Biomechanical–Structural Correlation of Chordae tendineae in Animal Models: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Biomechanical Examination
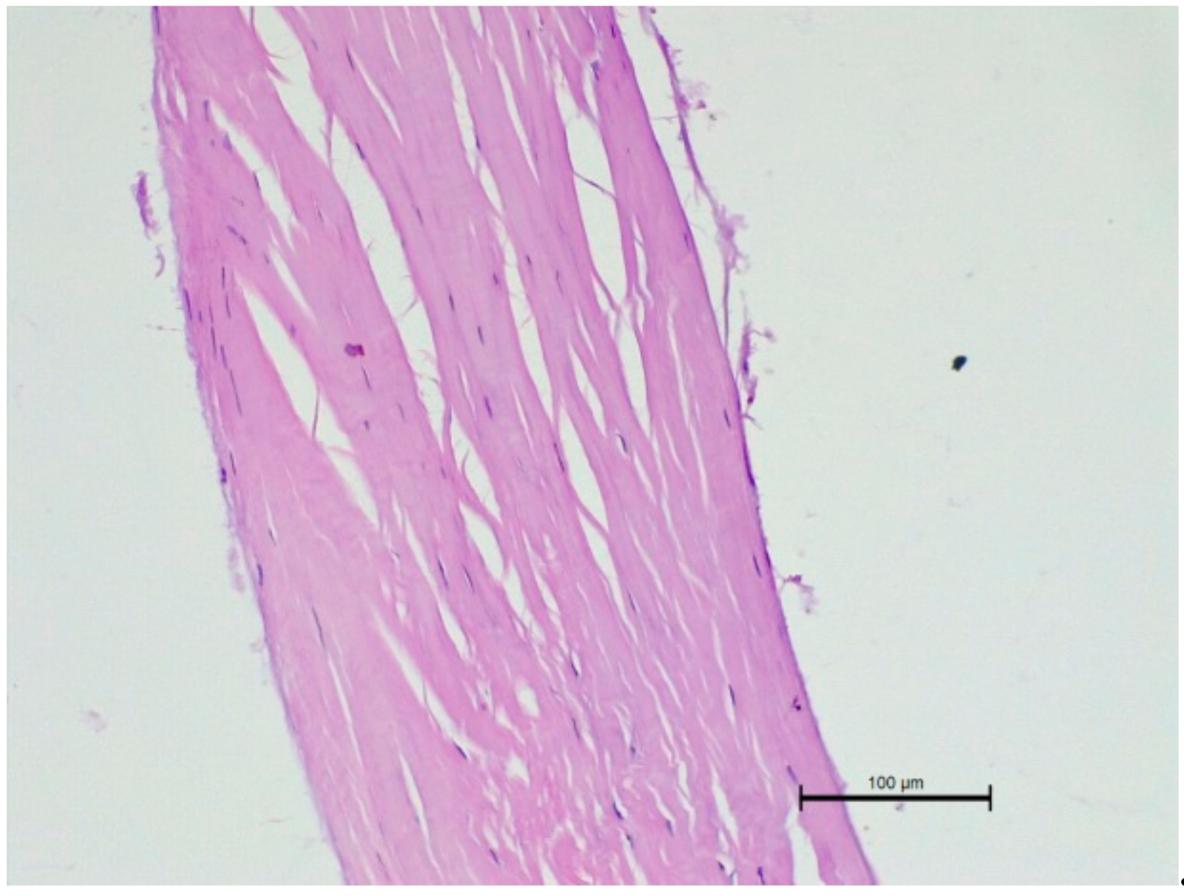
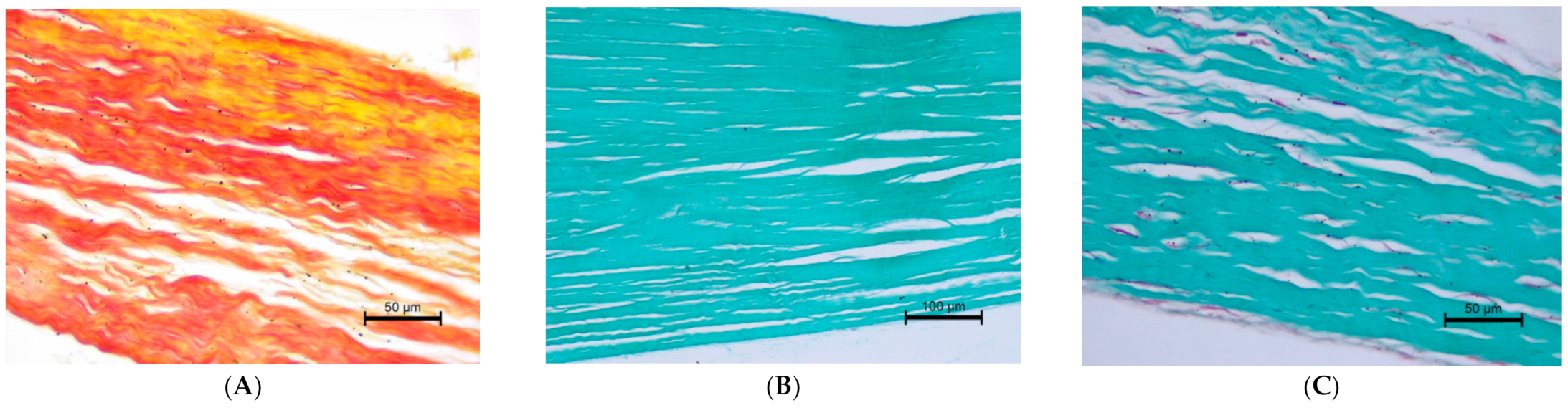
2.2. The Histopathological Examination
3. Results
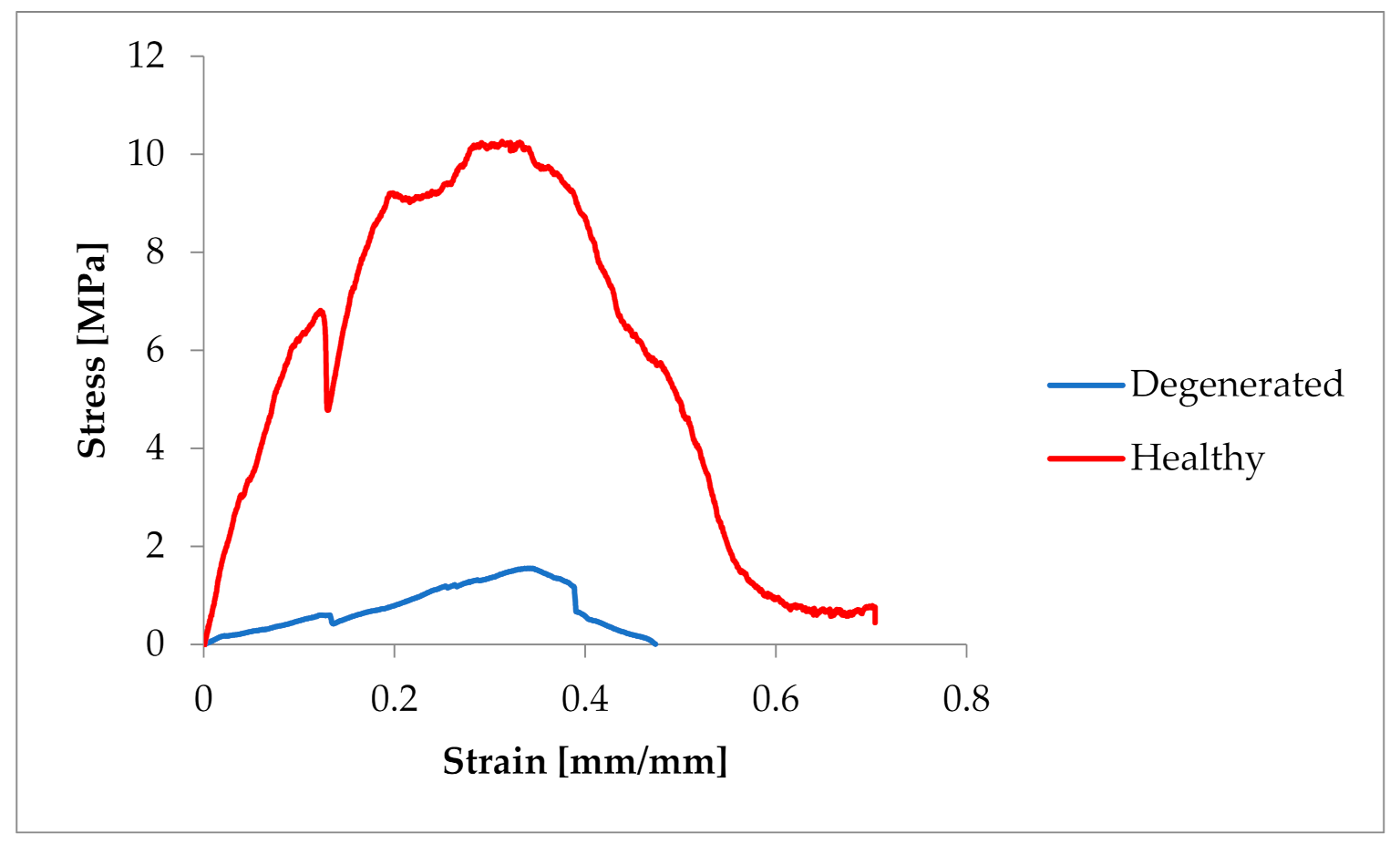
3.1. The Biomechanical Examination
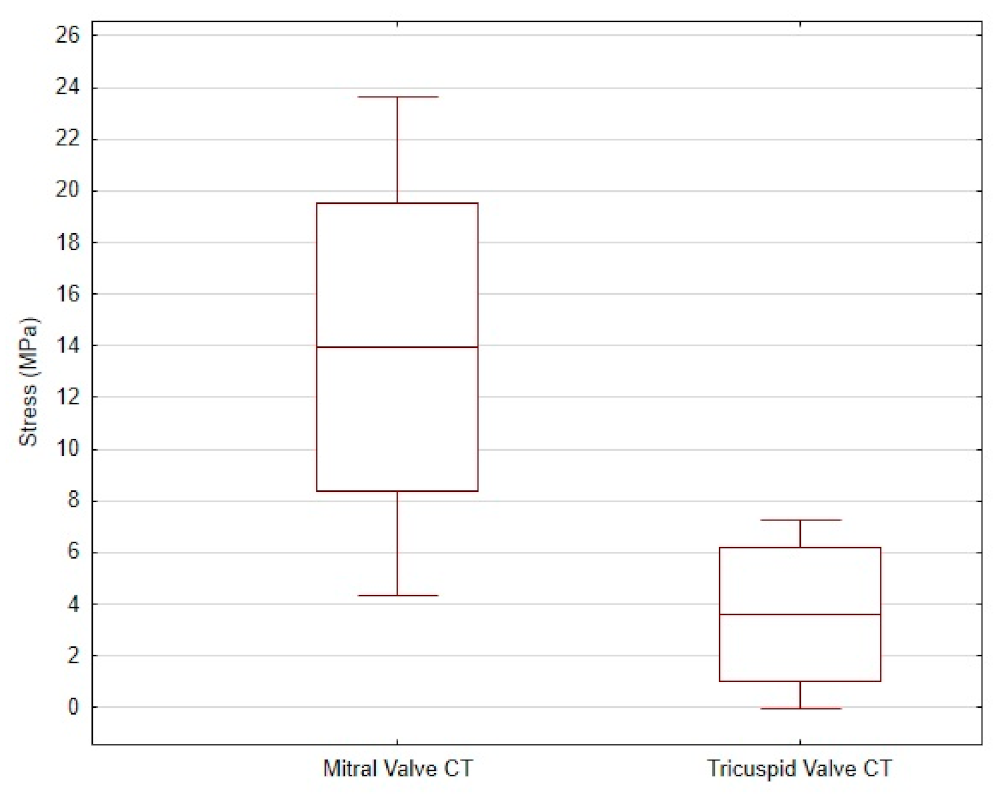
3.2. Comparison of CT from Mitral and Tricuspid Valve
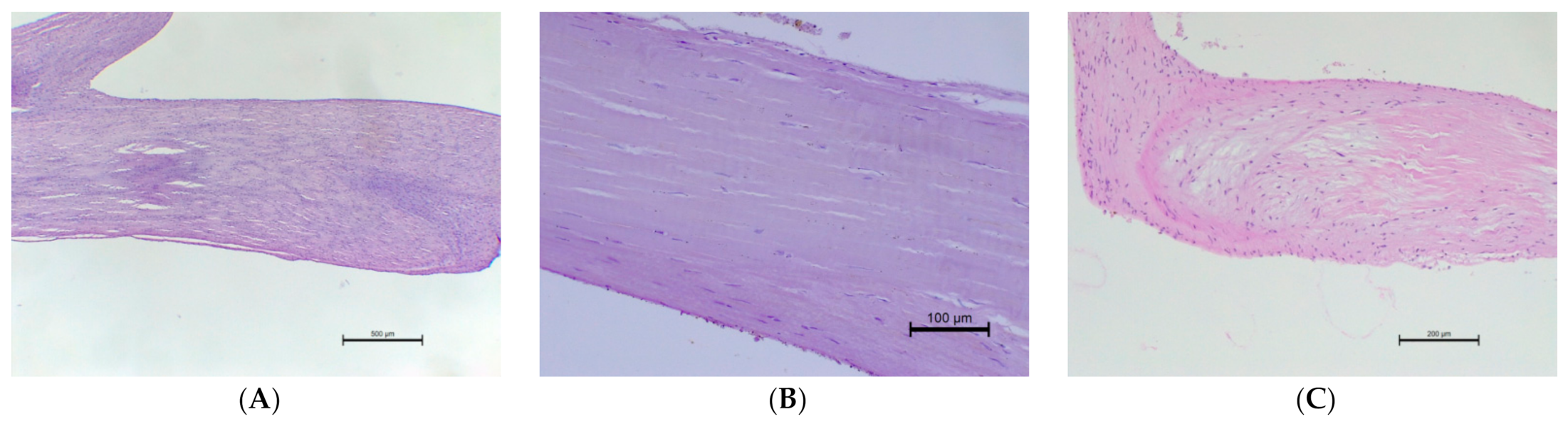
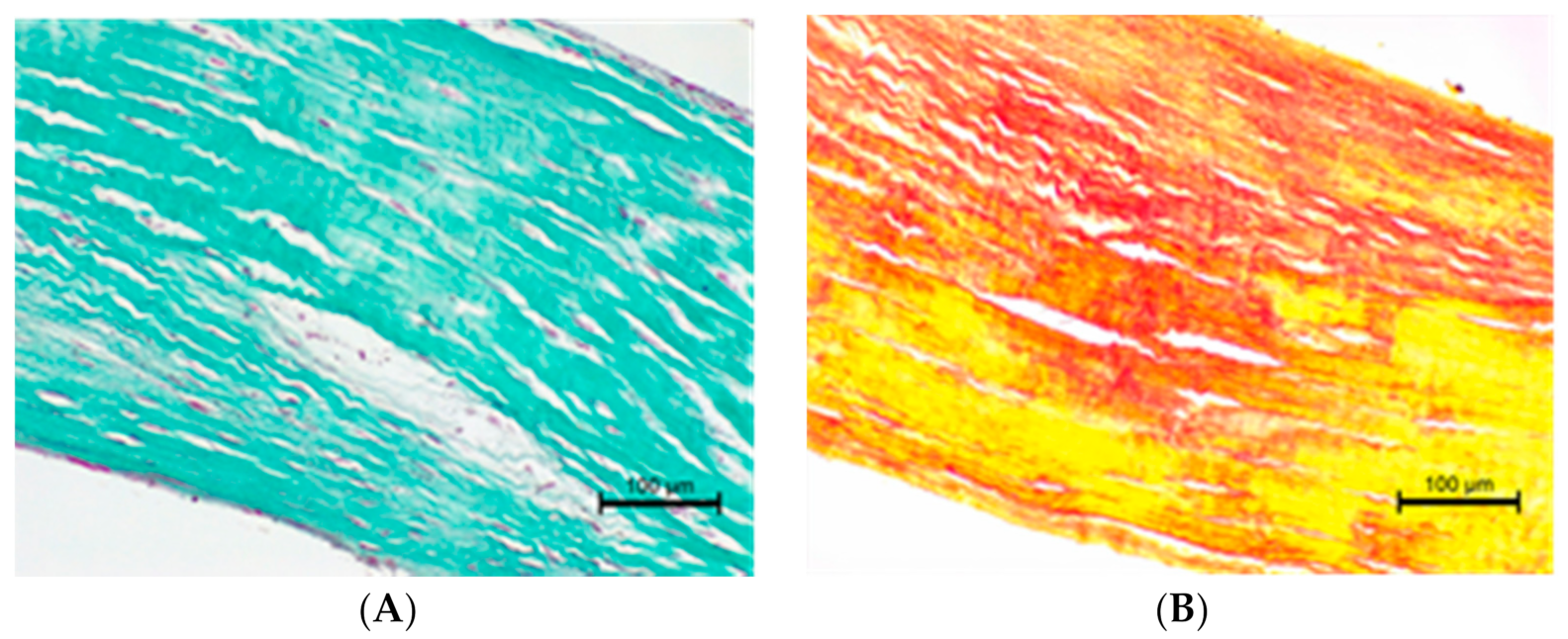
3.3. The Histopathological Examination
4. Discussion
4.1. The Way Forward
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Chordae tendineae |
| MMVD | myxomatous mitral valve disease |
| HE | hematoxylin–eosin |
References
- Gunnal, S.A.; Wabale, R.N.; Farooqui, M.S. Morphological study of chordae tendinae in human cadaveric hearts. Heart Views Off. J. Gulf Heart Assoc. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Serres, F.; Chetboul, V.; Tissier, R.; Sampedrano, C.C.; Gouni, V.; Nicolle, A.P.; Pouchelon, J.L. Chordae tendineae rupture in dogs with degenerative mitral valve disease: Prevalence, survival, and prognostic factors (114 cases, 2001–2006). J. Vet. Intern. Med. 2007, 21, 258–264. [Google Scholar] [CrossRef]
