Assessment of a Rapid Semi-Quantitative Immunochromatographic Test for the Evaluation of Transfer of Passive Immunity in Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. ELISA (Reference Method)
2.3. Brix Refractometry
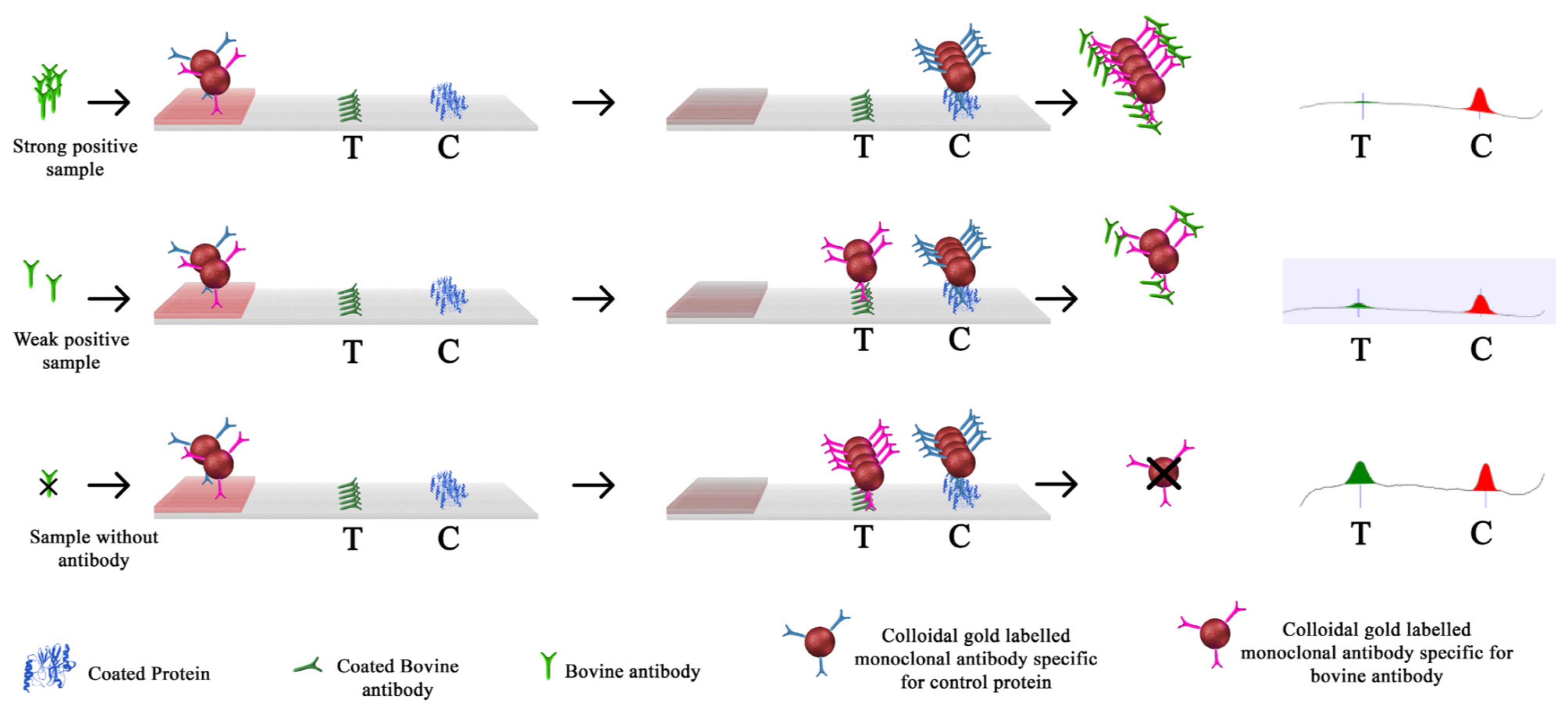
2.4. Immunochromatographic Assay Kits (SmartStrips™)
2.5. Statistical Analyses
2.5.1. Quantitative Analyses
2.5.2. Qualitative Analyses
3. Results
3.1. Quantitative Analyses
3.1.1. Descriptive Analysis
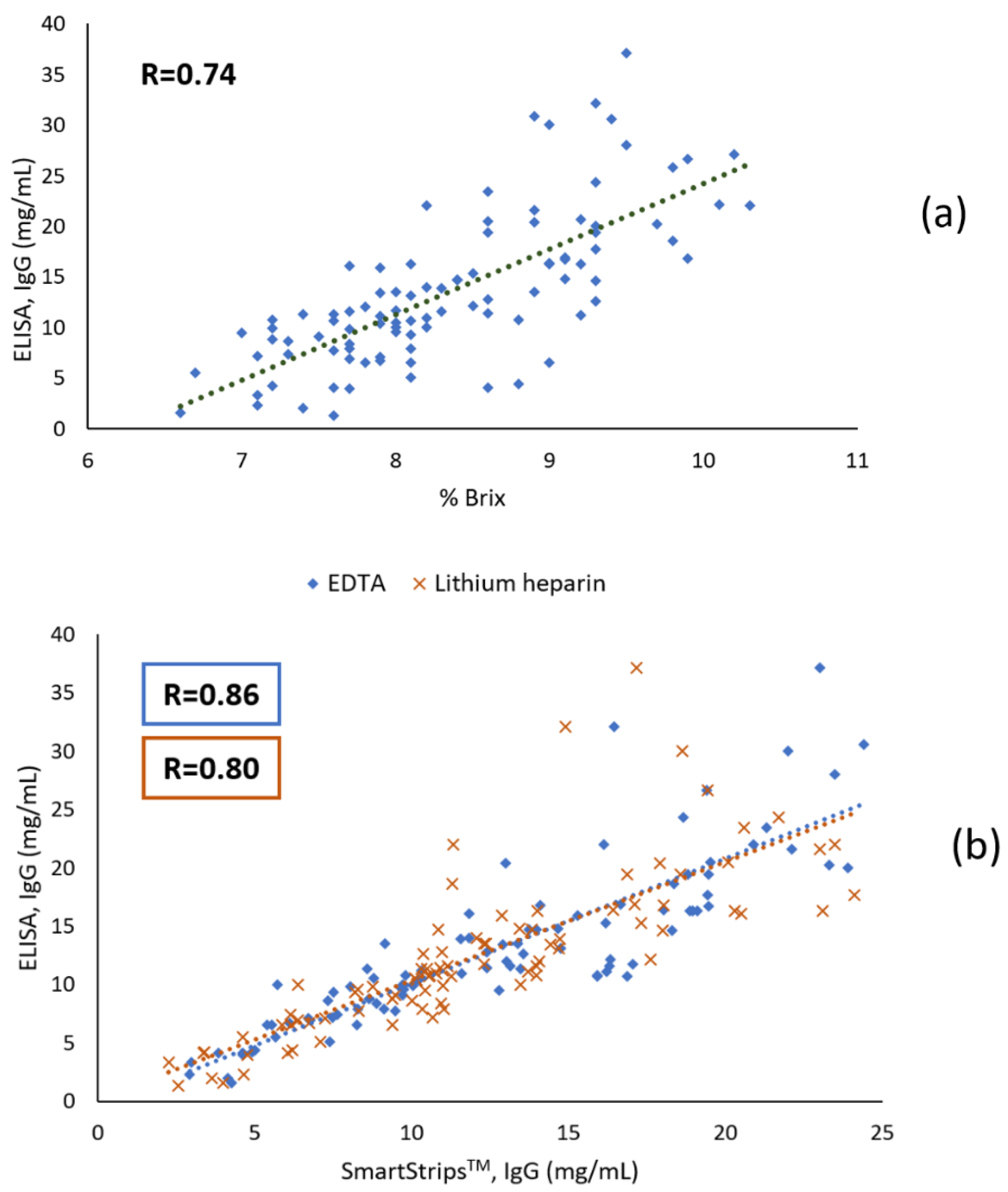
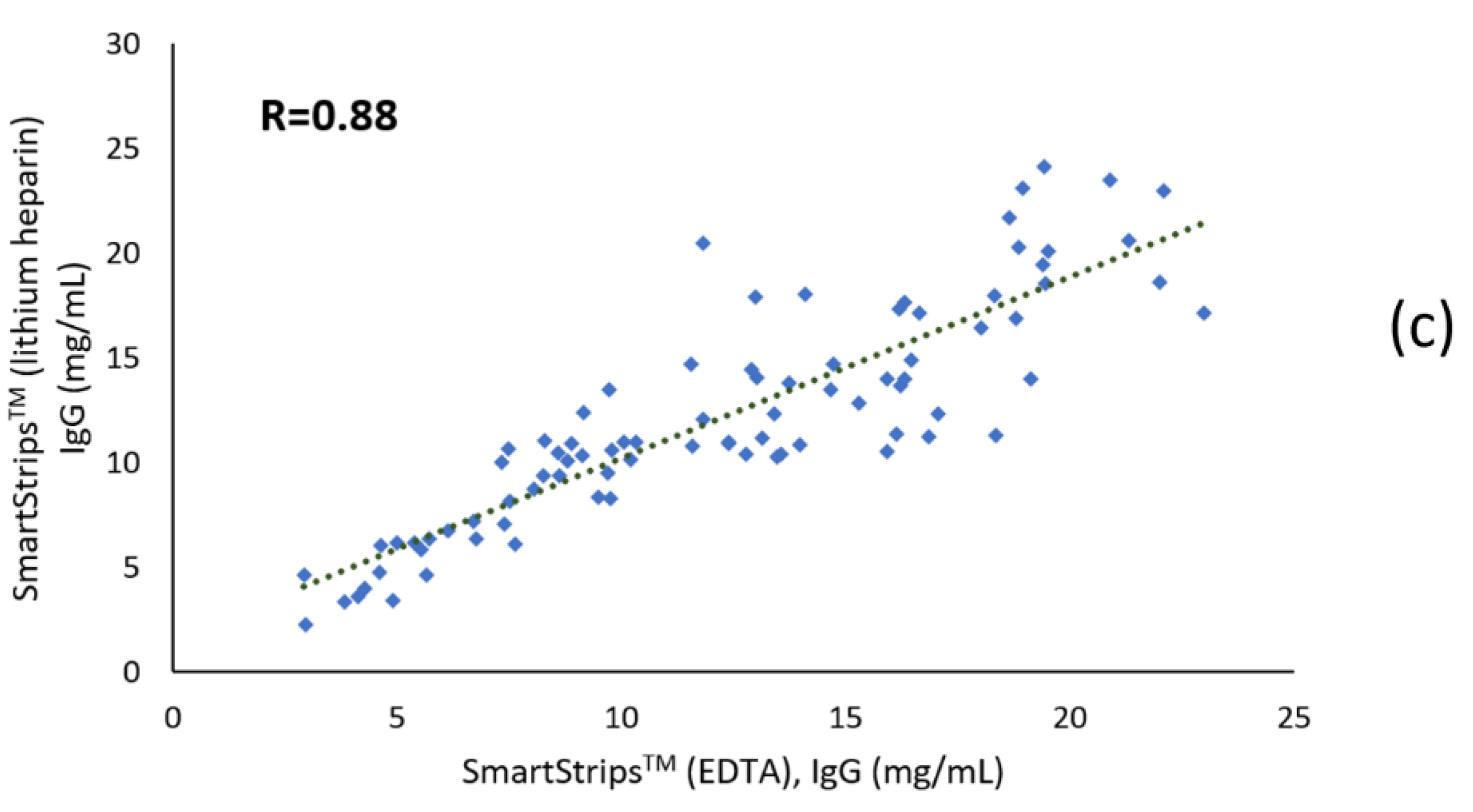
3.1.2. Correlation Coefficients
3.1.3. Bland-Altman Plots (Agreement)
3.2. Qualitative Analyses
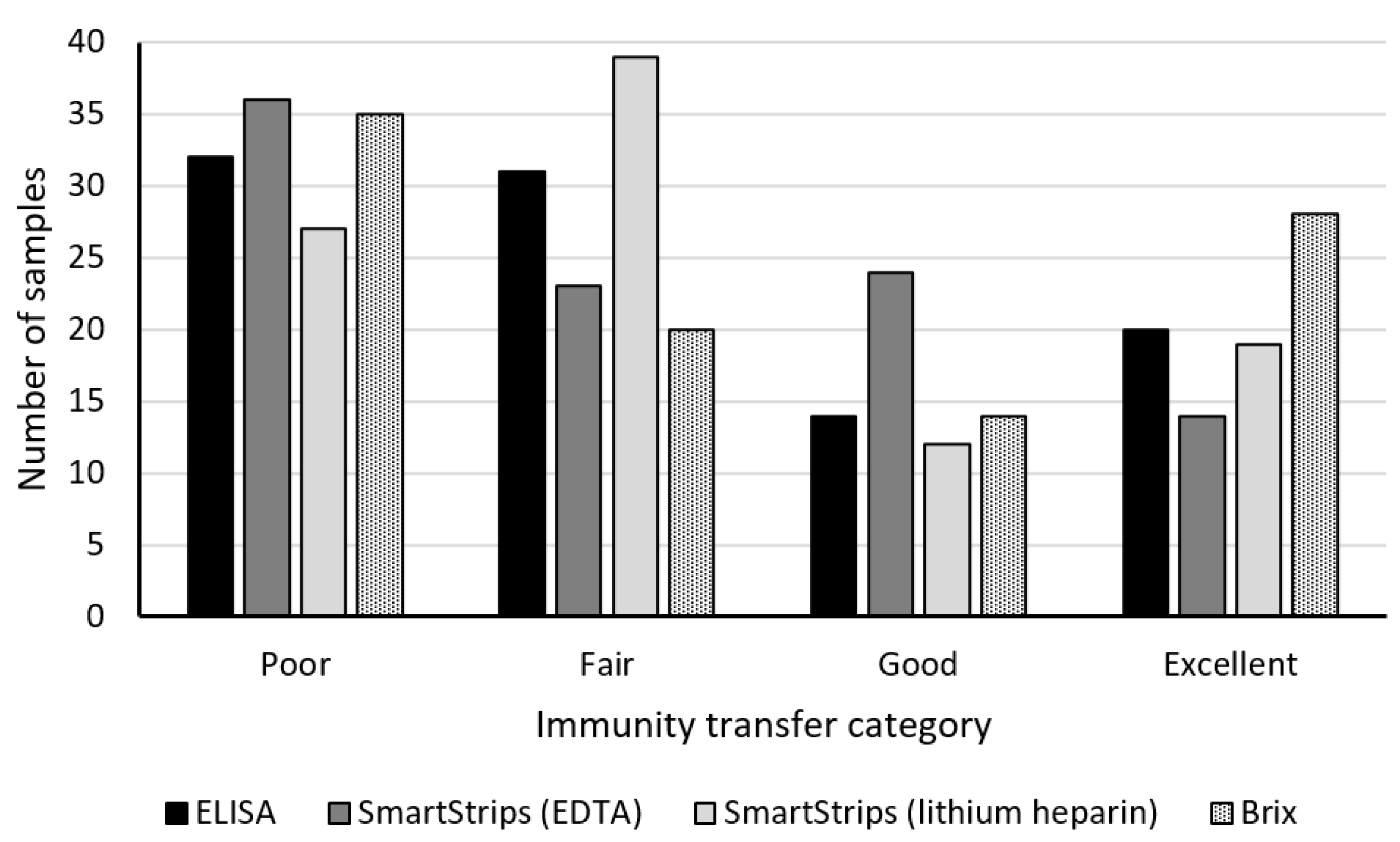
3.2.1. Descriptive Analysis
3.2.2. Diagnostic Test Characteristics
3.2.3. Weighted Kappa (Agreement)
4. Discussion
4.1. Quantitative Analyses
4.2. Qualitative Analyses
4.3. Effect of the Anticoagulant Type
4.4. Practical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, A.T. Bovine Placenta: A Review on Morphology, Components, and Defects from Terminology and Clinical Perspectives. Theriogenology 2013, 80, 693–705. [Google Scholar] [CrossRef]
- Godden, S. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef]
- Bush, L.J.; Staley, T.E. Absorption of Colostral Immunoglobulins in Newborn Calves. J. Dairy Sci. 1980, 63, 672–680. [Google Scholar] [CrossRef]
- Lombard, J. Consensus Recommendations on Calf- and Herd-Level Passive Immunity in Dairy Calves in the United States. J. Dairy Sci. 2020, 103, 7611–7624. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E. Bovine Immunoglobulins: A Review. J. Dairy Sci. 1969, 52, 1895–1909. [Google Scholar] [CrossRef]
