Physiological and Behavioral Mechanisms of Thermoregulation in Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Skin’s Role in Thermoregulation
2.1. Reception of Thermal Responses
2.2. Temperature Modulation through Cutaneous Circulation
Temperature Modulation Mediated by Sweating
3. Hypothalamic Control of Deep Body Temperature
4. Neurophysiological Responses for Controlling Hyperthermia
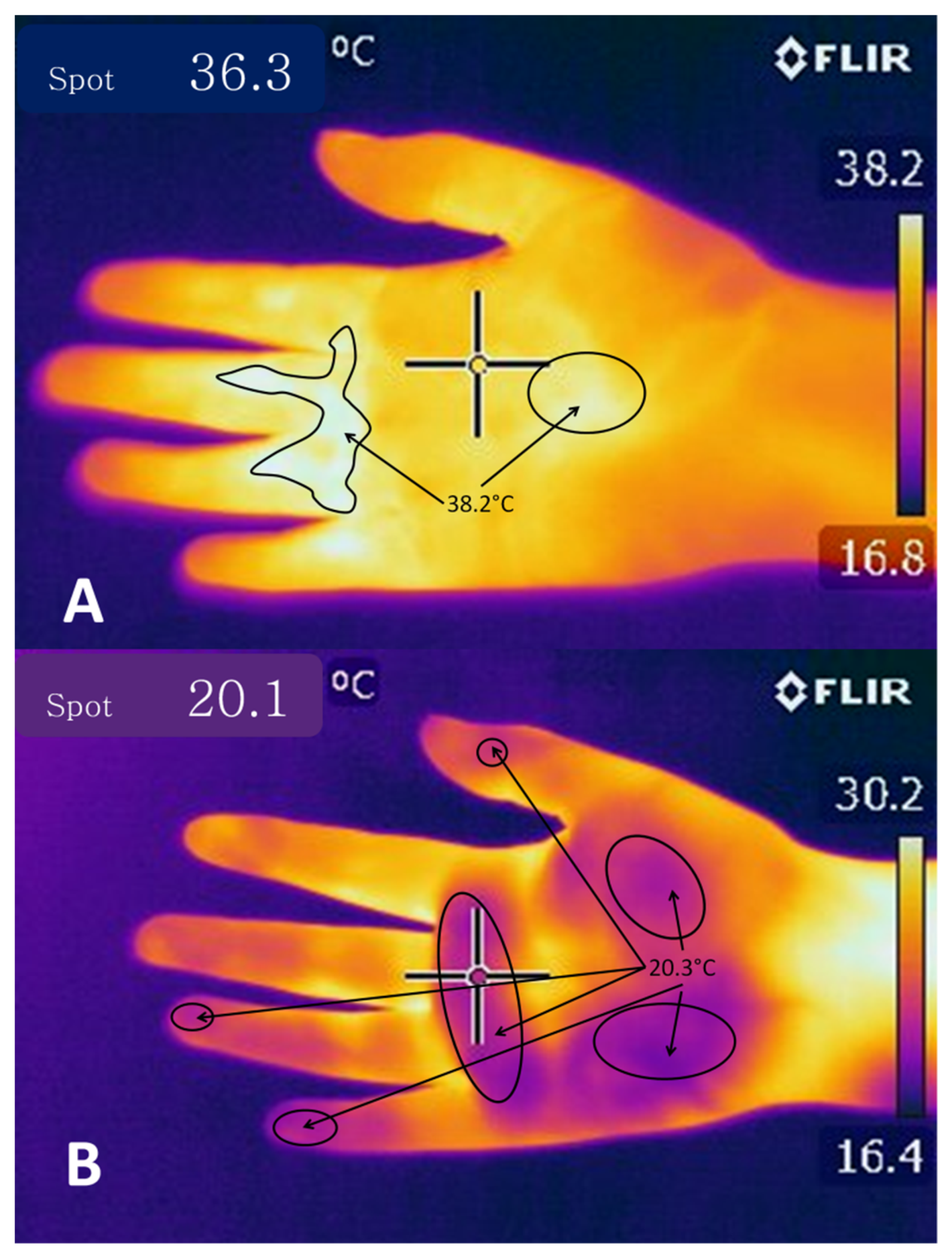
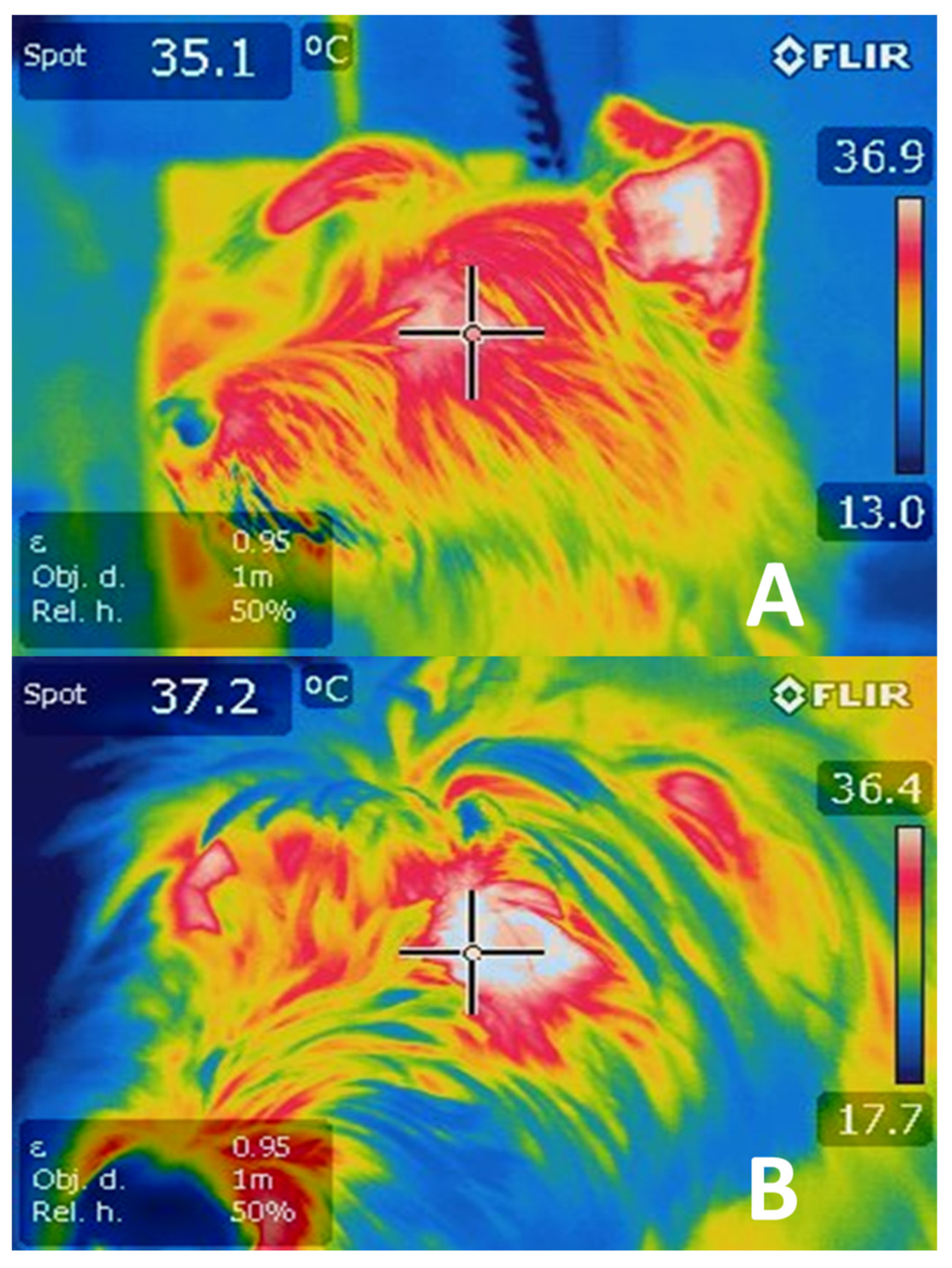
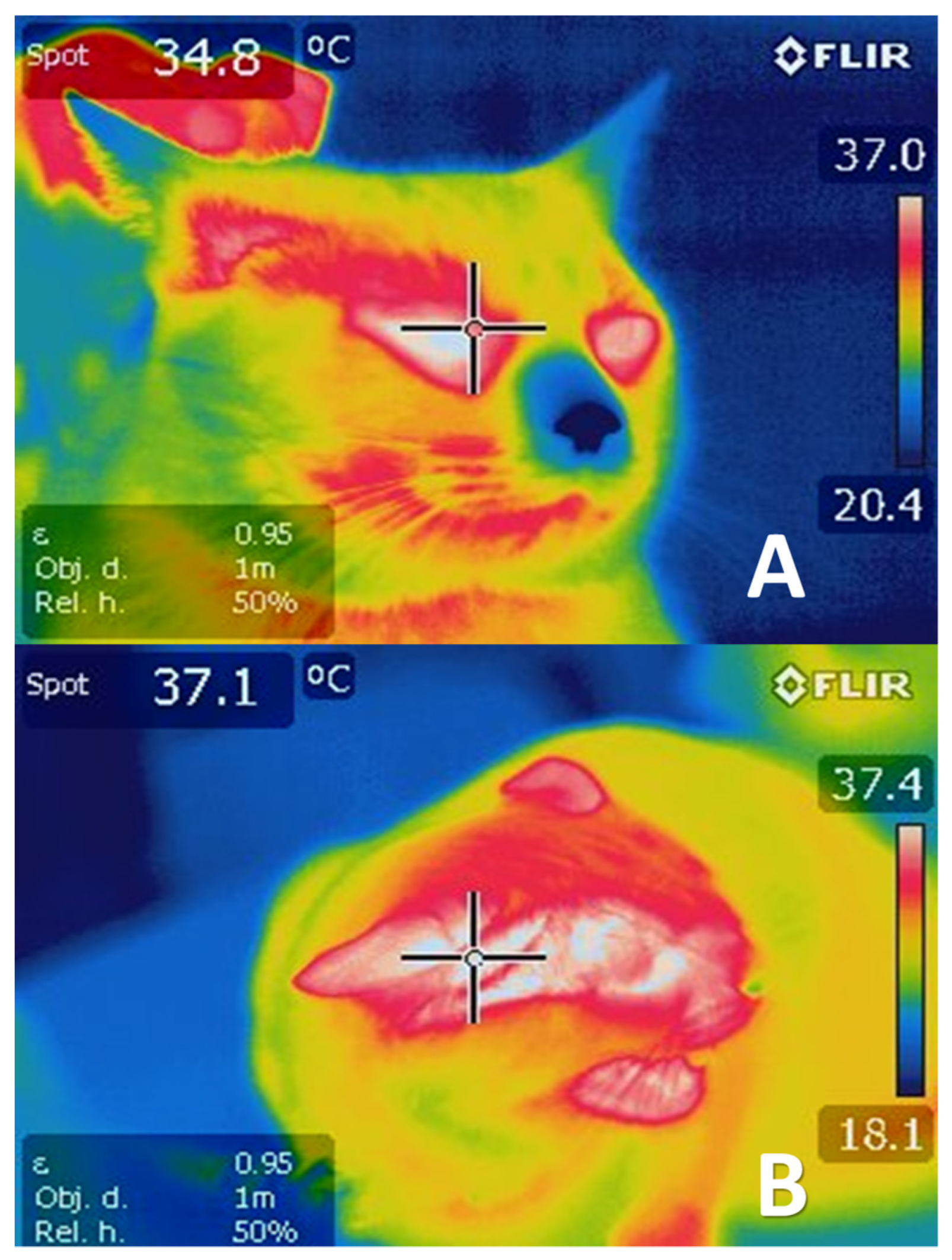
4.1. Cutaneous Vasodilatation
4.2. Evaporative Cooling
4.3. Response to Exercise
4.4. Fear Response
5. Neurophysiological Responses for Controlling Hypothermia
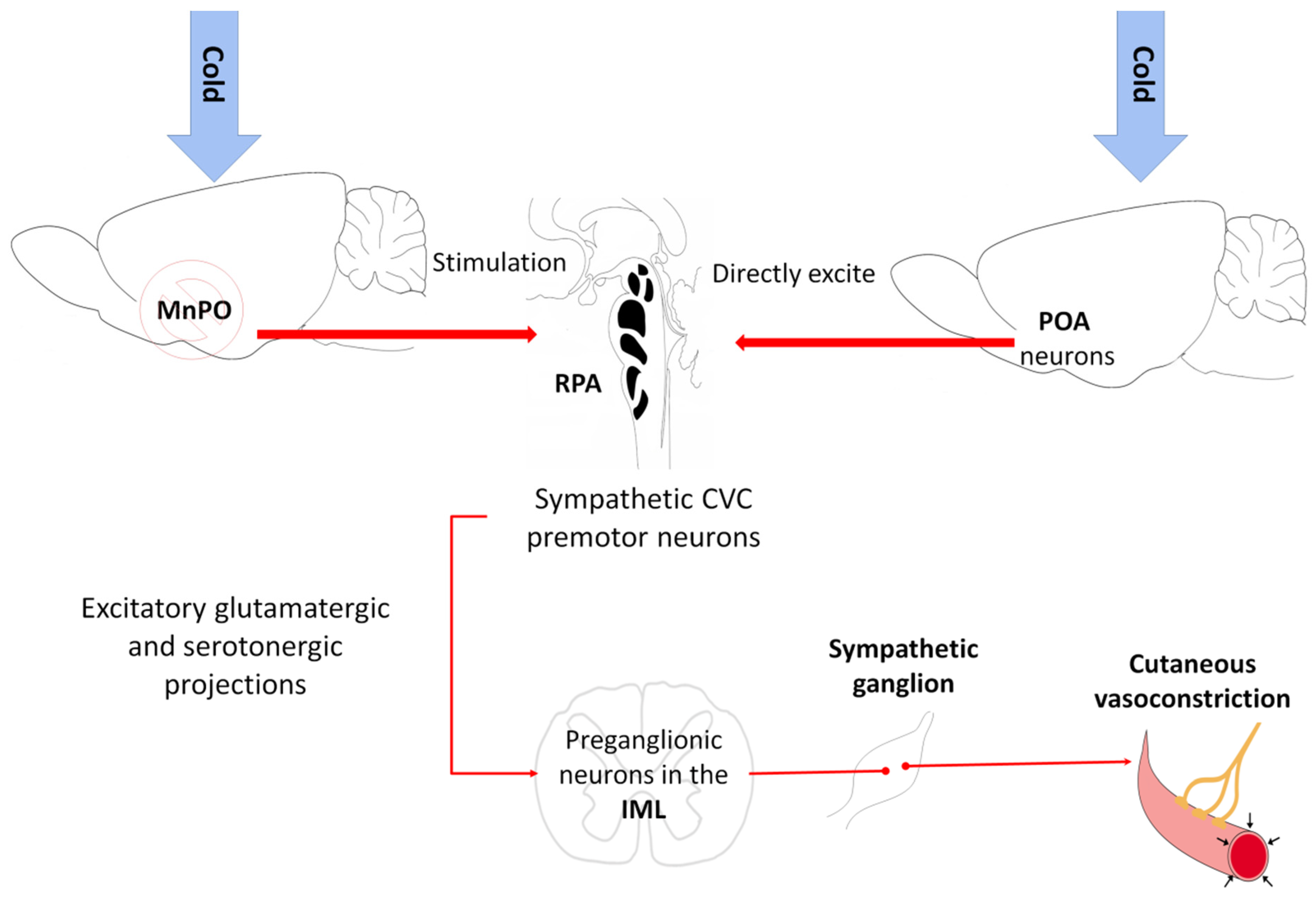
5.1. Vasoconstriction
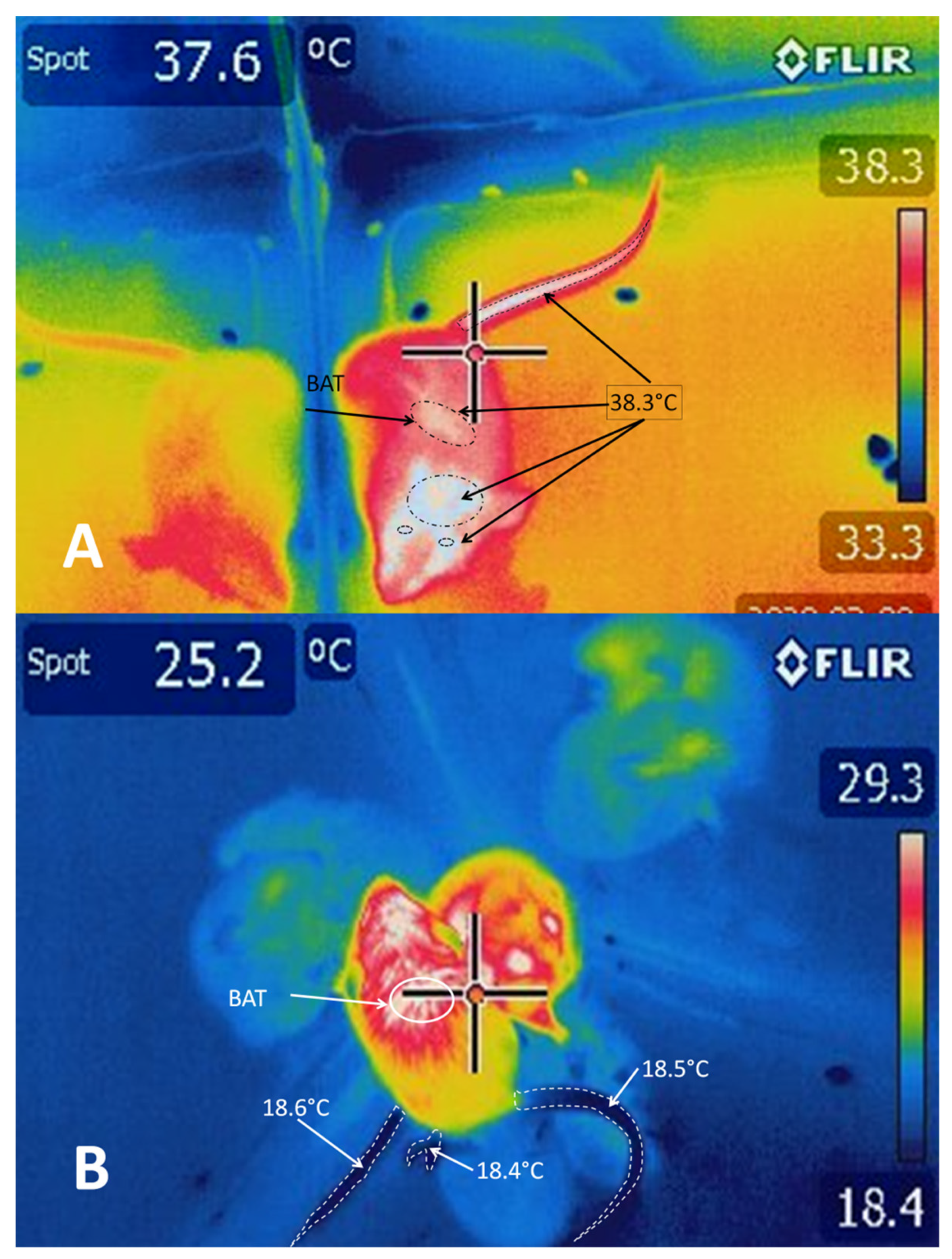
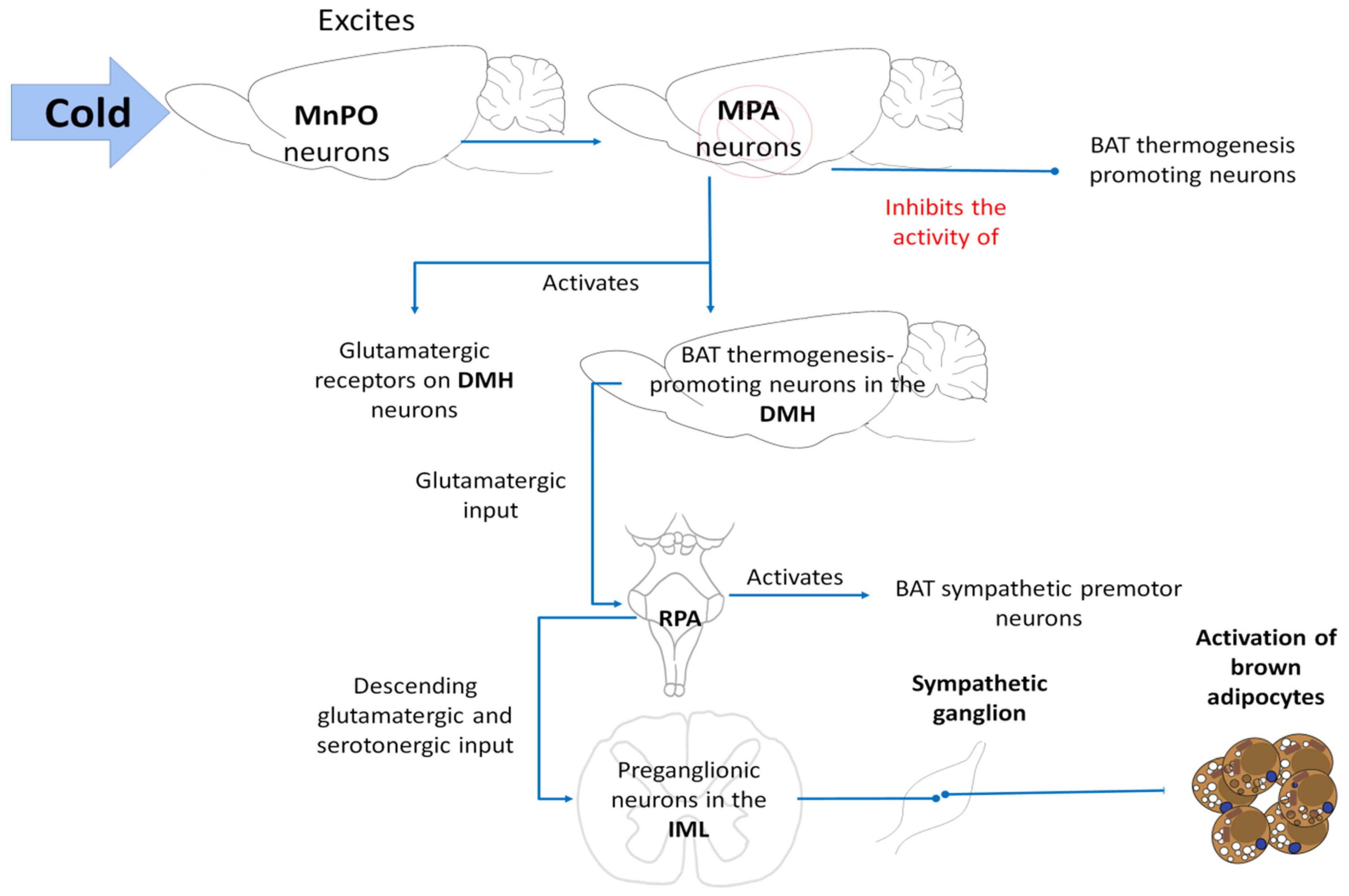
5.2. BAT Thermogenesis
5.3. Shivering
6. Thermoregulating Behavior in Mammals
7. Areas of Opportunity and Practical Applications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Morrison, S.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 2011, 589, 3641–3658. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Hao, S.; Zhang, M.; Zhang, X.; Wang, D. Aquaporins, evaporative water loss and thermoregulation in heat-acclimated Mongolian gerbils (Meriones unguiculatus). J. Therm. Biol. 2020, 91, 102641. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Bo, T.; Zhang, X.; Wang, Z.; Wang, D. Thermo-TRPs and gut microbiota are involved in thermogenesis and energy metabolism during low temperature exposure of obese mice. J. Exp. Biol. 2020, 223, 218974. [Google Scholar] [CrossRef]
- Pereira, A.M.F.; Titto, E.A.L.; Almeida, J.A.A. Adaptation of Ruminants to Hot Climates; Appris: Curitiba, Brazil, 2019. [Google Scholar]
- Ward, D. The Biology of Desert; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Terrien, J. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef]
- Legendre, L.J.; Davesne, D. The evolution of mechanisms involved in vertebrate endothermy. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190136. [Google Scholar] [CrossRef] [Green Version]
