Babesia microti in Rodents from Different Habitats of Lithuania
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
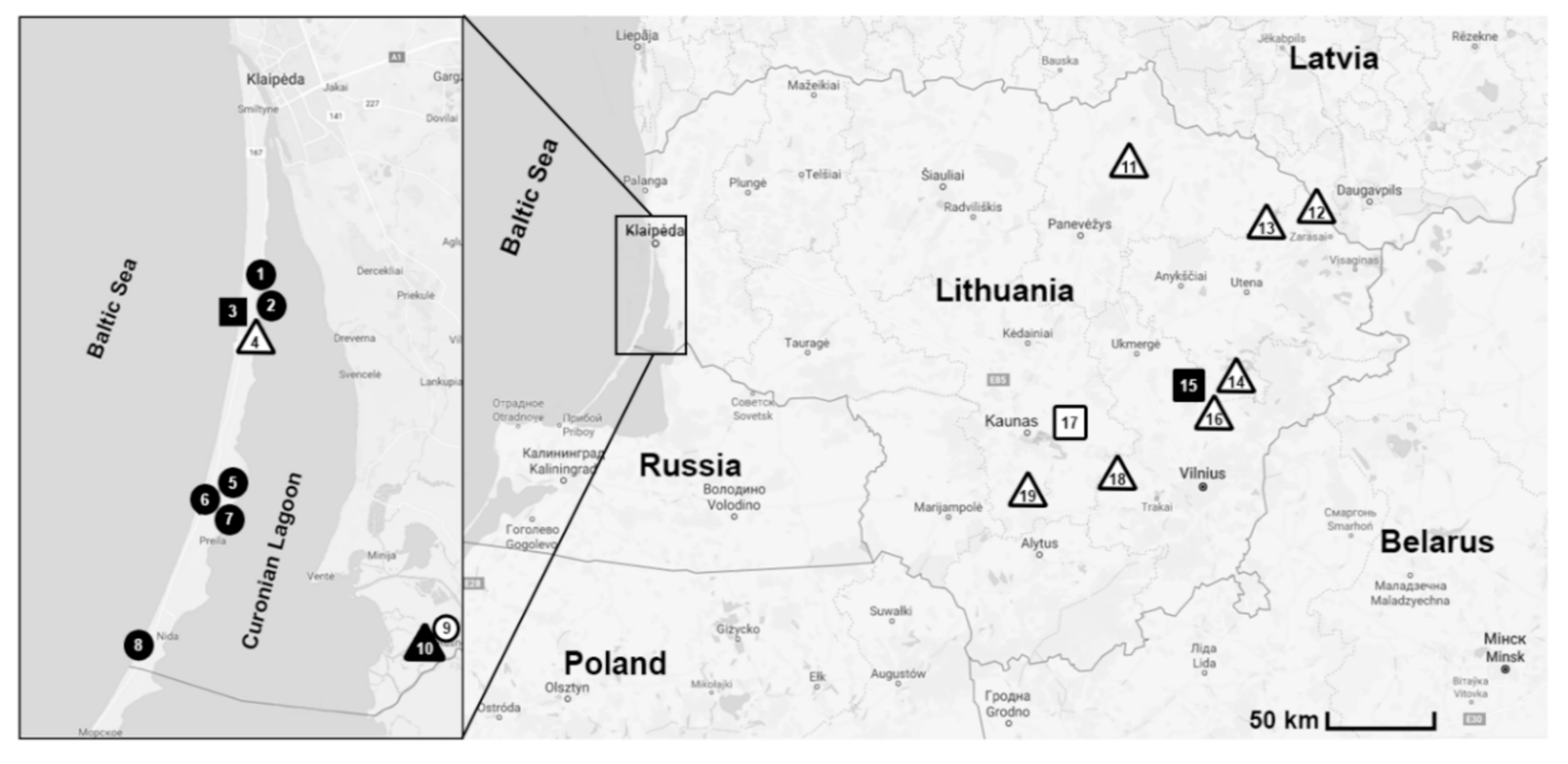
2.1. Study Sites
2.2. Rodent Trapping
2.3. Molecular Analyses
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Babesia Parasites in Various Rodent Species
3.2. Habitat-Based Differences
3.3. Molecular Characterization of Babesia Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, K.M.; Corrin, T.; Wilhelm, B.; Uhland, C.; Greig, J.; Mascarenhas, M.; Waddell, L.A. Zoonotic Babesia: A scoping review of the global evidence. PLoS ONE 2019, 14, e0226781. [Google Scholar] [CrossRef]
- Westblade, L.F.; Simon, M.S.; Mathison, B.A.; Kirkman, L.A. Babesia microti: From mice to ticks to an increasing number of highly susceptible humans. J. Clin. Microbiol. 2017, 55, 2903–2912. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Gray, J.S.; Hunfeld, K.P. Human Babesiosis in Europe: What clinicians need to know. Infection 2013, 41, 1057–1072. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Hunfeld, K.P.; Baier, M.; Krumbholz, A.; Sachse, S.; Lorenzen, T.; Kiehntopf, M.; Fricke, H.-J.; Straube, E. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Welc-Falęciak, R.; Pawełczyk, A.; Radkowski, M.; Pancewicz, S.A.; Zajkowska, J.; Siński, E. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann. Agric. Environ. Med. 2015, 22, 51–54. [Google Scholar] [CrossRef][Green Version]
- Moniuszko-Malinowska, A.; Swiecicka, I.; Dunaj, J.; Zajkowska, J.; Czupryna, P.; Zambrowski, G.; Czupryna, P.; Garkowski, A. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect. Dis. 2016, 48, 537–543. [Google Scholar] [CrossRef]
- Blaňarová, L.; Stanko, M.; Miklisová, D.; Víchová, B.; Mošanský, L.; Kraljik, J.; Bona, M.; Derdáková, M. Presence of Candidatus Neoehrlichia mikurensis and Babesia microti in rodents and two tick species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick Borne Dis. 2016, 7, 319–326. [Google Scholar] [CrossRef]
- Tołkacz, K.; Bednarska, M.; Alsarraf, M.; Dwużnik, D.; Grzybek, M.; Welc-Falęciak, R.; Behnke, J.M.; Bajer, A. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae). Parasites Vectors 2017, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Obiegala, A.; Pfeffer, M.; Pfister, K.; Karnath, C.; Silaghi, C. Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick Borne Dis. 2015, 6, 445–449. [Google Scholar] [CrossRef]