- Dunn, A.; Duffy, C.; Gordon, A.; Morrison, S.; Argűello, A.; Welsh, M.; Earley, B. Comparison of Single Radial Immunodiffusion and ELISA for the Quantification of Immunoglobulin G in Bovine Colostrum, Milk and Calf Sera. J. Appl. Anim. Res. 2018, 46, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive Transfer of Colostral Immunoglobulins in Calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.L.; Lombard, J.E.; Kopral, C.A.; Garber, L.P.; Winter, A.L.; Hicks, J.A.; Schlater, J.L. Prevalence of Failure of Passive Transfer of Immunity in Newborn Heifer Calves and Associated Management Practices on US Dairy Operations. J. Dairy Sci. 2009, 92, 3973–3980. [Google Scholar] [CrossRef] [Green Version]
- Cuttance, E.; Mason, W.; Denholm, K.; Laven, R. Comparison of Diagnostic Tests for Determining the Prevalence of Failure of Passive Transfer in New Zealand Dairy Calves. N. Z. Vet. J. 2017, 65, 6–13. [Google Scholar] [CrossRef]
- Abuelo, A.; Havrlant, P.; Wood, N.; Hernandez-Jover, M. An Investigation of Dairy Calf Management Practices, Colostrum Quality, Failure of Transfer of Passive Immunity, and Occurrence of Enteropathogens among Australian Dairy Farms. J. Dairy Sci. 2019, 102, 8352–8366. [Google Scholar] [CrossRef] [PubMed]
- Furman-Fratczak, K.; Rzasa, A.; Stefaniak, T. The Influence of Colostral Immunoglobulin Concentration in Heifer Calves’ Serum on Their Health and Growth. J. Dairy Sci. 2011, 94, 5536–5543. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, D.; Trillat, P.; Cahuzac, C. Failure of Passive Immune Transfer in Calves: A Meta-Analysis on the Consequences and Assessment of the Economic Impact. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef]