- Klein, B.G. Cunningham: Fisiología Veterinaria, 5th ed.; Elsevier: Madrid, Spain, 2013. [Google Scholar]
- Fuller-Jackson, J.-P.; Clarke, I.J.; Henry, B.A. Chapter 12: Animal Models for Manipulation of Thermogenesis. In Animal Models for the Study of Human Disease; Conn, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 281–312. [Google Scholar]
- Shilco, P.; Roitblat, Y.; Buchris, N.; Hanai, J.; Cohensedgh, S.; Frig-Levinson, E.; Burger, J.; Shterenshis, M. Normative surface skin temperature changes due to blood redistribution: A prospective study. J. Therm. Biol. 2019, 80, 82–88. [Google Scholar] [CrossRef]
- Takahashi, T.M.; Sunagawa, G.A.; Soya, S.; Abe, M.; Sakurai, K.; Ishikawa, K.; Yanagisawa, M.; Hama, H.; Hasegawa, E.; Miyawaki, A.; et al. A discrete neuronal circuit induces a hibernation-like state in rodents. Nat. Cell Biol. 2020, 583, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Carlton, P.L.; Marks, R.A. Cold Exposure and Heat Reinforced Operant Behavior. Science 1958, 128, 1344. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Laties, V.G. Behavioral Thermoregulation: Behavior is a remarkably sensitive mechanism in the regulation of body temperature. Science 1961, 133, 1338–1344. [Google Scholar] [CrossRef]
- Batchelder, P.; Kinney, R.O.; Demlow, L.; Lynch, C.B. Effects of temperature and social interactions on huddling behavior in Mus musculus. Physiol. Behav. 1983, 31, 97–102. [Google Scholar] [CrossRef]
- Wang, T.A.; Teo, C.F.; Åkerblom, M.; Chen, C.; Fontaine, M.T.-L.; Greiner, V.J.; Diaz, A.; McManus, M.T.; Jan, Y.N.; Jan, L.Y. Thermoregulation via Temperature-Dependent PGD2 Production in Mouse Preoptic Area. Neuron 2019, 103, 309–322.e7. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, S.; Leng, J.; Xu, S.; Tang, S.; Liu, C.; Gao, Y.; Mao, H. Impacts of shade on physiological and behavioural pattern of Dehong buffalo calves under high temperature. Appl. Anim. Behav. Sci. 2016, 177, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Van De Ven, T.M.F.N.; Fuller, A.; Clutton-Brock, T.H. Effects of climate change on pup growth and survival in a cooperative mammal, the meerkat. Funct. Ecol. 2020, 34, 194–202. [Google Scholar] [CrossRef]