- Morse, D.E.; Hamlett, W.C.; Noble, C.W. Morphogenesis of chordae tendineae. I: Scanning electron microscopy. Anat. Rec. 1984, 210, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.J.; Zheng, J.; Ma, L.; Wu, Y.; Lee, C.H. Mechanics and Microstructure of the Atrioventricular Heart Valve Chordae Tendineae: A Review. Bioengineering 2020, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.M.; Farrar, E.J.; Kornreich, B.G.; Moïse, N.S.; Butcher, J.T. The mechanobiology of mitral valve function, degeneration, and repair. J. Vet. Cardiol. Off. J. Eur. Soc. Vet. Cardiol. 2012, 14, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Borgarelli, M.; Haggstrom, J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Darke, P.G. Valvular incompetence in cavalier King Charles spaniels. Vet. Rec. 1987, 120, 365–366. [Google Scholar] [CrossRef]
- Detweiler, D.K.; Patterson, D.F. The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci. 1965, 127, 481–516. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.W. Chronic valvular disease (endocardiosis) in dogs. Adv. Vet. Sci. Comp. Med. 1977, 21, 75–106. [Google Scholar] [PubMed]
- Menciotti, G.; Borgarelli, M. Review of Diagnostic and Therapeutic Approach to Canine Myxomatous Mitral Valve Disease. Vet. Sci. 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, D.K.; Luginbu¨hl, H.; Buchanan, J.W.; Patterson, D.F. The natural history of acquired cardiac disability of the dog. Ann. N. Y. Acad. Sci. 1968, 147, 318–329. [Google Scholar] [CrossRef]