- Cuttance, E.L.; Mason, W.A.; Laven, R.A.; Phyn, C.V.C. The Relationship between Failure of Passive Transfer and Mortality, Farmer-Recorded Animal Health Events and Body Weights of Calves from Birth until 12 Months of Age on Pasture-Based, Seasonal Calving Dairy Farms in New Zealand. Vet. J. 2018, 236, 4–11. [Google Scholar] [CrossRef]
- Todd, C.G.; McGee, M.; Tiernan, K.; Crosson, P.; O’Riordan, E.; McClure, J.; Lorenz, I.; Earley, B. An Observational Study on Passive Immunity in Irish Suckler Beef and Dairy Calves: Tests for Failure of Passive Transfer of Immunity and Associations with Health and Performance. Prev. Vet. Med. 2018, 159, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Jaekal, J.; Bae, C.-S.; Chung, B.-H.; Yun, S.-C.; Gwak, M.-J.; Noh, G.-J.; Lee, D.-H. Enzyme-Linked Immunosorbent Assay, Single Radial Immunodiffusion, and Indirect Methods for the Detection of Failure of Transfer of Passive Immunity in Dairy Calves. J. Vet. Intern. Med. 2008, 22, 212–218. [Google Scholar] [CrossRef]
- Zakian, A.; Nouri, M.; Rasooli, A.; Ghorbanpour, M.; Constable, P.D.; Mohammad-Sadegh, M. Evaluation of 5 Methods for Diagnosing Failure of Passive Transfer in 160 Holstein Calves. Vet. Clin. Pathol. 2018, 47, 275–283. [Google Scholar] [CrossRef]
- Sutter, F.; Rauch, E.; Erhard, M.; Sargent, R.; Weber, C.; Heuwieser, W.; Borchardt, S. Evaluation of Different Analytical Methods to Assess Failure of Passive Transfer in Neonatal Calves. J. Dairy Sci. 2020, 103, 5387–5397. [Google Scholar] [CrossRef]
- Mancini, G.; Carbonara, A.O.; Heremans, J.F. Immunochemical Quantitation of Antigens by Single Radial Immunodiffusion. Immunochemistry 1965, 2, 235–254. [Google Scholar] [CrossRef]
