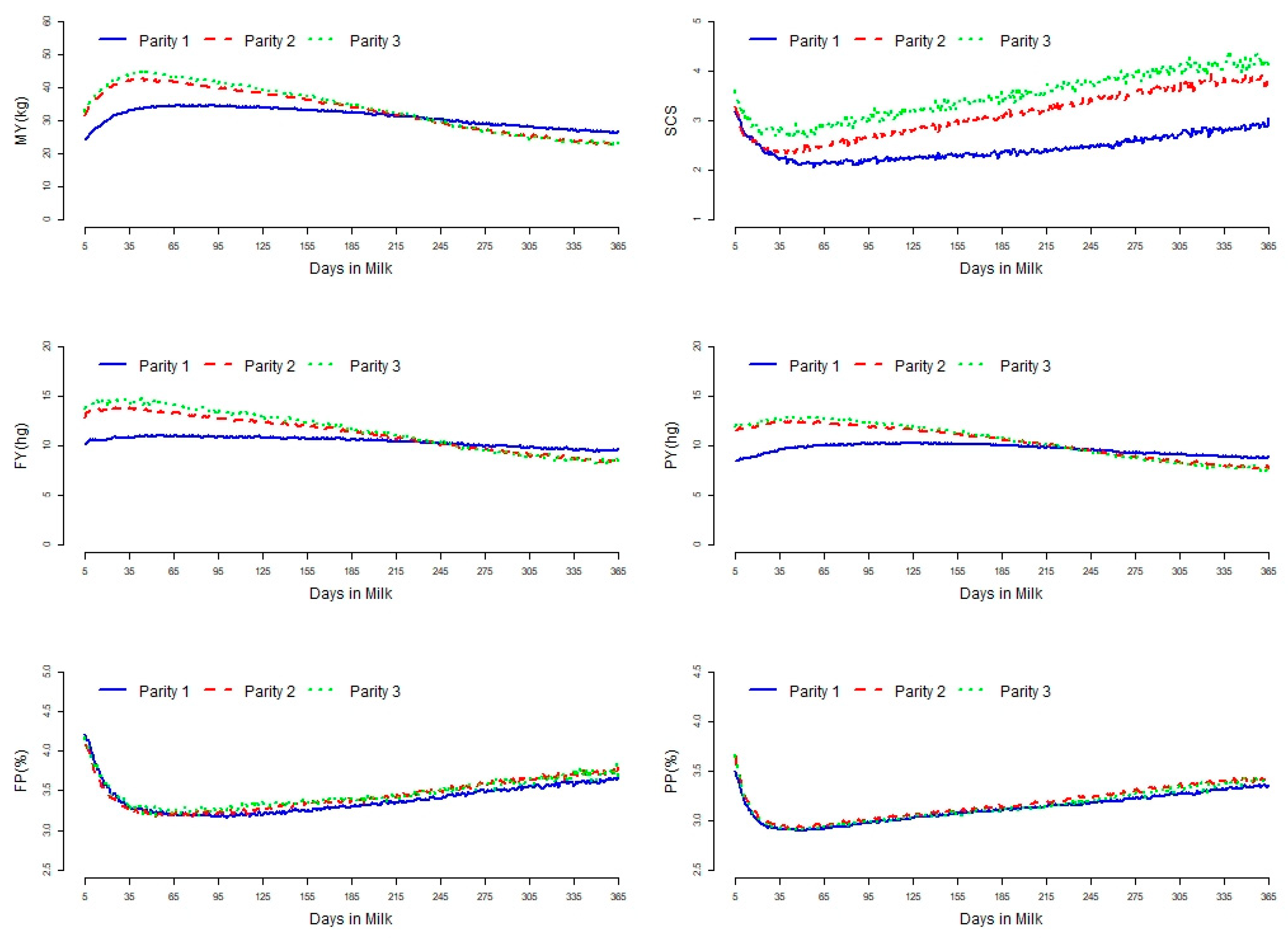
3. Results
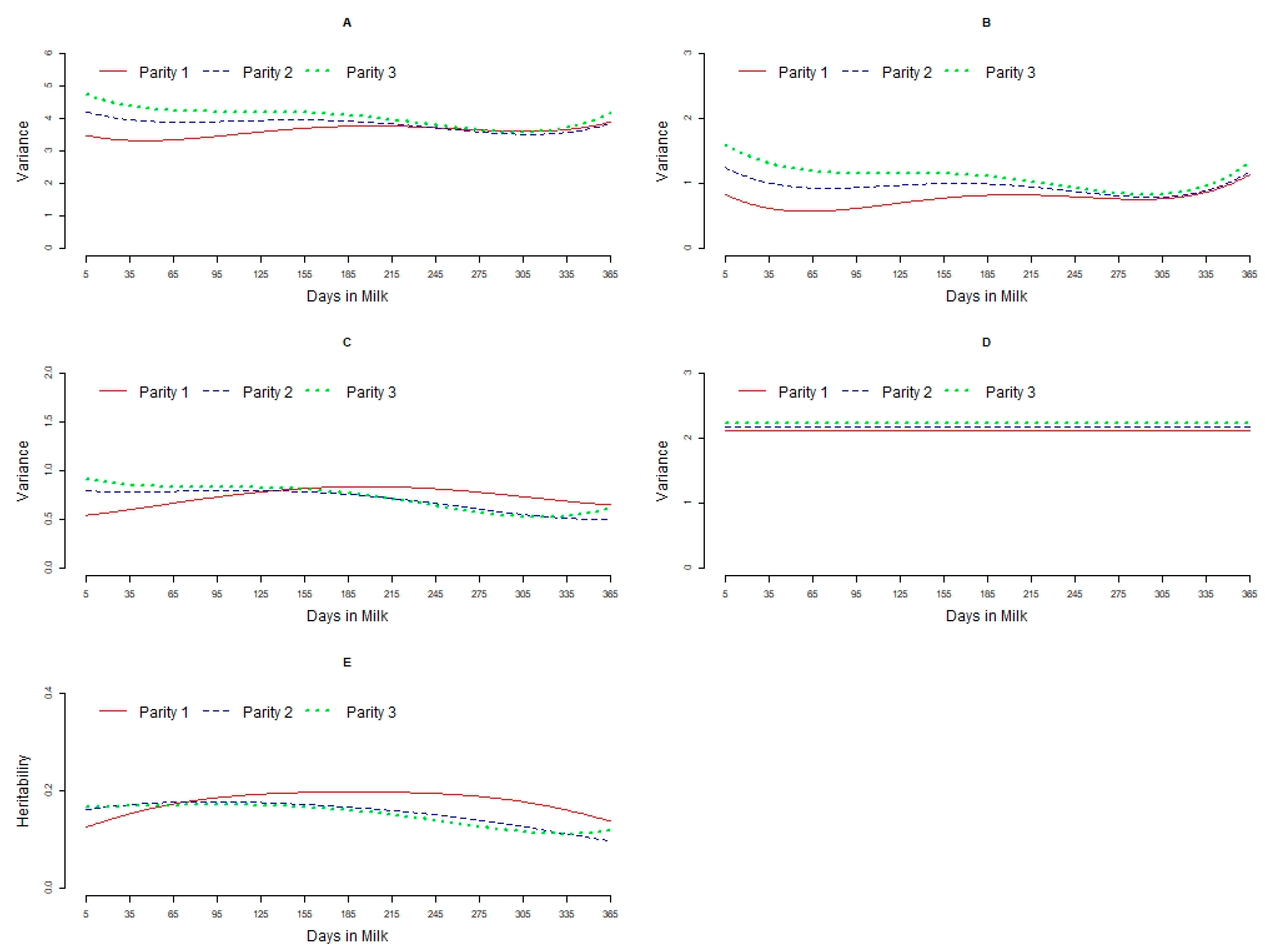
3.1. Variance Components
3.2. Heritabilities
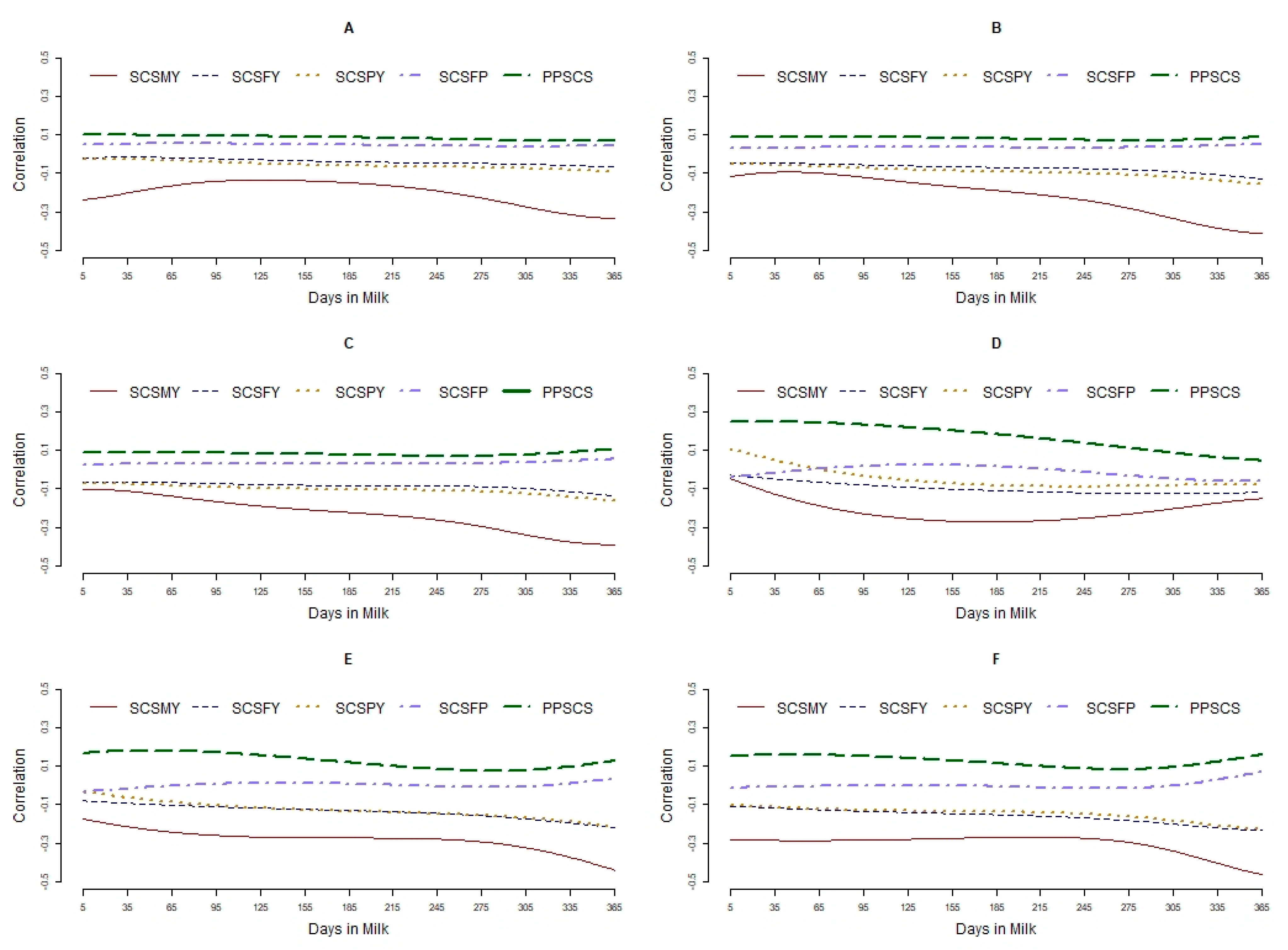
3.3. Relationships between Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atashi, H.; Zamiri, M.J.; Sayyadnejad, M.B.; Akhlaghi, A. Trends in the reproductive performance of Holstein dairy cows in Iran. Trop. Anim. Health Prod. 2012, 44, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Sefidmazgi, A.; Moradi-Shahrbabak, M.; Nejati-Javaremi, A.; Shadparvar, A. Estimation of economic values in three breeding perspectives for longevity and milk production traits in Holstein dairy cattle in Iran. Ital. J. Anim. Sci. 2009, 8, 359–375. [Google Scholar] [CrossRef][Green Version]