- Das, K.M.; Tashjian, R.J. Chronic mitral valve disease in the dog. Vet. Med. Small Anim. Clin. 1965, 60, 1209–1216. [Google Scholar]
- Fox, P.R.; Sisson, D.; Moise, N.S. Textbook of Canine and Feline Cardiology. Principles and Clinical Practice, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 1999; pp. 536–565. [Google Scholar]
- Olsen, L.H.; Martinussen, T.; Pedersen, H.D. Early echocardiographic predictors of myxomatous mitral valve disease in Dachshunds. Vet. Rec. 2003, 152, 293–297. [Google Scholar] [CrossRef]
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. Off. J. Eur. Soc. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.D.; Häggström, J.; Falk, T.; Mow, T.; Olsen, L.H.; Iversen, L.; Jensen, A.L. Auscultation in mild mitral regurgitation in dogs: Observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J. Vet. Intern. Med. 1999, 13, 56–64. [Google Scholar] [CrossRef]
- Pokutta-Paskaleva, A.; Sulejmani, F.; DelRocini, M.; Sun, W. Comparative mechanical, morphological, and microstructural characterization of porcine mitral and tricuspid leaflets and chordae tendineae. Acta Biomater. 2019, 85, 241–252. [Google Scholar] [CrossRef]
- Zuo, K.; Pham, T.; Li, K.; Martin, C.; He, Z.; Sun, W. Characterization of biomechanical properties of aged human and ovine mitral valve chordae tendineae. J. Mech. Behav. Biomed. Mater. 2016, 62, 607–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Sari, C.R.; Gao, H.; Lei, Y.; Segers, P.; De Beule, M.; Wang, G.; Ma, X. Mechanical and morphometric study of mitral valve chordae tendineae and related papillary muscle. J. Mech. Behav. Biomed. Mater. 2020, 111, 104011. [Google Scholar] [CrossRef] [PubMed]