- Burton, J.L.; Kennedy, B.W.; Burnside, E.B.; Wilkie, B.N.; Burton, J.H. Variation in Serum Concentrations of Immunoglobulins G, A, and M in Canadian Holstein-Friesian Calves. J. Dairy Sci. 1989, 72, 135–149. [Google Scholar] [CrossRef]
- Hogan, I.; Doherty, M.; Fagan, J.; Kennedy, E.; Conneely, M.; Brady, P.; Ryan, C.; Lorenz, I. Comparison of Rapid Laboratory Tests for Failure of Passive Transfer in the Bovine. Ir. Vet J 2015, 68, 18. [Google Scholar] [CrossRef] [Green Version]
- Bielmann, V.; Gillan, J.; Perkins, N.R.; Skidmore, A.L.; Godden, S.; Leslie, K.E. An Evaluation of Brix Refractometry Instruments for Measurement of Colostrum Quality in Dairy Cattle. J. Dairy Sci. 2010, 93, 3713–3721. [Google Scholar] [CrossRef]
- Deelen, S.M.; Ollivett, T.L.; Haines, D.M.; Leslie, K.E. Evaluation of a Brix Refractometer to Estimate Serum Immunoglobulin G Concentration in Neonatal Dairy Calves. J. Dairy Sci. 2014, 97, 3838–3844. [Google Scholar] [CrossRef]
- Lemberskiy-Kuzin, L.; Lavie, S.; Katz, G.; Merin, U.; Leitner, G. Determination of Immunoglobulin Levels in Colostrum by Using an Online Milk Analyzer. Can. J. Anim. Sci. 2019, 99, 631–633. [Google Scholar] [CrossRef]
- Alley, M.L.; Haines, D.M.; Smith, G.W. Short Communication: Evaluation of Serum Immunoglobulin G Concentrations Using an Automated Turbidimetric Immunoassay in Dairy Calves. J. Dairy Sci. 2012, 95, 4596–4599. [Google Scholar] [CrossRef]
- Elsohaby, I.; Keefe, G.P. Preliminary Validation of a Calf-Side Test for Diagnosis of Failure of Transfer of Passive Immunity in Dairy Calves. J. Dairy Sci. 2015, 98, 4754–4761. [Google Scholar] [CrossRef]