- Becerril-Herrera, M.; Alonso-Spilsbury, M.; Lemus-Flores, C.; Guerrero-Legarreta, I.; Olmos-Hernández, A.; Ramírez-Necoechea, R.; Mota-Rojas, D. CO2 stunning may compromise swine welfare compared with electrical stunning. Meat Sci. 2009, 81, 233–237. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Becerril-Herrera, M.; Roldan-Santiago, P.; Alonso-Spilsbury, M.; Flores-Peinado, S.; Ramírez-Necoechea, R.; Ramírez-Telles, J.; Mora-Medina, P.; Pérez, M.; Molina, E.; et al. Effects of long distance transportation and CO2 stunning on critical blood values in pigs. Meat Sci. 2012, 90, 893–898. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Marai, I.; Haeeb, A. Buffalo’s biological functions as affected by heat stress—A review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Miranda-Cortés, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato-Funes, L.; Hernández-Ávalos, I. Neurobiología y modulación de la hipertermia inducida por estrés agudo y fiebre en los animales. Abanico Veter. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Ghezzi, M.D.; Napolitano, F.; Rosmini, M.R.; Guerrero-Legarreta, I.; Martínez-Burnes, J.; Lezama-García, K.; Miranda-Cortés, A.; de la Vega, L.T.; Mora-Medina, P.; et al. Quality of death in the river buffalo (Bubalus bubalis). J. Anim. Behav. Biometeorol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal biology in river buffalo in the humid tropics: Neurophysiological and behavioral responses assessed by infrared thermography. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Bertoni, A.; Napolitano, F.; Mota-Rojas, D.; Sabia, E.; Álvarez-Macías, A.; Mora-Medina, P.; Morales-Canela, A.; Berdugo-Gutiérrez, J.; Legarreta, I.G.-; Agrarie, F.S.D.S. Similarities and Differences between River Buffaloes and Cattle: Health, Physiological, Behavioral and Productivity Aspects. J. Buffalo Sci. 2020, 9, 92–109. [Google Scholar] [CrossRef]