- Versteegden, L.R.; van Kampen, K.A.; Janke, H.P.; Tiemessen, D.M.; Hoogenkamp, H.R.; Hafmans, T.G.; Roozen, E.A.; Lomme, R.M.; van Goor, H.; Oosterwijk, E.; et al. Tubular collagen scaffolds with radial elasticity for hollow organ regeneration. Acta Biomater. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Perron, E.; Gauvin, R.; Bernard, G.; Ouellet, G.; Cattan, V.; Bolduc, S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng. Part A 2010, 16, 1539–1548. [Google Scholar] [CrossRef]
- Liao, J.; Vesely, I. Relationship between collagen fibrils, glycosaminoglycans, and stress relaxation in mitral valve chordae tendineae. Ann. Biomed. Eng. 2004, 32, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yagihara, T.; Yamamoto, F.; Uemura, H.; Yamashita, K.; Ishizaka, T. Artificial chordae for mitral valve reconstruction in children. Ann. Thorac. Surg. 1998, 65, 1377–1380. [Google Scholar] [CrossRef]
- Smedira, N.G.; Selman, R.; Cosgrove, D.M.; McCarthy, P.M.; Lytle, B.W.; Taylor, P.C.; Apperson-Hansen, C.; Stewart, R.W.; Loop, F.D. Repair of anterior leaflet prolapse: Chordal transfer is superior to chordal shortening. J. Thorac. Cardiovasc. Surg. 1996, 112, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Klekiel, T.; Mackiewicz, A.; Kaczmarek- Pawelska, A.; Skonieczna, J.; Kurowiak, J.; Piasecki, T.; Noszczyk-Nowak, A.; Bedzinski, R. Novel design of sodium alginate based absorbable stent for the use in urethral stricture disease. J. Mater. Res. Technol. 2020, 9. [Google Scholar] [CrossRef]
- Rudyk, R.; Malinowski, M.; Mackiewicz, A.; Będziński, R.; Noszczyk-Nowak, A.; Skonieczna, J.; Madej, J.P. Numerical analysis of deformation and flow in the proximal area of the urethra. Int. J. Appl. Mech. Eng. 2020, 25, 130–141. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Hansen, S.B.; Nielsen, K.O.; Nygaard, H.; Paulsen, P.K.; Hasenkam, J.M. Imbalanced chordal force distribution causes acute ischemic mitral regurgitation: Mechanistic insights from chordae tendineae force measurements in pigs. J. Thorac. Cardiovasc. Surg. 2005, 129, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackiewicz, A.; Kaczmarek-Pawelska, A.; Kurowiak, J.; Malinowski, M.; Zaręba, Ł.; Noszczyk-Nowak, A.; Pasławska, U.; Madej, J.; Skonieczna, J.; Michałek, M.; et al. Biomechanical investigation of animal urethra model. In International Conference of the Polish Society of Biomechanics—BIOMECHANICS 2018: Abstracts Book; Division of Biomedical Engineering, Department of Mechanical Engineering, University of Zielona Góra: Zielona Góra, Poland, 2018; pp. 143–144. [Google Scholar]