- Mugnier, A.; Pecceu, K.; Schelcher, F.; Corbiere, F. A Parallel Evaluation of 5 Indirect Cost-Effective Methods for Assessing Failure of Passive Immunity Transfer in Neonatal Calves. JDS Commun. 2020, 1, 10–14. [Google Scholar] [CrossRef]
- Thornhill, J.; Krebs, G.; Petzel, C. Evaluation of the Brix Refractometer as an On-Farm Tool for the Detection of Passive Transfer of Immunity in Dairy Calves. Aust. Vet. J. 2015, 93, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Nydam, D.V.; Godden, S.M.; Bristol, L.S.; Kryzer, A.; Ranum, J.; Schaefer, D. Brix Refractometry in Serum as a Measure of Failure of Passive Transfer Compared to Measured Immunoglobulin G and Total Protein by Refractometry in Serum from Dairy Calves. Vet. J. 2016, 211, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Dzantiev, B.B.; Byzova, N.A.; Urusov, A.E.; Zherdev, A.V. Immunochromatographic Methods in Food Analysis. Trends Anal. Chem. 2014, 55, 81–93. [Google Scholar] [CrossRef]
- Ronzoni, A. Investigation of Biological Markers to Evaluate the Adaptation of the Newborn Belgian Blue Calf to the Extrauterine Life: A Pilot Study; Université de Liège: Liège, Belgium, 2012. [Google Scholar]
- De Marchin, E.; Theron, L. Projet GPS Veau, Résultats d’essai en Wallonie; 2013; p. 10, in press. [Google Scholar]
- Coons, D.M.; Thompson, K.A.; Lamberski, N.; Chigerwe, M. Quantitative Indirect ELISA-Based Method for the Measurement of Serum IgG in Springbok Calves. Justifying Use Elisa Ref. Method Present Study 2012, 10, 142–146. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bland, J.M.; Altman, D.G. Applying the Right Statistics: Analyses of Measurement Studies. Ultrasound Obs. Gynecol. 