- Krawczyk, A.I.; van Duijvendijk, G.L.A.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasites Vectors 2020, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Azagi, T.; Jaarsma, R.I.; Docters van Leeuwen, A.; Fonville, M.; Maas, M.; Franssen, F.F.J.; Kik, M.; Rijks, J.M.; Montizaan, M.G.; Groenevelt, M.; et al. Circulation of Babesia Species and Their Exposure to Humans through Ixodes ricinus. Pathogens 2021, 10, 386. [Google Scholar] [CrossRef]
- Duh, D.; Petrovec, M.; Trilar, T.; Avsic-Zupanc, T. The molecular evidence of Babesia microti infection in small mammals collected in Slovenia. Parasitology 2003, 126, 113–117. [Google Scholar] [CrossRef]
- Nefedova, V.V.; Korenberg, E.I.; Kovalevskii, Y.V.; Samokhvalov, M.V.; Gorelova, N.B. The role of Ixodes trianguliceps tick larvae in circulation of Babesia microti in the Middle Urals. Entomol. Rev. 2013, 93, 258–266. [Google Scholar] [CrossRef]
- Bown, K.J.; Lambin, X.; Telford, G.R.; Ogden, N.; Telfer, S.; Woldehiwet, Z.; Birtles, R. Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl. Environ. Microbiol. 2008, 74, 7118–7125. [Google Scholar] [CrossRef]
- Cayol, C.; Jääskeläinen, A.; Koskela, E.; Kyröläinen, S.; Mappes, T.; Siukkola, A.; Kallio, E.R. Sympatric Ixodes-tick species: Pattern of distribution and pathogen transmission within wild rodent populations. Sci. Rep. 2018, 8, 16660. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Florin-Christensen, M.; Cardoso, L.; Schnittger, L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasites Vectors 2015, 8, 207. [Google Scholar] [CrossRef]
- Rar, V.; Yakimenko, V.; Makenov, M.; Tikunov, A.; Epikhina, T.; Tancev, A.; Bobrova, O.; Tikunova, N. High prevalence of Babesia microti ‘Munich’ type in small mammals from an Ixodes persulcatus/Ixodes trianguliceps sympatric area in the Omsk region, Russia. Parasitol. Res. 2016, 115, 3619–3629. [Google Scholar] [CrossRef]
- Usluca, S.; Celebi, B.; Karasartova, D.; Gureser, A.S.; Matur, F.; Oktem, M.A.; Sozen, M.; Karatas, A.; Babur, C.; Mumcuoglu, K.Y.; et al. Molecular Survey of Babesia microti (Aconoidasida: Piroplasmida) in Wild Rodents in Turkey. J. Med. Entomol. 2019, 56, 1605–1609. [Google Scholar] [CrossRef]
- Beck, R.; Vojta, L.; Ćurković, S.; Mrljak, V.; Margaletić, J.; Habrun, B. Molecular survey of Babesia microti in wild rodents in central Croatia. Vector Borne Zoonotic Dis. 2011, 11, 81–83. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Bajer, A.; Paziewska-Harris, A.; Baumann-Popczyk, A.; Siński, E. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv. Med. Sci. 2012, 57, 364–369. [Google Scholar] [CrossRef]