- Mackiewicz, A.; Klekiel, T.; Kurowiak, J.; Piasecki, T.; Bedzinski, R. Determination of Stent Load Conditions in New Zealand White Rabbit Urethra. J. Funct. Biomater. 2020, 11, 70. [Google Scholar] [CrossRef]
- Whitney, J.C. Observations on the effect of age on the severity of heart valve lesions in the dog. J. Small Anim. Pract. 1974, 15, 511–522. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, A.H.; Drach, A.; Bloodworth, C.H., 4th; Pierce, E.L.; Yoganathan, A.P.; Gorman, R.C.; Gorman, J.H., III; Sacks, M.S. Mitral Valve Chordae Tendineae: Topological and Geometrical Characterization. Ann. Biomed. Eng. 2017, 45, 378–393. [Google Scholar] [CrossRef]
- Arts, T.; Meerbaum, S.; Reneman, R.; Corday, E. Stresses in the closed mitral valve: A model study. J. Biomech. 1983, 16, 539–547. [Google Scholar] [CrossRef]
- McQueen, D.M.; Peskin, C.S.; Yellin, E.L. Fluid dynamics of the mitral valve: Physiological aspects of a mathematical model. Am. J. Physiol. 1982, 242, H1095–H1110. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Yeo, J.H.; Duran, C.M. Three-dimensional asymmetrical modeling of the mitral valve: A finite element study with dynamic boundaries. J. Heart Valve Dis. 2005, 14, 386–392. [Google Scholar] [PubMed]
- Fatemifar, F.; Feldman, M.; Oglesby, M.; Han, H.C. Comparison of Biomechanical Properties and Microstructure of Trabeculae Carneae, Papillary muscles, and Myocardium in Human Heart. J. Biomech. Eng. 2018, 141, 0210071. [Google Scholar] [CrossRef] [PubMed]