2003, 22, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Morrill, K.M.; Polo, J.; Lago, A.; Campbell, J.; Quigley, J.; Tyler, H. Estimate of Serum Immunoglobulin G Concentration Using Refractometry with or without Caprylic Acid Fractionation. J. Dairy Sci. 2013, 96, 4535–4541. [Google Scholar] [CrossRef]
- Grandini, M.; Bagli, E.; Visani, G. Metrics for Multi-Class Classification: An Overview. arXiv 2020, arXiv:2008.05756 [stat.ML]. [Google Scholar]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common Pitfalls in Statistical Analysis: Measures of Agreement. Perspect. Clin. Res. 2017, 8, 187–191. [Google Scholar] [CrossRef]
- Dawes, M.E.; Tyler, J.W.; Hostetler, D.; Lakritz, J.; Tessman, R. Evaluation of a Commercially Available Immunoassay for Assessing Adequacy of Passive Transfer in Calves. J. Am. Vet. Med. Assoc. 2002, 220, 791–793. [Google Scholar] [CrossRef]
- McVicker, J.K.; Rouse, G.C.; Fowler, M.A.; Perry, B.H.; Miller, B.L.; Johnson, T.E. Evaluation of a Lateral-Flow Immunoassay for Use in Monitoring Passive Transfer of Immunoglobulins in Calves. Am. J. Vet. Res. 2002, 63, 247–250. [Google Scholar] [CrossRef]
- Elsohaby, I.; McClure, J.T.; Waite, L.A.; Cameron, M.; Heider, L.C.; Keefe, G.P. Using Serum and Plasma Samples to Assess Failure of Transfer of Passive Immunity in Dairy Calves. J. Dairy Sci. 2019, 102, 567–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCracken, M.M.; Morrill, K.M.; Fordyce, A.L.; Tyler, H.D. Technical Note: Evaluation of Digital Refractometers to Estimate Serum Immunoglobulin G Concentration and Passive Transfer in Jersey Calves. J. Dairy Sci. 2017, 100, 8438–8442. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.; Earley, B. Review: Passive Immunity in Beef-Suckler Calves. Animal 2019, 13, 810–825. [Google Scholar] [CrossRef] [Green Version]