- Miglior, F.; Muir, B.; Van Doormaal, B. Selection indices in Holstein cattle of various countries. J. Dairy Sci. 2005, 88, 1255–1263. [Google Scholar] [CrossRef]
- Van Raden, P. Selection of dairy cattle for lifetime profit. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002; pp. 127–130. [Google Scholar]
- Mark, T.; Fikse, W.; Emanuelson, U.; Philipsson, J. International genetic evaluations of Holstein sires for milk somatic cell and clinical mastitis. J. Dairy Sci. 2002, 85, 2384–2392. [Google Scholar] [CrossRef]
- Schaeffer, L.; Jamrozik, J.; Kistemaker, G.; Van Doormaal, J. Experience with a test-day model. J. Dairy Sci. 2000, 83, 1135–1144. [Google Scholar] [CrossRef]
- Lidauer, M.H.; Pösö, J.; Pedersen, J.; Lassen, J.; Madsen, P.; Mäntysaari, E.A.; Nielsen, U.S.; Eriksson, J.-Å.; Johansson, K.; Pitkänen, T. Across-country test-day model evaluations for Holstein, Nordic Red Cattle, and Jersey. J. Dairy Sci. 2015, 98, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Miglior, F.; Gong, W.; Wang, Y.; Kistemaker, G.; Sewalem, A.; Jamrozik, J. Genetic parameters of production traits in Chinese Holsteins using a random regression test-day model. J. Dairy Sci. 2009, 92, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- ICAR. ICAR (International Committee for Animal Recording). International Agreement of Recording Practices. 2012. Available online: http://aberdeenangus.ro/wp-content/uploads/2014/03/ICAR.pdf (accessed on 26 May 2021).
