Female Scent Activated Expression of Arginase1 and Inducible NO-Synthetase in Lung of BALB/c Male Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing Condition of Mice
2.2. Female Bedding Collection
2.3. Experimental Groups
2.4. Sniffing Test to Female Scent
2.5. Cytometry of Blood Cells
2.6. Testosterone and Corticosterone Immunoassays
2.7. Expression of Nos2, Arg1, and Foxp3 in Lungs
2.8. Statistical Analysis
3. Results
3.1. The Effect of Female Scent on Endocrine and Behavioral Factors of Males
3.2. The Effect of Female Scent on the Number of White Blood Cells
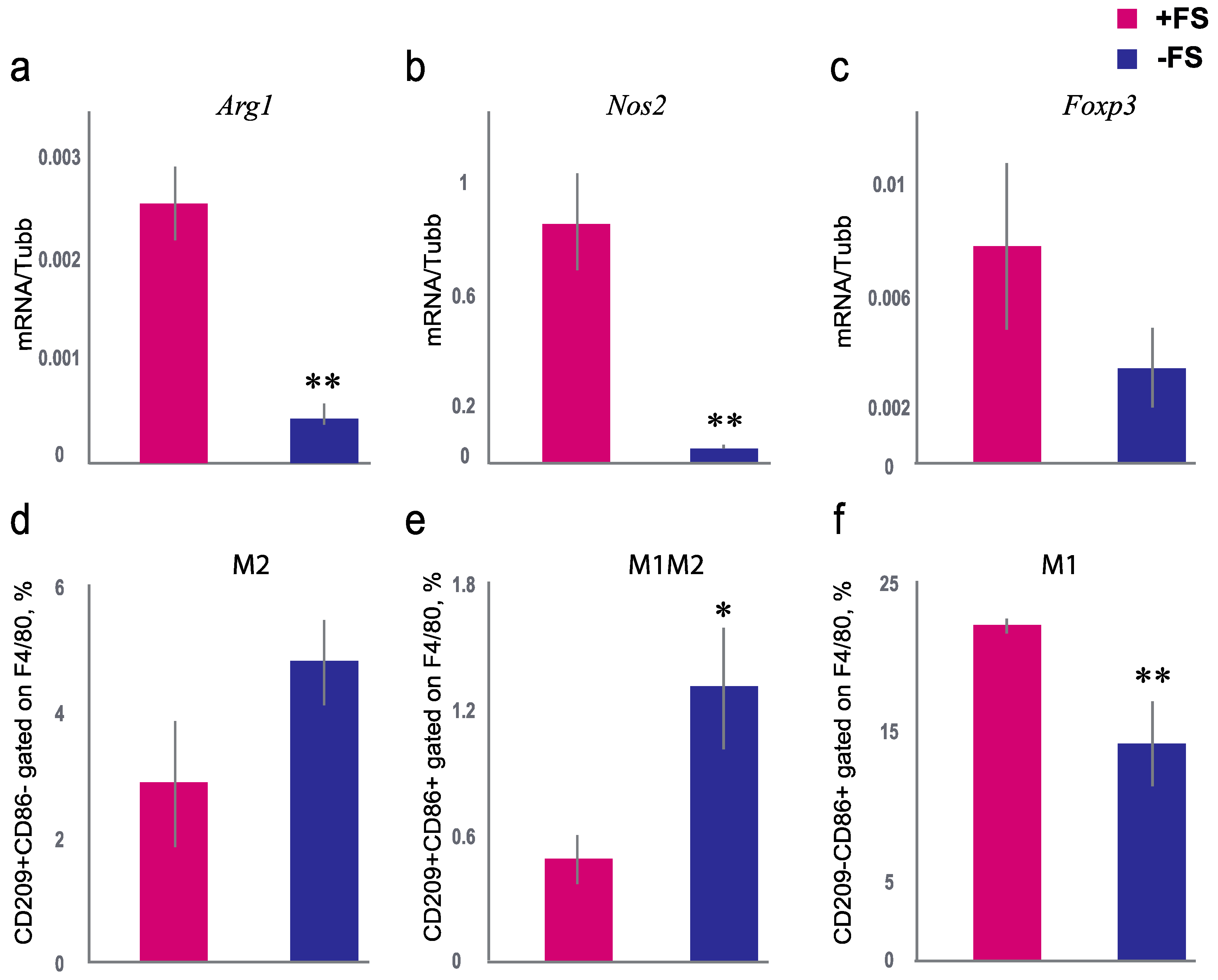
3.3. The Effect of Female Scent on the Expression of Nos2, Arg1, and Foxp3 in Lungs and the Percentage of M1 and M2 Macrophages in Bronchoalveolar lavage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apfelbach, R.; Blanchard, C.D.; Blanchard, R.J.; Hayes, R.A.; McGregor, I.S. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci. Biobehav. Rev. 2005, 29, 1123–1144. [Google Scholar] [CrossRef]
- Vandenbergh, J.G. Male odor accelerates female sexual maturation in mice. Endocrinology 1969, 84, 658–660. [Google Scholar] [CrossRef]
- Jemiolo, B.; Novotny, M. Inhibition of sexual maturation in juvenile female and male mice by a chemosignal of female origin. Physiol. Behav. 1994, 55, 519–522. [Google Scholar] [CrossRef]
- Petrulis, A. Chemosignals and hormones in the neural control of mammalian sexual behavior. Front. Neuroendocr. 2013, 34, 255–267. [Google Scholar] [CrossRef]
- Surinov, B.P.; Isaeva, V.G.; Tokarev, O.U. Allelopathic activity of volatile secretions of irradiated animals. Radiats. Biol. Radioecol. 2001, 6, 645–649. [Google Scholar]
- Moshkin, M.P.; Kolosova, I.E.; Novikov, E.A.; Litvinova, E.A.; Mershieva, L.V.; Mak, V.V.; Petrovskii, D.V. Co-Modulation of the Immune function and the reproductive chemosignals. Asian Australas J. Anim. Sci. 2001, 14, 43–51. [Google Scholar]
- Moshkin, M.P.; Akinchina, L.V.; Kudaeva, O.T.; Kolosova, V.A.; Kozlov, V.A. The influence of female odor on immunity, endocrine status, and aggressive behavior of male laboratory mice. Immunologia 2004, 6, 350–354. [Google Scholar]
- Litvinova, E.A.; Garms, A.I.; Zaidman, A.M.; Korel, A.V.; Gerlinskaya, L.A.; Moshkin, M.P. Adaptive redistribution of immune defense in response to sexual chemosignals. Zhurnal Obs. Biol. 2009, 70, 21–29. [Google Scholar]
- Litvinova, E.A.; Moshkin, M.P.; Gerlinskaya, L.A.; Nagatomi, R.; Zhang, X.; Matsuo, K.; Shikano, S. Female scent mobilizes leukocytes to airways in BALB/c male mice. Integr. Zool. 2009, 4, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Zaas, A.K.; Schwartz, D.A. Innate immunity and the lung: Defense at the interface between host and environment. Trends Cardiovasc. Med. 2005, 15, 195–202. [Google Scholar] [CrossRef]
- Ho, A.W.; Prabhu, N.; Betts, R.J.; Ge, M.Q.; Dai, X.; Hutchinson, P.E.; Lew, F.C.; Wong, K.L.; Hanson, B.J.; Macary, P.A.; et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J. Immunol. 2011, 187, 6011–6021. [Google Scholar] [CrossRef] [Green Version]