- Lendez, P.A.; Cuesta, L.M.; Farias, M.V.N.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Alterations in TNF-α and its receptors expression in cows undergoing heat stress. Veter. Immunol. Immunopathol. 2021, 235, 110232. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.A.; Garcia, A.R.; Filho, S.T.R.; da Silva, J.A.R.; de Melo, D.N.; Guimarães, T.C.; Tavares, H.R.; Silva, T.V.G.; de Souza, E.B.; Santos, S.D.S.D.; et al. Scrotal thermoregulation and sequential sperm abnormalities in buffalo bulls (Bubalus bubalis) under short-term heat stress. J. Therm. Biol. 2021, 96, 102842. [Google Scholar] [CrossRef]
- Sevegnani, K.B.; Fernandes, D.P.B.; Da Silva, S.H.M.-G. Evaluation of thermorregulatory capacity of dairy buffaloes using infrared thermography. Engenharia Agrícola 2016, 36, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lendez, P.A.; Farias, M.V.N.; Cuesta, L.M.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Heat Stress: Its Effect on the Immune Status of Dairy Cows. Rev. Medica Vet. 2020, 101, 7–13. [Google Scholar]
- Flores-Peinado, S.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Mora-Medina, P.; Cruz-Monterrosa, R.; Gómez-Prado, J.; Hernández, M.G.; Cruz-Playas, J.; Martínez-Burnes, J. Physiological responses of pigs to preslaughter handling: Infrared and thermal imaging applications. Int. J. Veter. Sci. Med. 2020, 8, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Caldara, F.R.; Dos Santos, L.S.; Machado, S.T.; Moi, M.; Nääs, I.D.A.; Foppa, L.; Garcia, R.G.; Santos, R.D.K.S.D. Piglets’ Surface Temperature Change at Different Weights at Birth. Asian Australas. J. Anim. Sci. 2014, 27, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Yáñez, P.A.; Mota-Rojas, D.; Ramírez-Necoechea, R.; Castillo-Rivera, M.; Roldan, P.; Mora-Medina, P.; González, M. Application of infrared thermography to assess the effect of different types of environmental enrichment on the ocular, auricular pavilion and nose area temperatures of weaned piglets. Comput. Electron. Agric. 2019, 156, 33–42. [Google Scholar] [CrossRef]
- Weschenfelder, A.V.; Saucier, L.; Maldague, X.; Rocha, L.M.; Schaefer, A.L.; Faucitano, L. Use of infrared ocular thermography to assess physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci. 2013, 95, 616–620. [Google Scholar] [CrossRef]