- Aguilar, I.; Tsuruta, S.; Masuda, Y.; Lourenco, D.; Legarra, A.; Misztal, I. BLUPF90 suite of programs for animal breeding with focus on genomics. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 10–15 February 2018; pp. 11–16. [Google Scholar]
- Jamrozik, J.; Bohmanova, J.; Schaeffer, L. Relationships between milk yield and somatic cell score in Canadian Holsteins from simultaneous and recursive random regression models. J. Dairy Sci. 2010, 93, 1216–1233. [Google Scholar] [CrossRef] [PubMed]
- Haile-Mariam, M.; Goddard, M.; Bowman, P. Estimates of genetic parameters for daily somatic cell count of Australian dairy cattle. J. Dairy Sci. 2001, 84, 1255–1264. [Google Scholar] [CrossRef]
- Alam, M.; Cho, C.; Choi, T.; Park, B.; Choi, J.; Choy, Y.; Lee, S.; Cho, K. Estimation of genetic parameters for Somatic Cell Scores of Holsteins using multi-trait lactation models in Korea. Asian-Australas. J. Anim. Sci. 2015, 28, 303. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Silvestre, A.; Petim-Batista, M.; Colaço, J. Somatic cell score genetic parameter estimates of dairy cattle in Portugal using fractional polynomials. J. Anim. Sci. 2011, 89, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Yang, F.; Luo, J.; Wang, X.; Liu, L.; Li, H. Influences of season, parity, lactation, udder area, milk yield, and clinical symptoms on intramammary infection in dairy cows. J. Dairy Sci. 2016, 99, 6484–6493. [Google Scholar] [CrossRef] [PubMed]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vete. Res. 2016, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Girma, S.; Mammo, A.; Bogele, K.; Sori, T.; Tadesse, F.; Jibat, T. Study on prevalence of bovine mastitis and its major causative agents in West Harerghe zone, Doba district, Ethiopia. J. Vet. Med. Anim. Health 2012, 4, 116–123. [Google Scholar]
- Costa, A.; Lopez-Villalobos, N.; Visentin, G.; De Marchi, M.; Cassandro, M.; Penasa, M. Heritability and repeatability of milk lactose and its relationships with traditional milk traits, somatic cell score and freezing point in Holstein cows. Animal 2019, 13, 909–916. [Google Scholar] [CrossRef]
- Soumri, N.; Carabaño, M.J.; González-Recio, O.; Bedhiaf-Romdhani, S. Genetic parameters of somatic cell scores using random regression test-day models with Legendre polynomials in Tunisian dairy cattle. Livest. Sci. 2020, 241, 104178. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.; Gröhn, Y.; McCulloch, C.; Guard, C. Effects of clinical mastitis on milk yield in dairy cows. J. Dairy Sci. 1999, 82, 1213–1220. [Google Scholar] [CrossRef]
- Franzoi, M.; Manuelian, C.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Koivula, M.; Mäntysaari, E.; Negussie, E.; Serenius, T. Genetic and phenotypic relationships among milk yield and somatic cell count before and after clinical mastitis. J. Dairy Sci. 2005, 88, 827–833. [Google Scholar] [CrossRef]