- Conrady, C.D.; Zheng, M.; Mandal, N.A.; van Rooijen, N.; Carr, D.J. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013, 6, 45–55. [Google Scholar] [CrossRef]
- Litvinova, E.A.; Goncharova, E.P.; Zaydman, A.M.; Zenkova, M.A.; Moshkin, M.P. Female scent signals enhance the resistance of male mice to influenza. PLoS ONE 2010, 5, e9473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wensveen, F.M.; Šestan, M.; Wensveen, T.T.; Polić, B. ‘Beauty and the beast’ in infection: How immune-endocrine interactions regulate systemic metabolism in the context of infection. Eur. J. Immunol. 2019, 49, 982–995. [Google Scholar] [CrossRef] [Green Version]
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From pheromones to behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef] [PubMed]
- Dulac, C.; Axel, L. A novel family of genes encoding putative pheromone receptors in mammals. Cell 1995, 83, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Herrada, G.; Dulac, C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell 1997, 90, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Matsunami, H.; Buck, L.B. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 1997, 90, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Ryba, N.J.; Tirindelli, R. A new multigene family of putative pheromone receptors. Neuron 1997, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Leinders-Zufall, T.; Lane, A.P.; Puche, A.C.; Ma, W.; Novotny, M.V.; Shipley, M.T.; Zufall, F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 2000, 405, 792–796. [Google Scholar] [CrossRef]
- Pantages, E.; Dulac, C. A novel family of candidate pheromone receptors in mammals. Neuron 2000, 28, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Liberles, S.D.; Horowitz, L.F.; Kuang, D.; Contos, J.J.; Wilson, K.L.; Siltberg-Liberles, J.; Liberles, D.A.; Buck, L.B. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc. Natl. Acad. Sci. USA 2009, 106, 9842–9847. [Google Scholar] [CrossRef] [Green Version]
- Riviere, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Liberles, S.D.; Buck, L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature 2006, 442, 645–650. [Google Scholar] [CrossRef]
- Greenstein, B.D.; Fitzpatrick, F.T.; Adcock, I.M.; Kendall, M.D.; Wheeler, M.J. Reappearance of the thymus in old rats after orchidectomy: Inhibition of regeneration by testosterone. J. Endocrinol. 1986, 110, 417–422. [Google Scholar] [CrossRef]
- Batticane, N.; Morale, M.C.; Gallo, F.; Farinella, Z.; Marchetti, B. Luteinizing hormone-releasing hormone signaling at the lymphocyte involves stimulation of interleukin-2 receptor expression. Endocrinology 1991, 129, 277–286. [Google Scholar] [CrossRef]
- Wilson, C.A.; Mrose, S.A.; Thomas, D.W. Enhanced production of B lymphocytes after castration. Blood 1995, 85, 1535–1539. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.Y.; Muller, N.; Herold, M.J.; van den Brandt, J.; Reichardt, H.M. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 2007, 122, 47–53. [Google Scholar] [CrossRef]
- Bahr, E.C.; Yorty, J.L. Rapid decrease in CD8+ T cells following treatment of mice with exogenous corticosterone. BIOS 2013, 84, 148–157. [Google Scholar] [CrossRef]