- Pérez-Pedraza, E.; Mota-Rojas, D.; González-Lozano, M.; Guerrero-Legarreta, I.; Martinez-Burnes, J.; Mora-Medina, P.; Cruz-Monterrosa, R.; Ramírez-Necoechea, R. Infrared Thermography and Metabolic Changes in Castrated Piglets due to the Effects of Age and the Number of Incisions in the Testicles. Am. J. Anim. Veter. Sci. 2018, 13, 104–114. [Google Scholar] [CrossRef]
- Andersen, I.L.; Berg, S.; Bøe, K.E.; Edwards, S. Positive handling in late pregnancy and the consequences for maternal behaviour and production in sows. Appl. Anim. Behav. Sci. 2006, 99, 64–76. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of Human-Animal Relationship on Animal Productivity and Welfare. J. Anim. Behav. Biometeorol. 2020, 8, 196–205. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Ghezzi, M.; Hernández-Ávalos, I.; Mora-Medina, P.; Whittaker, A. Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation. Animals 2021, 11, 1085. [Google Scholar] [CrossRef]
- Khongdee, T.; Sripoon, S.; Vajrabukka, C. The effects of high temperature and wallow on physiological responses of swamp buffaloes (Bubalus bubalis) during winter season in Thailand. J. Therm. Biol. 2011, 36, 417–421. [Google Scholar] [CrossRef]
- Narayan, E.; Perakis, A.; Meikle, W. Using Thermal Imaging to Monitor Body Temperature of Koalas (Phascolarctos cinereus) in A Zoo Setting. Animals 2019, 9, 1094. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Legarreta, I.; Napolitano, F.; Mota-Rojas, D.; Orihuela, A. El Búfalo de Agua en las Américas, Enfoques Prácticos y Experimentales, 2nd ed.; BM Editores: Mexico City, Mexico, 2019; pp. 1–881. [Google Scholar]
- Napolitano, F.; Mota-Rojas, D.; Guerrero Legarreta, I.; Orihuela, A. The Latin American River Buffalo, Recent Findings, 3rd ed.; BM Editores Press: Mexico City, Mexico, 2020; pp. 1–1558. Available online: https://www.lifescienceglobal.com/journals/journal-of-buffalo-science/97-abstract/jbs/4550-el-bufalo-de-agua-en-latinoamerica-hallazgos-recientes (accessed on 14 January 2021).