- Kosiński, A.; Grzybiak, M.; Dubaniewicz, A.; Kędziora, K.; Makarewicz, W.; Kozłowski, D. False chordae tendineae in right ventricle of adult human hearts—Morphological aspects. Arch. Med. Sci. 2012, 8, 834–840. [Google Scholar] [CrossRef]


| Animal Model | Sex | CT No. | Type of Valve | Before Biomechanical Test | Post Biomechanical Test | ||
|---|---|---|---|---|---|---|---|
| Length [mm] | Diameter [mm] | Surface Area [mm2] | Length [mm] | ||||
| Dog 1 | Male | 1 | Mitral | 8.638 | 0.294 | 0.271 | 16.926 |
| 2 | Mitral | 6.584 | 0.562 | 0.991 | 16.254 | ||
| 3 | Mitral | 8.218 | 0.445 | 0.623 | 16.708 | ||
| 4 | Tricuspid | 9.551 | 0.402 | 0.507 | 11.422 | ||
| 5 | Tricuspid | 9.748 | 0.271 | 0.230 | 11.934 | ||
| Dog 2 | Female | 1 | Mitral | 9.577 | 0.421 | 0.557 | 13.126 |
| 2 | Mitral | 7.489 | 0.367 | 0.422 | 12.222 | ||
| 3 | Mitral | 7.488 | 0.310 | 0.302 | 11.976 | ||
| Dog 3 | Male | 1 | Mitral | 8.306 | 0.382 | 0.458 | 15.894 |
| 2 | Mitral | 8.093 | 0.252 | 0.199 | 18.290 | ||
| 3 | Mitral | 9.360 | 0.984 | 3.039 | 16.875 | ||
| 4 | Mitral | 8.309 | 0.382 | 0.458 | 21.193 | ||
| Dog 4 | Male | 1 | Mitral | 25.458 | 0.768 | 1.852 | 18.255 |
| 2 | Mitral | 18.810 | 0.343 | 0.37 | 16.049 | ||
| 3 | Mitral | 14.072 | 0.369 | 0.428 | 19.087 | ||
| Dog 5 | Male | 1 | Mitral | 10.078 | 0.505 | 0.800 | 16.994 |