| First Parity | Second Parity | Third Parity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Traits | Mean | SD | CV (%) | Mean | SD | CV (%) | Mean | SD | CV (%) |
| Milk yield | 31.6 | 7.6 | 24 | 34.7 | 10.4 | 30 | 35.8 | 11.3 | 32 |
| Fat yield 1 | 10.49 | 3.34 | 32 | 11.63 | 4.34 | 37 | 12.09 | 4.72 | 39 |
| Protein yield 2 | 9.70 | 2.38 | 24 | 10.73 | 3.10 | 29 | 10.96 | 3.35 | 31 |
| Fat percentage | 3.36 | 0.83 | 25 | 3.40 | 0.88 | 26 | 3.42 | 0.89 | 26 |
| Protein percentage | 3.09 | 0.39 | 13 | 3.13 | 0.41 | 13 | 3.10 | 0.41 | 13 |
| SCS 3 | 2.39 | 1.97 | 82 | 2.99 | 2.06 | 68 | 3.37 | 2.11 | 63 |
| First Parity | Second Parity | Third Parity | |
|---|---|---|---|
| Milk yield | −0.19 (−0.00 to −0.00) | −0.25 (−0.00 to −0.00) | −0.27 (−0.00 to −0.00) |
| Fat yield | −0.04 (−0.00 to −0.00) | −0.08 (−0.00 to −0.00) | −0.09 (−0.00 to −0.00) |
| Protein yield | −0.06 (−0.00 to −0.00) | −0.10 (−0.00 to −0.00) | −0.11 (−0.00 to −0.00) |
| Fat percentage | 0.05 (−0.00 to −0.00) | 0.04 (−0.00 to −0.00) | 0.03 (−0.00 to −0.00) |
| Protein percentage | 0.08 (−0.00 to −0.00) | 0.08 (−0.00 to −0.00) | 0.08 (−0.00 to −0.00) |
| First Parity | Second Parity | Third Parity | |
|---|---|---|---|
| Milk yield | −0.21 (−0.27 to −0.04) | −0.28 (−0.47 to −0.17) | −0.30 (−0.45 to −0.27) |
| Fat yield | −0.10 (−0.13 to −0.03) | −0.14 (−0.23 to −0.08) | −0.16 (−0.22 to −0.11) |
| Protein yield | −0.05 (−0.09 to 0.11) | −0.13 (−0.23 to −0.04) | −0.15 (−0.22 to −0.10) |
| Fat percentage | −0.01 (−0.06 to 0.02) | 0.00 (−0.03 to 0.05) | 0.00 (−0.0 to 0.09) |
| Protein percentage | 0.17 (0.05 to 0.25) | 0.12 (0.07 to 0.17) | 0.13 (0.09 to 0.18) |
| First Parity | Second Parity | Third Parity | |
|---|---|---|---|
| Milk yield | −0.16(−0.29 to −0.10) | −0.21(−0.42 to −0.10) | −0.24(−0.42 to −0.10) |
| Fat yield | −0.04(−0.11 to −0.00) | −0.10(−0.21 to −0.01) | −0.11(−0.21 to −0.03) |
| Protein yield | −0.06(−0.11 to −0.02) | −0.09(−0.21 to −0.02) | −0.10(−0.19 to −0.02) |
| Fat percentage | 0.06(0.02 to 0.09) | 0.02(0.00 to 0.04) | 0.02(−0.01 to 0.05) |
| Protein percentage | 0.05(0.03 to 0.08) | 0.07(0.05 to 0.08) | 0.03(0.05 to 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atashi, H.; Hostens, M. Genetic Aspects of Somatic Cell Count in Holstein Dairy Cows in Iran. Animals 2021, 11, 1637. https://doi.org/10.3390/ani11061637
Atashi H, Hostens M. Genetic Aspects of Somatic Cell Count in Holstein Dairy Cows in Iran. Animals. 2021; 11(6):1637. https://doi.org/10.3390/ani11061637
Chicago/Turabian StyleAtashi, Hadi, and Miel Hostens. 2021. "Genetic Aspects of Somatic Cell Count in Holstein Dairy Cows in Iran" Animals 11, no. 6: 1637. https://doi.org/10.3390/ani11061637
APA StyleAtashi, H., & Hostens, M. (2021). Genetic Aspects of Somatic Cell Count in Holstein Dairy Cows in Iran. Animals, 11(6), 1637. https://doi.org/10.3390/ani11061637