- Tortora, G.J.; Derrickson, B. Principios de Anatomía y Fisiología, 13th ed.; Editorial Médica Panamericana: Madrid, Spain, 2013; pp. 1048–1051. [Google Scholar]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Chapter 1: Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- McGowan, N.E.; Scantlebury, D.M.; Bennett, N.C.; Maule, A.G.; Marks, N.J. Thermoregulatory differences in African mole-rat species from disparate habitats: Responses and limitations. J. Therm. Biol. 2020, 88, 102495. [Google Scholar] [CrossRef]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; HogenEsch, J.B.; McIntyre, P.; et al. A Heat-Sensitive TRP Channel Expressed in Keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel that Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.-H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nat. Cell Biol. 2016, 536, 460–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, K. Central Mechanisms for Thermoregulation in a Hot Environment. Ind. Heal. 2006, 44, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Plotczyk, M.; Higgins, C.A. Skin biology. In Biomaterials for Skin Repair and Regeneration; Woodhead Publishing: Sawston, UK, 2019; pp. 3–25. [Google Scholar]
- Liu, Y.; Ma, Q. Generation of somatic sensory neuron diversity and implications on sensory coding. Curr. Opin. Neurobiol. 2011, 21, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, A.D.; Krout, K.; Andrew, D. Quantitative Response Characteristics of Thermoreceptive and Nociceptive Lamina I Spinothalamic Neurons in the Cat. J. Neurophysiol. 2001, 86, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.A.; Minson, C.T. Chapter 12: Cutaneous active vasodilation as a heat loss thermoeffector. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 193–205. [Google Scholar]
- Yahiro, T.; Kataoka, N.; Nakamura, Y.; Nakamura, K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaun, F.M. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104. [Google Scholar] [CrossRef] [Green Version]
- Charkoudian, N. Skin Blood Flow in Adult Human Thermoregulation: How It Works, When It Does Not, and Why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Mallette, M.M.; Hodges, G.J.; McGarr, G.; Gabriel, D.A.; Cheung, S.S. Spectral analysis of reflex cutaneous vasodilatation during passive heat stress. Microvasc. Res. 2017, 111, 42–48. [Google Scholar] [CrossRef] [PubMed]
- DiMario, F.J.; Burleson, J.A. Cutaneous blood flow and thermoregulation in prader-willi syndrome patients. Pediatr. Neurol. 2002, 26, 130–133. [Google Scholar] [CrossRef]
- Drake, R.L.; Vogl, W.; Mitchell, A.M. Gray: Anatomía Básica, 2nd ed.; Elsevier: Madrid, Spain, 2018. [Google Scholar]
- Nakayama, T. Thermosensitive neurons in the brain. Jpn. J. Physiol. 1985, 35, 375–389. [Google Scholar] [CrossRef]
- Kanosue, K.; Crawshaw, L.I.; Nagashima, K.; Yoda, T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 109, 5–11. [Google Scholar] [CrossRef]
- Muta, K.; Matsen, M.E.; Acharya, N.K.; Stefanovski, D.; Bergman, R.N.; Schwartz, M.W.; Morton, G.J. Glucoregulatory responses to hypothalamic preoptic area cooling. Brain Res. 2019, 1710, 136–145. [Google Scholar] [CrossRef]
- Yu, S.; Qualls-Creekmore, E.; Rezai-Zadeh, K.; Jiang, Y.; Berthoud, H.-R.; Morrison, C.; Derbenev, A.V.; Zsombok, A.; Münzberg, H. Glutamatergic Preoptic Area Neurons That Express Leptin Receptors Drive Temperature-Dependent Body Weight Homeostasis. J. Neurosci. 2016, 36, 5034–5046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.D.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Kashio, M.; Tominaga, M. The TRPM2 channel: A thermo-sensitive metabolic sensor. Channels 2017, 11, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Schlader, Z.J.; Coleman, G.L.; Sackett, J.R.; Sarker, S.; Chapman, C.L.; Johnson, B.D. Activation of autonomic thermoeffectors preceding the decision to behaviourally thermoregulate in resting humans. Exp. Physiol. 2016, 101, 1218–1229. [Google Scholar] [CrossRef] [Green Version]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Kamm, G.B.; Siemens, J. The TRPM2 channel in temperature detection and thermoregulation. Temperature 2016, 4, 21–23. [Google Scholar] [CrossRef] [Green Version]
- Romanovsky, A.A.; Almeida, M.C.; Garami, A.; Steiner, A.A.; Norman, M.H.; Morrison, S.F.; Nakamura, K.; Burmeister, J.J.; Nucci, T.B. The Transient Receptor Potential Vanilloid-1 Channel in Thermoregulation: A Thermosensor It Is Not. Pharmacol. Rev. 2009, 61, 228–261. [Google Scholar] [CrossRef]
- Oka, T. Stress-induced hyperthermia and hypothermia. Handb. Clin. Neurol. 2018, 157, 599–621. [Google Scholar] [CrossRef]
- Kathryn, E.S.; Haynes, K.; Jackson, A.C. Diagnosis of hypothermia in the European hedgehog, Erinaceus europaeus, using infrared thermography. J. Therm. Biol. 2020, 90, 102574. [Google Scholar] [CrossRef]
- Smith, C.J.; Johnson, J.M. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton. Neurosci. 2016, 196, 25–36. [Google Scholar] [CrossRef]
- Ott, I. The Heat Center in the Brain. J. Nerv. Ment. Dis. 1887, 14, 152–162. [Google Scholar] [CrossRef]
- Gisolfi, C.V.; Owen, M.D.; Wall, P.; Kregel, K.C. Effects of changing hypothalamic temperature on eccrine sweating in the patas monkey. Brain Res. Bull. 1988, 20, 179–182. [Google Scholar] [CrossRef]
- Wu, Y.; Nieuwenhoff, M.; Huygen, F.; van der Helm, F.; Niehof, S.; Schouten, A. Characterizing human skin blood flow regulation in response to different local skin temperature perturbations. Microvasc. Res. 2017, 111, 96–102. [Google Scholar] [CrossRef]
- Hodges, G.J.; Kosiba, W.A.; Zhao, K.; Johnson, J.M. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am. J. Physiol. Circ. Physiol. 2009, 296, H51–H56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Nagashima, K.; McAllen, R.M.; Kanosue, K. Role of the medullary raphé in thermoregulatory vasomotor control in rats. J. Physiol. 2002, 540, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ootsuka, Y.; McAllen, R.M. Interactive drives from two brain stem premotor nuclei are essential to support rat tail sympathetic activity. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R1107–R1115. [Google Scholar] [CrossRef] [Green Version]
- Ootsuka, Y.; Tanaka, M. Control of cutaneous blood flow by central nervous system. Temperature 2015, 2, 392–405. [Google Scholar] [CrossRef]
- Sugenoya, J.; Iwase, S.; Mano, T.; Sugiyama, Y.; Ogawa, T.; Nishiyama, T.; Nishimura, N.; Kimura, T. Vasodilator component in sympathetic nerve activity destined for the skin of the dorsal foot of mildly heated humans. J. Physiol. 1998, 507, 603–610. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Wendelschafer-Crabb, G.; Brelje, T.C. Innervation and vasculature of human sweat glands: An immunohistochemistry-laser scanning confocal fluorescence microscopy study. J. Neurosci. 1994, 14, 6825–6833. [Google Scholar] [CrossRef]