- Desimoni, E.; Brunetti, B. About Estimating the Limit of Detection of Heteroscedastic Analytical Systems. Anal. Chim. Acta 2009, 655, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gamsjäger, L.; Elsohaby, I.; Pearson, J.M.; Levy, M.; Pajor, E.A.; Windeyer, M.C. Evaluation of 3 Refractometers to Determine Transfer of Passive Immunity in Neonatal Beef Calves. J. Vet. Intern. Med. 2021, 35, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Mohri, M.; Shakeri, H.; Lotfollah Zadeh, S. Effects of Common Anticoagulants (Heparin, Citrate and EDTA) on Routine Plasma Biochemistry of Cattle. Comp. Clin. Pathol. 2007, 16, 207–209. [Google Scholar] [CrossRef]
- Gray, E.; Hogwood, J.; Mulloy, B. he Anticoagulant and Antithrombotic Mechanisms of Heparin. In Heparin—A Century of Progress; Springer: Berlin/Heidelberg, Germany, 2012; Volume 207, pp. 43–61. [Google Scholar]


| TPI Category | IgG Category (mg/mL) | Equivalent Brix % |
|---|---|---|
| Excellent | ≥20.0 | ≥9.0 |
| Good | 15.0–19.9 | 8.5–8.9 |
| Fair | 10.0–14.9 | 8.0–8.4 |
| Poor | <10.0 | <8.0 |
| Method | Measure | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| ELISA | IgG (mg/mL) | 97 | 13.6 | 7.5 | 1.3 | 37.1 |
| Brix refractometer | Brix (%) | 97 | 8.4 | 0.9 | 6.6 | 10.3 |
| Quick test (SmartStripsTM) (EDTA) | IgG (mg/mL) | 91 | 12.8 | 5.7 | 2.9 | 24.4 |
| Quick test (SmartStripsTM) (lithium heparin) | IgG (mg/mL) | 87 | 12.0 | 5.3 | 2.3 | 24.1 |
| Method | Macro F1 Score | Overall Accuracy | TPI Categories | Precision | Sensitivity | F1 Score |