- Kallio, E.R.; Begon, M.; Birtles, R.J.; Bown, K.J.; Koskela, E.; Mappes, T.; Watts, P.C. First report of Anaplasma phagocytophilum and Babesia microti in rodents in Finland. Vector Borne Zoonotic Dis. 2014, 14, 389–393. [Google Scholar] [CrossRef]
- Jouglin, M.; Perez, G.; Butet, A.; Malandrin, L.; Bastian, S. Low prevalence of zoonotic Babesia in small mammals and Ixodes ricinus in Brittany, France. Vet. Parasitol. 2017, 238, 58–60. [Google Scholar] [CrossRef]
- Balčiauskas, L. Methods of Investigation of Terrestrial Ecosystems. Part I. Animal Surveys; Vilnius University: Vilnius, Lithuania, 2004; p. 183. [Google Scholar]
- Prūsaitė, J.; Mažeikytė, R.; Pauža, D.; Paužienė, N.; Baleišis, R.; Juškaitis, R.; Mickus, A.; Grušas, A.; Skeiveris, R.; Bluzma, P.M. Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; p. 295. [Google Scholar]
- Rar, V.A.; Fomenko, N.V.; Dobrotvorsky, A.K.; Livanova, N.N.; Rudakova, S.A.; Fedorov, E.G.; Astanin, V.B.; Morozova, O.V. Tick-borne pathogen detection, western Siberia, Russia. Emerg. Infect. Dis. 2005, 11, 1708. [Google Scholar] [CrossRef]
- Rar, V.A.; Epikhina, T.I.; Livanova, N.N.; Panov, V.V. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology 2011, 138, 175–182. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Brown, L.D.; Cat, T.T.; DasGupta, A. Interval Estimation for a Proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Abramson, J.H. WINPEPI Updated: Computer Programs for Epidemiologists, and their Teaching Potential. Epidemiol. Perspect. Innov. 2011, 8, 1. [Google Scholar] [CrossRef]
- Thomas, J.R.; Salazar, W.; Landers, D.M. What is Missing in p <0.05? Effect Size. Res. Q. Exerc. Sport 1991, 62, 344–348. [Google Scholar] [CrossRef]
- Šebek, Z.; Sixl, W.; Stünzner, D.; Valová, M.; Hubálek, Z.; Troger, H. Zur Kenntnis der Blutparasiten wildlebender Kleinsäuger in der Steiermark und im Burgenland [Blood parasites of small wild mammals in Steiermark and Burgenland]. Folia Parasitol. 1980, 27, 295–301. (In German) [Google Scholar]
- Šebek, Z. Blutparasiten der wildlebenden Kleinsäuger in der Tschechoslowakei [Blood parasites of small wild mammals in Czechoslovakia]. Folia Parasitol. 1975, 22, 11–20. (In German) [Google Scholar]
- Welc-Falęciak, R.; Bajer, A.; Behnke, J.M.; Siński, E. Effects of host diversity and the community composition of hard ticks (Ixodidae) on Babesia microti infection. Int. J. Med. Microbiol. 2008, 298, 235–242. [Google Scholar] [CrossRef]
- Tadin, A.; Turk, N.; Korva, M.; Margaletić, J.; Beck, R.; Vucelja, M.; Habuš, J.; Svoboda, P.; Županc, T.A.; Henttonen, H.; et al. Multiple co-infections of rodents with hantaviruses, Leptospira, and Babesia in Croatia. Vector Borne Zoonotic Dis. 2012, 12, 388–392. [Google Scholar] [CrossRef]