- Gagnon, D.; Crandall, C.G. Chapter 13: Sweating as a heat loss thermoeffector. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 211–232. [Google Scholar]
- Shafton, A.D.; McAllen, R.M. Location of cat brain stem neurons that drive sweating. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R804–R809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, J.-Å.; Molokwu, M.N.; Olsson, O. Body Temperature Regulation in Hot Environments. PLoS ONE 2016, 11, e0161481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kpodo, K.R.; Duttlinger, A.W.; Radcliffe, J.S.; Johnson, J.S. Time course determination of the effects of rapid and gradual cooling after acute hyperthermia on body temperature and intestinal integrity in pigs. J. Therm. Biol. 2020, 87, 102481. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Kellogg, D.L.; Liu, Y.; McAllister, K.; Friel, C.; Pergola, P.E. Bradykinin does not mediate cutaneous active vasodilation during heat stress in humans. J. Appl. Physiol. 2002, 93, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, S.; Kuwahara, T.; Zhang, Y.; Koga, S.; Inoue, Y.; Kondo, N. Intensity-dependent thermoregulatory responses at the onset of dynamic exercise in mildly heated humans. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R200–R207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibasaki, M.; Kondo, N.; Crandall, C.G. Evidence for metaboreceptor stimulation of sweating in normothermic and heat-stressed humans. J. Physiol. 2001, 534, 605–611. [Google Scholar] [CrossRef]
- Nybo, L. Hyperthermia and fatigue. J. Appl. Physiol. 2008, 104, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Tanda, G. Skin temperature measurements by infrared thermography during running exercise. Exp. Therm. Fluid Sci. 2016, 71, 103–113. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Kaneps, A.J.; Geor, R.J. Equine Sports Medicine and Surgery; Elsevier: London, UK, 2004; pp. 1–1364. [Google Scholar]
- Soroko, M.; Howell, K.; Dudek, K.; Wilk, I.; Zastrzeżyńska, M.; Janczarek, I. A Pilot Study into the Utility of Dynamic Infrared Thermography for Measuring Body Surface Temperature Changes During Treadmill Exercise in Horses. J. Equine Veter. Sci. 2018, 62, 44–46. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Youngblood, J.P.; Neel, L.K.; VandenBrooks, J.M. The neuroscience of adaptive thermoregulation. Neurosci. Lett. 2019, 692, 127–136. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.L.; Cooke, E.K.; Leib, D.; Lin, Y.-C.; Daly, G.E.; Zimmerman, C.A.; Knight, Z.A. Warm-Sensitive Neurons that Control Body Temperature. Cell 2016, 167, 47–59.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, S.M.; Schafe, G.E.; LeDoux, J.E. Molecular Mechanisms Underlying Emotional Learning and Memory in the Lateral Amygdala. Neuron 2004, 44, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianna, D.M.L.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Avalos, I.; Flores-Gasca, E.; Mota-Rojas, D.; Casas-Alvarado, A.; Miranda-Cortés, A.E.; Domínguez-Oliva, A. Neurobiology of anesthetic-surgical stress and induced behavioral changes in dogs and cats: A review. Veter. World 2021, 14, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Larrondo, C.; Orihuela, A.; Strappini, A.; Acosta-Jamett, G.; Mota-Rojas, D.; Gallo, C. Provision of straw and the presence of undocked lambs reduce the behavioural and physiological expressions of pain and stress associated with tail docking in lambs: A preliminary study. Anim. Prod. Sci. 2021, 61, 423. [Google Scholar] [CrossRef]
- Lezama-García, K. Facial expressions and emotions in domestic animals. CAB Rev. Perspect. Agric. Veter. Sci. Nutr. Nat. Resour. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Tanaka, M.; McKinley, M.J.; McAllen, R.M. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1248–R1257. [Google Scholar] [CrossRef] [Green Version]
- Abbott, S.B.G.; Saper, C.B. Median preoptic glutamatergic neurons promote thermoregulatory heat loss and water consumption in mice. J. Physiol. 2017, 595, 6569–6583. [Google Scholar] [CrossRef]
- Blessing, W.W.; Yu, Y.H.; Nalivaiko, E. Raphe pallidus and parapyramidal neurons regulate ear pinna vascular conductance in the rabbit. Neurosci. Lett. 1999, 270, 33–36. [Google Scholar] [CrossRef]
- Nakamura, K.; Matsumura, K.; Kaneko, T.; Kobayashi, S.; Katoh, H.; Negishi, M. The Rostral Raphe Pallidus Nucleus Mediates Pyrogenic Transmission from the Preoptic Area. J. Neurosci. 2002, 22, 4600–4610. [Google Scholar] [CrossRef] [Green Version]
- Rathner, J.A.; Madden, C.J.; Morrison, S.F. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R343–R354. [Google Scholar] [CrossRef]
- Tanaka, M.; McKinley, M.J.; McAllen, R.M. Preoptic-Raphe Connections for Thermoregulatory Vasomotor Control. J. Neurosci. 2011, 31, 5078–5088. [Google Scholar] [CrossRef] [Green Version]
- McAllen, R.M.; May, C.N. Effects of preoptic warming on subretrofacial and cutaneous vasoconstrictor neurons in anaesthetized cats. J. Physiol. 1994, 481, 719–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ootsuka, Y.; Terui, N. Functionally different neurons are organized topographically in the rostral ventrolateral medulla of rabbits. J. Auton. Nerv. Syst. 1997, 67, 67–78. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central Control of Brown Adipose Tissue Thermogenesis. Front. Endocrinol. 2012, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 2016, 196, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, S.F.; Sved, A.F.; Passerin, A.M. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R290–R297. [Google Scholar] [CrossRef]
- Zaretskaia, M.V.; Zaretsky, D.V.; Shekhar, A.; DiMicco, J.A. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002, 928, 113–125. [Google Scholar] [CrossRef]
- Kataoka, N.; Hioki, H.; Kaneko, T.; Nakamura, K. Psychological Stress Activates a Dorsomedial Hypothalamus-Medullary Raphe Circuit Driving Brown Adipose Tissue Thermogenesis and Hyperthermia. Cell Metab. 2014, 20, 346–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madden, C.J.; Morrison, S.F. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R320–R325. [Google Scholar] [CrossRef]