| 2 | Mitral | 10.370 | 0.758 | 1.803 | 15.966 | ||
| Pig 1 | Female | 1 | Mitral | 10.013 | 0.704 | 1.557 | 20.289 |
| 2 | Mitral | 15.746 | 0.772 | 1.872 | 22.760 | ||
| 3 | Mitral | 8.935 | 0.804 | 2.030 | 21.865 | ||
| Pig 2 | Female | 1 | Mitral | 10.725 | 0.805 | 2.033 | 24.245 |
| 2 | Mitral | 8.246 | 0.762 | 1.823 | 12.582 | ||
| 3 | Mitral | 8.735 | 0.657 | 1.355 | 17.768 | ||
| 4 | Mitral | 10.947 | 0.540 | 0.915 | 15.725 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gach, J.; Janus, I.; Mackiewicz, A.; Klekiel, T.; Noszczyk-Nowak, A. Biomechanical–Structural Correlation of Chordae tendineae in Animal Models: A Pilot Study. Animals 2021, 11, 1678. https://doi.org/10.3390/ani11061678
Gach J, Janus I, Mackiewicz A, Klekiel T, Noszczyk-Nowak A. Biomechanical–Structural Correlation of Chordae tendineae in Animal Models: A Pilot Study. Animals. 2021; 11(6):1678. https://doi.org/10.3390/ani11061678
Chicago/Turabian StyleGach, Justyn, Izabela Janus, Agnieszka Mackiewicz, Tomasz Klekiel, and Agnieszka Noszczyk-Nowak. 2021. "Biomechanical–Structural Correlation of Chordae tendineae in Animal Models: A Pilot Study" Animals 11, no. 6: 1678. https://doi.org/10.3390/ani11061678
APA StyleGach, J., Janus, I., Mackiewicz, A., Klekiel, T., & Noszczyk-Nowak, A. (2021). Biomechanical–Structural Correlation of Chordae tendineae in Animal Models: A Pilot Study. Animals, 11(6), 1678. https://doi.org/10.3390/ani11061678