- Hamšíková, Z.; Kazimírová, M.; Haruštiaková, D.; Mahríková, L.; Slovák, M.; Berthová, L.; Kocianová, E.; Schnittger, L. Babesia spp. in ticks and wildlife in different habitat types of Slovakia. Parasites Vectors 2016, 9, 292. [Google Scholar] [CrossRef]
- Baltrūnaitė, L.; Kitrytė, N.; Križanauskienė, A. Blood parasites (Babesia, Hepatozoon and Trypanosoma) of rodents, Lithuania: Part I. Molecular and traditional microscopy approach. Parasitol. Res. 2020, 119, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Radzijevskaja, J.; Kaminskienė, E.; Lipatova, I.; Mardosaitė-Busaitienė, D.; Balčiauskas, L.; Stanko, M.; Paulauskas, A. Prevalence and diversity of Rickettsia species in ectoparasites collected from small rodents in Lithuania. Parasites Vectors 2018, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Saito-Ito, A.; Kawai, A.; Ohmori, S.; Nagano-Fujii, M. Continuous in vivo culture and indirect fluorescent antibody test for zoonotic protozoa of Babesia microti. Parasitol. Int. 2016, 65, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Capligina, V.; Berzina, I.; Bormane, A.; Salmane, I.; Vilks, K.; Kazarina, A.; Bandere, D.; Baumanis, V.; Ranka, R. Prevalence and phylogenetic analysis of Babesia spp. in Ixodes ricinus and Ixodes persulcatus ticks in Latvia. Exp. Appl. Acarol. 2016, 68, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Wang, X.M.; Wang, Z.S.; Wang, Z.H.; Guan, Z.Z.; Zhang, L.H.; Dou, X.F.; Wang, H. High prevalence of Babesia microti in small mammals in Beijing. Infect. Dis. Poverty 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Katargina, O.; Geller, J.; Vasilenko, V.; Kuznetsova, T.; Järvekülg, L.; Vene, S.; Lundkvist, Å.; Golovljova, I. Detection and characterization of Babesia species in Ixodes ticks in Estonia. Vector-Borne Zoonotic Dis. 2011, 11, 923–928. [Google Scholar] [CrossRef]
- Radzijevskaja, J.; Mardosaitė-Busaitienė, D.; Aleksandravičienė, A.; Paulauskas, A. Investigation of Babesia spp. in sympatric populations of Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania and Latvia. Ticks Tick Borne Dis. 2018, 9, 270–274. [Google Scholar] [CrossRef]
- Rudolf, I.; Golovchenko, M.; Sikutová, S.; Rudenko, N. Babesia microti (Piroplasmida: Babesiidae) in nymphal Ixodes ricinus (Acari: Ixodidae) in the Czech Republic. Folia Parasitol. 2005, 52, 274. [Google Scholar] [CrossRef] [PubMed]