- Zhang, Y.; Kerman, I.A.; Laque, A.; Nguyen, P.; Faouzi, M.; Louis, G.W.; Jones, J.C.; Rhodes, C.; Münzberg, H. Leptin-Receptor-Expressing Neurons in the Dorsomedial Hypothalamus and Median Preoptic Area Regulate Sympathetic Brown Adipose Tissue Circuits. J. Neurosci. 2011, 31, 1873–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, G.T.; Worth, A.A.; Nunn, N.; Korpal, A.K.; Bechtold, D.A.; Allison, M.B.; Myers, M.G.; Statnick, M.A.; Luckman, S.M. The Thermogenic Effect of Leptin Is Dependent on a Distinct Population of Prolactin-Releasing Peptide Neurons in the Dorsomedial Hypothalamus. Cell Metab. 2014, 20, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seoane-Collazo, P.; Martínez-Sánchez, N.; Milbank, E.; Contreras, C. Incendiary Leptin. Nutrients 2020, 12, 472. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.X.; D’Souza, S.; Upton, B.A.; Kernodle, S.; Vemaraju, S.; Nayak, G.; Gaitonde, K.D.; Holt, A.L.; Linne, C.D.; Smith, A.N.; et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nat. Cell Biol. 2020, 585, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.L.; Abbott, S.B.; Resch, J.M.; Zhu, L.; Arrigoni, E.; Lowell, B.B.; Fuller, P.M.; Fontes, M.A.; Saper, C.B. A Glutamatergic Hypothalamomedullary Circuit Mediates Thermogenesis, but Not Heat Conservation, during Stress-Induced Hyperthermia. Curr. Biol. 2018, 28, 2291–2301.e5. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.T.; Andersson, B.; Gale, C. Central Control of Cold Defense Mechanisms and the Release of “Endopyrogen” in the Goat. Acta Physiol. Scand. 1962, 54, 159–174. [Google Scholar] [CrossRef]
- Hammel, H.T.; Hardy, J.D.; Fusco, M.M. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am. J. Physiol. Content 1960, 198, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.; Kawamura, Y.; Hemingway, A. Activation and suppression of shivering during septal and hypothalamic stimulation. Exp. Neurol. 1961, 4, 485–506. [Google Scholar] [CrossRef]
- Tanaka, M.; Owens, N.C.; Nagashima, K.; Kanosue, K.; McAllen, R.M. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphé. J. Physiol. 2006, 572, 569–583. [Google Scholar] [CrossRef]
- Wright, P. Why do elephants flap their ears? South Afr. J. Zool. 1984, 19, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Phillips, P.; Heath, J. Heat loss in Dumbo: A theoretical approach. J. Therm. Biol. 2001, 26, 117–120. [Google Scholar] [CrossRef]
- Cabanac, M.; Serres, P. Peripheral heat as a reward for heart rate response in the curarized rat. J. Comp. Physiol. Psychol. 1976, 90, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D. Functional architecture of behavioural thermoregulation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.D.; Kwan, C.L.; Crawley, A.P.; Mikulis, D.J. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J. Neurophysiol. 1998, 80, 1533–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, M.C.; Vizin, R.C.L.; Carrettiero, D.C. Current understanding on the neurophysiology of behavioral thermoregulation. Temperature 2015, 2, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanosue, K.; Nakayama, T.; Tanaka, H.; Yanase, M.; Yasuda, H. Modes of action of local hypothalamic and skin thermal stimulation on salivary secretion in rats. J. Physiol. 1990, 424, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Magoun, H.W.; Harrison, F.; Brobeck, J.R.; Ranson, S.W. Activation of heat loss mechanisms by local heating of the brain. J. Neurophysiol. 1938, 1, 101–114. [Google Scholar] [CrossRef]
- Whyte, D.G.; Johnson, A.K. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (av3v) during acute heat stress in the rat. Clin. Exp. Pharmacol. Physiol. 2005, 32, 457–461. [Google Scholar] [CrossRef]
- Stricker, E.M.; Hainsworth, F.R. Evaporative cooling in the rat: Effects of hypothalamic lesions and chorda tympani damage. Can. J. Physiol. Pharmacol. 1970, 48, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Flynn, F.W.; Evey, L.A.; Mitchell, J.C. Heat-induced saliva secretion and thermoregulation in female rats with ventromedial hypothalamic lesions. Physiol. Behav. 1981, 26, 779–782. [Google Scholar] [CrossRef]
- Saper, C.; Loewy, A. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980, 197, 291–317. [Google Scholar] [CrossRef]
- Tanaka, H.; Kanosue, K.; Nakayama, T.; Shen, Z. Grooming, body extension, and vasomotor responses induced by hypothalamic warming at different ambient temperatures in rats. Physiol. Behav. 1986, 38, 145–151. [Google Scholar] [CrossRef]
- Roberts, W.W.; Mooney, R.D. Brain areas controlling thermoregulatory grooming, prone extension, locomotion, and tail vasodilation in rats. J. Comp. Physiol. Psychol. 1974, 86, 470–480. [Google Scholar] [CrossRef]
- Roberts, W.W.; Martin, J.R. Effects of lesions in central thermosensitive areas on thermoregulatory responses in rat. Physiol. Behav. 1977, 19, 503–511. [Google Scholar] [CrossRef]
- Whyte, D.G.; Brennan, T.J.; Johnson, A.K. Thermoregulatory behavior is disrupted in rats with lesions of the anteroventral third ventricular area (AV3V). Physiol. Behav. 2006, 87, 493–499. [Google Scholar] [CrossRef]
- Almeida, M.C.; Steiner, A.A.; Branco, L.G.S.; Romanovsky, A.A. Neural Substrate of Cold-Seeking Behavior in Endotoxin Shock. PLoS ONE 2006, 1, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlisle, H.J. Effect of preoptic and anterior hypothalamic lesions on behavioral thermoregulation in the cold. J. Comp. Physiol. Psychol. 1969, 69, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D.; Bushnell, M.C.; Zhang, E.-T.; Blomqvist, A. A thalamic nucleus specific for pain and temperature sensation. Nat. Cell Biol. 1994, 372, 770–773. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Wanner, S.P.; Almeida, M.C.; Shimansky, Y.P.; Oliveira, D.L.; Eales, J.R.; Coimbra, C.C.; Romanovsky, A.A. Cold-Induced Thermogenesis and Inflammation-Associated Cold-Seeking Behavior Are Represented by Different Dorsomedial Hypothalamic Sites: A Three-Dimensional Functional Topography Study in Conscious Rats. J. Neurosci. 2017, 37, 6956–6971. [Google Scholar] [CrossRef]
- Konishi, M.; Kanosue, K.; Kano, M.; Kobayashi, A.; Nagashima, K. The median preoptic nucleus is involved in the facilitation of heat-escape/cold-seeking behavior during systemic salt loading in rats. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R150–R159. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Dallman, M.F. Hypothalamic Obesity: Multiple Routes Mediated by Loss of Function in Medial Cell Groups1. Endocrinology 1999, 140, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. https://doi.org/10.3390/ani11061733
Mota-Rojas D, Titto CG, Orihuela A, Martínez-Burnes J, Gómez-Prado J, Torres-Bernal F, Flores-Padilla K, Carvajal-de la Fuente V, Wang D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals. 2021; 11(6):1733. https://doi.org/10.3390/ani11061733
Chicago/Turabian StyleMota-Rojas, Daniel, Cristiane Gonçalves Titto, Agustín Orihuela, Julio Martínez-Burnes, Jocelyn Gómez-Prado, Fabiola Torres-Bernal, Karla Flores-Padilla, Verónica Carvajal-de la Fuente, and Dehua Wang. 2021. "Physiological and Behavioral Mechanisms of Thermoregulation in Mammals" Animals 11, no. 6: 1733. https://doi.org/10.3390/ani11061733
APA StyleMota-Rojas, D., Titto, C. G., Orihuela, A., Martínez-Burnes, J., Gómez-Prado, J., Torres-Bernal, F., Flores-Padilla, K., Carvajal-de la Fuente, V., & Wang, D. (2021). Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals, 11(6), 1733. https://doi.org/10.3390/ani11061733