|---|---|---|---|---|---|---|
| SmartStripsTM (EDTA) | 0.75 | 0.76 | Excellent | 0.70 | 1.00 | 0.82 |
| Good | 0.86 | 0.50 | 0.63 | |||
| Fair | 0.58 | 0.78 | 0.67 | |||
| Poor | 0.94 | 0.83 | 0.88 | |||
| SmartStripsTM (lithium heparin) | 0.72 | 0.76 | Excellent | 0.70 | 0.74 | 0.72 |
| Good | 0.43 | 0.50 | 0.46 | |||
| Fair | 0.90 | 0.72 | 0.80 | |||
| Poor | 0.81 | 0.96 | 0.88 | |||
| Brix refractometer | 0.49 | 0.55 | Excellent | 0.70 | 0.50 | 0.58 |
| Good | 0.14 | 0.14 | 0.14 | |||
| Fair | 0.42 | 0.65 | 0.51 | |||
| Poor | 0.75 | 0.69 | 0.72 |
| ELISA | Brix Refractometer | SmartStripsTM (EDTA) | SmartStripsTM (Lithium Heparin) | |
|---|---|---|---|---|
| ELISA | ||||
| Brix refractometer | 0.57 | |||
| SmartStripsTM (EDTA) | 0.80 | 0.50 | ||
| SmartStripsTM (lithium heparin) | 0.78 | 0.54 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delhez, P.; Meurette, E.; Knapp, E.; Theron, L.; Daube, G.; Rao, A.-S. Assessment of a Rapid Semi-Quantitative Immunochromatographic Test for the Evaluation of Transfer of Passive Immunity in Calves. Animals 2021, 11, 1641. https://doi.org/10.3390/ani11061641
Delhez P, Meurette E, Knapp E, Theron L, Daube G, Rao A-S. Assessment of a Rapid Semi-Quantitative Immunochromatographic Test for the Evaluation of Transfer of Passive Immunity in Calves. Animals. 2021; 11(6):1641. https://doi.org/10.3390/ani11061641
Chicago/Turabian StyleDelhez, Pauline, Elise Meurette, Emilie Knapp, Léonard Theron, Georges Daube, and Anne-Sophie Rao. 2021. "Assessment of a Rapid Semi-Quantitative Immunochromatographic Test for the Evaluation of Transfer of Passive Immunity in Calves" Animals 11, no. 6: 1641. https://doi.org/10.3390/ani11061641