- Paulauskas, A.; Radzijevskaja, J.; Turčinavičienė, J.; Ambrasienė, D.; Galdikaitė, E. Data on some Ixodid tick species (Acari, Ixodidae) in the Baltic countries. New Rare Lith. Insect Species 2010, 22, 43–51. [Google Scholar]



| No | Habitat | A. flavicollis | A. agrarius | M. musculus | M. minutus | C. glareolus | M. oeconomus | M. agrestis | M. arvalis | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | coastal meadow | 4/59 (6.8) | 1/40 (2.5) | 1/9 | 1/4 | 0/1 | 7/113 (6.2) | |||
| 2 | coastal meadow | 2/2 | 2/2 | |||||||
| 3 | forest-meadow ecotone | 2/192 (1.1) | 0/2 | 0/36 | 1/2 | 0/1 | 3/233 (1.3) | |||
| 4 | mixed forest | 0/29 | 0/29 | |||||||
| 5 | coastal meadow | 0/33 | 0/26 | 1/5 | 0/2 | 1/66 (1.5) | ||||
| 6 | coastal meadow | 2/54 (3.7) | 0/1 | 1/3 | 8/18 (44.5) | 0/1 | 11/77 (14.3) | |||
| 7 | coastal meadow | 3/37 (8.1) | 0/4 | 0/8 | 0/2 | 3/51 (5.9) | ||||
| 8 | coastal meadow | 0/5 | 1/1 | 1/6 | ||||||
| 9 | flooded meadows | 0/52 | 0/3 | 0/19 | 0/40 | 0/12 | 0/126 | |||
| 10 | flooded forest | 0/5 | 0/7 | 0/1 | 1/14 (2.5) | 0/2 | 0/9 | 1/38 (2.6) | ||
| 11 | mixed forest | 0/1 | 0/13 | 0/14 | ||||||
| 12 | deciduous forest | 0/12 | 0/6 | 0/52 | 0/70 | |||||
| 13 | mixed forest | 0/3 | 0/7 | 0/10 | ||||||
| 14 | mixed forest | 0/3 | 0/11 | 0/14 | ||||||
| 15 | forest-meadow ecotone | 0/20 | 4/117 (3.5) | 4/137 (2.9) | ||||||
| 16 | mixed forest | 0/6 | 0/14 | 0/20 | ||||||
| 17 | forest-meadow ecotone | 0/17 | 0/10 | 0/12 | 0/4 | 0/43 | ||||
| 18 | deciduous forest | 0/2 | 0/7 | 0/88 | 0/97 | |||||
| 19 | mixed forest | 0/22 | 0/12 | 0/34 | ||||||
| Total | 11/499 (2.2) | 0/82 | 0/12 | 1/77 (1.3) | 9/396 (2.3) | 10/69 (14.5) | 2/28 (7.1) | 0/17 | 33/1180 (2.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardosaitė-Busaitienė, D.; Radzijevskaja, J.; Balčiauskas, L.; Paulauskas, A. Babesia microti in Rodents from Different Habitats of Lithuania. Animals 2021, 11, 1707. https://doi.org/10.3390/ani11061707
Mardosaitė-Busaitienė D, Radzijevskaja J, Balčiauskas L, Paulauskas A. Babesia microti in Rodents from Different Habitats of Lithuania. Animals. 2021; 11(6):1707. https://doi.org/10.3390/ani11061707
Chicago/Turabian StyleMardosaitė-Busaitienė, Dalytė, Jana Radzijevskaja, Linas Balčiauskas, and Algimantas Paulauskas. 2021. "Babesia microti in Rodents from Different Habitats of Lithuania" Animals 11, no. 6: 1707. https://doi.org/10.3390/ani11061707
APA StyleMardosaitė-Busaitienė, D., Radzijevskaja, J., Balčiauskas, L., & Paulauskas, A. (2021). Babesia microti in Rodents from Different Habitats of Lithuania. Animals, 11(6), 1707. https://doi.org/10.3390/ani11061707