- Kissick, H.T.; Sanda, M.G.; Dunn, L.K.; Pelleqrini, K.L.; On, S.T.; Noel, J.K.; Arredouani, M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 9887–9892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.A.; Tolbert, M.D.; Singh, S.J.; Bost, K.L. Expression of neuronal trace-amine-associated receptor (Taar) mRNAs in leukocytes. J. Neuroimmunol. 2007, 192, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bufe, B.; Schumann, T.; Zufall, F. Formyl peptide receptors from immune and vomeronasal system exhibit distinct agonist properties. J. Biol. Chem. 2012, 287, 33644–33655. [Google Scholar] [CrossRef] [Green Version]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Li, J.J.; Tay, H.L.; Plank, M.; Essilfie, A.T.; Hansbro, P.M.; Foster, P.S.; Yang, M. Activation of olfactory receptors on mouse pulmonary macrophages promotes monocyte chemotactic protein-1 production. PLoS ONE 2013, 8, e80148. [Google Scholar] [CrossRef]
- Larosa, D.F.; Orange, J.S. Lymphocytes. Allergy Clin. Immunol. 2008, 121, 364–369. [Google Scholar] [CrossRef]
- Miyoshi, J.; Leone, V.; Nobutani, K.; Musch, M.W.; Martinez-Guryn, K.; Wang, Y.; Miyoshi, S.; Bobe, A.M.; Eren, A.M.; Chang, E.B. Minimizing confounders and increasing data quality in murine models for studies of the gut microbiome. Microbiology 2018, 6, e5166. [Google Scholar] [CrossRef]
- Yu, H.; Yue, P.; Sun, P.; Zhao, X. Self-grooming induced by sexual chemical signals in male root voles (Microtus oeconomus Pallas). Behav. Process. 2010, 83, 292–298. [Google Scholar] [CrossRef]
- Litvinova, E.A.; Kontsevaya, G.V.; Kozhevnikova, E.N.; Achasova, K.M.; Gerlinskaya LAFeofanova, N.A.; Moshkin, M.P. Modification of fecal bacteria counts and blood immune cells in the offspring of BALB/c and C57BL/6 obtained by interstrain mouse embryo transfer. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S. Primer effects by conspecific odors in house mice: A new perspective in the study of primer effects on reproductive activities. Horm. Behav. 2004, 46, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.W.; Croft, N.P.; Tscharke, D.C. Immunology by numbers: Quantitation of antigen presentation completes the quantitative milieu of systems immunology! Curr. Opin. Immunol. 2016, 40, 88–95. [Google Scholar] [CrossRef]
- Odobasic, D.; Jia, Y.; Kao, W.; Fan, H.; Wei, X.; Gu, R.; Ngo, D.; Kitching, R.A.; Holdworth, S.R.; Morand, E.F.; et al. Formyl peptide receptor activation inhibits the expansion of effector T cells and synovial fibroblasts and attenuates joint injury in models of rheumatoid arthritis. Int. Immunopharmacol. 2018, 61, 140–149. [Google Scholar] [CrossRef]
- Le, Y.; Murphy, P.M.; Wang, J.M. Formyl-peptide receptors revisited. Trends Immunol. 2002, 23, 541–548. [Google Scholar] [CrossRef]
- Achasova, K.M. The effect of sex chemosignals on the immunity and microflora of male laboratory mice of the C57BL/6 and BALB/s lines. In Proceedings of the 52nd International Students Scientific Conference, Biology/Novosibirsk State University, Novosibirsk, Russian, 11–18 April 2014; p. 119, [on the Russian]. [Google Scholar]
- Migeotte, I.; Communi, D.; Parmentier, M. Formyl peptide receptors: A promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006, 17, 501–519. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Baum, M.J.; Cherry, J.A. Olfactory sex discrimination persist, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J. Neurosci. 2004, 24, 9451–9457. [Google Scholar] [CrossRef] [Green Version]
- Akaike, T.; Maeda, H. Nitric oxide and virus infection. Immunology 2000, 101, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Pesce, J.T.; Ramalingam, T.R.; Mentink-Kane, M.M.; Wilson, M.S.; El Kasmi, K.C.; Smith, A.M.; Thompson, R.W.; Cheever, A.W.; Murray, P.J.; Wynn, T.A. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009, 5, e1000371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ckless, K.; van der Vliet, A.; Janssen-Heininger, Y. Oxidative-nitrosative stress and post-translational protein modifications: Implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2007, 36, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H. Molecular targets of FoxP3+ regulatory T cells. Mini Rev. Med. Chem. 2007, 7, 1136–1143. [Google Scholar] [CrossRef]
- Moshkin, M.P.; Kontsevaya, G.V.; Litvinova, E.A.; Gerlinskaya, L.A. IL-1β-independent activation of lung immunity in male mice by female odor. Brain Behav. Immun. 2013, 30, 150–155. [Google Scholar] [CrossRef]
- D’Alessio, F.R.; Tsushima, K.; Aggarwal, N.R.; West, E.E.; Willett, M.H.; Britos, M.F.; Pipeling, M.R.; Brower, R.G.; Tuder, R.M.; McDyer, J.F.; et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Investig. 2009, 111, 2898–2913. [Google Scholar] [CrossRef] [Green Version]


| Primer Name | Sequence 5′->3′ | Target |
|---|---|---|
| Nos2_F | CAGGGTCACAACTTTACAGGGA | Mouse Nos2 |
| Nos2_R | CACTTCTGCTCCAAATCCAACG | |
| Arg1_F | TCACCTGAGCTTTGATGTCGA | Mouse Arg1 |
| Arg1_R | TGAAAGGAGCCCTGTCTTGTA | Mouse Foxp3 |
| Foxp3_F | AGAGTTTCTCAAGCACTGCCA | |
| Foxp3_R | TCCCAGCTTCTCCTTTTCCA | |
| Tubb_F | TGAAGCCACAGGTGGCAAGTAT | Mouse Tubb5 |
| Tubb_R | CCAGACTGACCGAAAACGAAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdeva, O.V.; Achasova, K.M.; Litvinova, N.A.; Kozhevnikova, E.N.; Litvinova, E.A. Female Scent Activated Expression of Arginase1 and Inducible NO-Synthetase in Lung of BALB/c Male Mice. Animals 2021, 11, 1756. https://doi.org/10.3390/ani11061756
Gvozdeva OV, Achasova KM, Litvinova NA, Kozhevnikova EN, Litvinova EA. Female Scent Activated Expression of Arginase1 and Inducible NO-Synthetase in Lung of BALB/c Male Mice. Animals. 2021; 11(6):1756. https://doi.org/10.3390/ani11061756
Chicago/Turabian StyleGvozdeva, Olga V., Kseniya M. Achasova, Nadezhda A. Litvinova, Elena N. Kozhevnikova, and Ekaterina A. Litvinova. 2021. "Female Scent Activated Expression of Arginase1 and Inducible NO-Synthetase in Lung of BALB/c Male Mice" Animals 11, no. 6: 1756. https://doi.org/10.3390/ani11061756
APA StyleGvozdeva, O. V., Achasova, K. M., Litvinova, N. A., Kozhevnikova, E. N., & Litvinova, E. A. (2021). Female Scent Activated Expression of Arginase1 and Inducible NO-Synthetase in Lung of BALB/c Male Mice. Animals, 11(6), 1756. https://doi.org/10.3390/ani11061756







