Analysis of the Gastro-Intestinal Tract of Marine Mammals: A Multidisciplinary Approach with a New Multi-Sieves Tool
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- To ensure the best practicality of use of the device;
- To ensure a fast GIT evaluation and sample collection, even in the cases of large volume of GIT content;
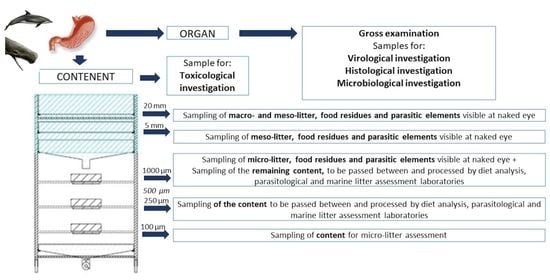
- To ensure the collection of samples suitable for the analysis in the different disciplines concerned (i.e., pathological evaluation, microbiological and virological analyses, algal biotoxin detection, diet and parasitological investigations, and marine litter presence assessment).Pathological evaluation and virological analysis are performed on the organs, microbiological analysis can be performed both on the organ or content before the separation though the sieves, algal biotoxins detection is performed on part of the content before the separation, while diet and parasitological analyses, and marine litter assessment are performed on the content. The presence of parasites that developed in the organ walls (e.g., Pholeter gastrophilus) is assessed during the pathological evaluation.
2.1. Animals and GITs Analyzed
2.2. The Multi-Sieves Tool
2.3. Protocol for a Multidisciplinary Samples Collection and Analysis of the GIT
- Thoroughly rinse the external part of the organ (i.e., stomachs or intestine);
- Weigh the organ still closed;
- Place each organ in a tank/container;
- Collect fecal sample from the rectum for copro-microscopic examination for the detection of parasitic elements (i.e., eggs, larvae, cysts and oocysts) [31];
- Sample the content and the surface of the mucosa for microbiological culture, with a swab under aseptic conditions if there is a suspect of a gastro-intestinal diseases and/or sample the tissue from any lesion suspected of microbiological origin observed during gross examination on the wall, after the removal of the content;
- Open the organ longitudinally, throughout the entire length, using scissors or scalpels;
- Collect gastric content sample for toxins detection, if marine algal biotoxins are suspected;
- Gently rinse the mucosa with current water in the tank to collect the content;
- Check and record any gross lesion and the presence of parasitic elements;
- Collect samples for histological investigations;
- Collect samples for virological analysis (molecular testing or isolation on cell culture);
- Rinse intensely the mucosa with current water to facilitate the complete exit of material from the organ and collect it in the tank;
- Weigh the organs without the content and subtract it from the weight of organ still closed to obtain the content wet weight;
- Transfer the organ contents from the tank/container into the first sieve (20 mm mesh) and rinse the material to make it proceed towards the next sieves;
- After an abundant rinse, extract the 20 mm and 5 mm sieves from the support and sample any marine litter item, parasite and alimentary residues in 3 different containers;
- Open partially the valve of the first collection tank positioned under the 5 mm sieve and made the flow continue through the round sieves;
- If marine debris, parasites and alimentary residues visible to naked eye are present in the 1000 µm, record its presence and amount and collect them;
- Collect the material present in the 1000 µm, 500 µm, 250 µm and 100 µm sieves in 4 different containers;
- These samples should be passed between and processed by all the laboratories interested in the GIT analysis, except for the 100 µm one which is exclusively for micro-litter assessment; the marine litter analysis must be the last one to be executed, due to its destructive process.
3. Results
3.1. The Multi-Sieves Tool
3.2. Protocol for a Multidisciplinary Samples Collection and Analysis of the GIT
3.3. Sample Suitability for the Investigations Concerned in the Multidisciplinary Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arbelo, M.; De Los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Jepson, P.D.; Fernández, A. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis. Aquat. Organ. 2013, 103, 87–99. [Google Scholar] [CrossRef]
- Di Guardo, G.; Di Francesco, C.E.; Eleni, C.; Cocumelli, C.; Scholl, F.; Casalone, C.; Peletto, S.; Mignone, W.; Tittarelli, C.; Di Nocera, F.; et al. Morbillivirus infection in cetaceans stranded along the Italian coastline: Pathological, immunohistochemical and biomolecular findings. Res. Vet. Sci. 2013, 94, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Plön, S.; De Wet, M.; Lane, E.; Wohlsein, P.; Siebert, U.; Thompson, P. A standardized necropsy protocol for health investigations of small cetaceans in southern Africa. Afr. J. Wildl. Res. 2015, 45, 332–341. [Google Scholar] [CrossRef]
- Van Bressem, M.F.; Raga, J.A.; Di Guardo, G.; Jepson, P.D.; Duignan, P.J.; Siebert, U.; Barrett, T.; De Oliveira Santos, M.C.; Moreno, I.B.; Siciliano, S.; et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 2009, 86, 143–157. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, O.; Paz, Y.; Zucca, D.; Groch, K.; et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Dierauf, L.; Gulland, F.M.D. CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Gulland, F.M.D.; Hall, A.J. Is marine mammal health deteriorating? Trends in the global reporting of marine mammal disease. Ecohealth 2007, 4, 135–150. [Google Scholar] [CrossRef]
- Ijsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. Best practice on cetacean post mortem investigation and tissue sampling. Jt. ACCOBAMS ASCOBANS Doc. 2019, 1–73. [Google Scholar]
- Jauniaux, T.; Petitjean, D.; Brenez, C.; Borrens, M.; Brosens, L.; Haelters, J.; Tavernier, T.; Coignoul, F. Post-mortem findings and causes of death of harbour porpoises (Phocoena phocoena) stranded from 1990 to 2000 along the coastlines of Belgium and Northern France. J. Comp. Pathol. 2002, 126, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Jepson, P.D.; Deaville, R.; Barber, J.L.; Aguilar, À.; Borrell, A.; Murphy, S.; Barry, J.; Brownlow, A.; Barnett, J.; Berrow, S.; et al. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Lane, E.P.; De Wet, M.; Thompson, P.; Siebert, U.; Wohlsein, P.; Plön, S. A systematic health assessment of Indian ocean bottlenose (Tursiops aduncus) and indo-pacific humpback (Sousa plumbea) dolphins incidentally caught in shark nets off the KwaZulu-Natal coast, South Africa. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Peltier, H.; Dabin, W.; Daniel, P.; Van Canneyt, O.; Dorémus, G.; Huon, M.; Ridoux, V. The significance of stranding data as indicators of cetacean populations at sea: Modelling the drift of cetacean carcasses. Ecol. Indic. 2012, 18, 278–290. [Google Scholar] [CrossRef]
- Panti, C.; Baini, M.; Lusher, A.; Hernandez-Milan, G.; Bravo Rebolledo, E.L.; Unger, B.; Syberg, K.; Simmonds, M.P.; Fossi, M.C. Marine litter: One of the major threats for marine mammals. Outcomes from the European Cetacean Society workshop. Environ. Pollut. 2019, 247, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Pedà, C.; Battaglia, P.; Scuderi, A.; Voliani, A.; Mancusi, C.; Andaloro, F.; Romeo, T. Cephalopod prey in the stomach contents of odontocete cetaceans stranded in the western Mediterranean Sea. Mar. Biol. Res. 2015, 11, 593–602. [Google Scholar] [CrossRef]
- Fossi, M.C.; Baini, M.; Panti, C.; Baulch, S. Impacts of Marine Litter on Cetaceans: A Focus on Plastic Pollution; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128122501. [Google Scholar]
- Fossi, M.C.; Baini, M.; Simmonds, M.P. Cetaceans as Ocean Health Indicators of Marine Litter Impact at Global Scale. Front. Environ. Sci. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Kühn, S.; van Oyen, A.; Booth, A.M.; Meijboom, A.; van Franeker, J.A. Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 2018, 213, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine mammals and microplastics: A systematic review and call for standardisation. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef]
- Philipp, C.; Unger, B.; Fischer, E.; Siebert, U. Handle with care—Detecting microplastic particles in faeces samples of seals from German waters. Sustainability 2020, 12, 10424. [Google Scholar] [CrossRef]
- Baulch, S.; Perry, C. Evaluating the impacts of marine debris on cetaceans. Mar. Pollut. Bull. 2014, 80, 210–221. [Google Scholar] [CrossRef]
- Pierantonio, N.; Simmonds, M.P. Relevant debris to be targeted for cetaceans: A review of available information. In Proceedings of the International Whaling Commission Scientific Committee/Scientific Committee Meeting Papers SC/67B/E/15, Bled, Slovenia, 24 April–6 May 2018. [Google Scholar]
- Puig-Lozano, R.; Bernaldo de Quirós, Y.; Díaz-Delgado, J.; García-Álvarez, N.; Sierra, E.; De la Fuente, J.; Sacchini, S.; Suárez-Santana, C.M.; Zucca, D.; Câmara, N.; et al. Retrospective study of foreign body-associated pathology in stranded cetaceans, Canary Islands (2000–2015). Environ. Pollut. 2018, 243, 519–527. [Google Scholar] [CrossRef]
- Simmonds, M.P. Cetaceans and Marine Debris: The Great Unknown. J. Mar. Biol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Filella, M. Questions of size and numbers in environmental research on microplastics: Methodological and conceptual aspects. Environ. Chem. 2015, 12, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmans, A.A. Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review. Environ. Sci. Technol. 2018, 52, 10230–10240. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; Berrow, S.; Rogan, E.; O’Connor, I. Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ. Pollut. 2018, 232, 467–476. [Google Scholar] [CrossRef]
- Banca Dati Spiaggiamenti. Available online: http://mammiferimarini.unipv.it/ (accessed on 30 March 2021).
- United Nations Environment Programm. Marine Litter: A Global Challenge; United Nations Environment Programm: Nairobi, Kenya, 2009. [Google Scholar]
- Directive, S.F. Guidance on Monitoring of Marine Litter in European Seas; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Aznar, F.J.; Agustí, C.; Littlewood, D.T.J.; Raga, J.A.; Olson, P.D. Insight into the role of cetaceans in the life cycle of the tetraphyllideans (Platyhelminthes: Cestoda). Int. J. Parasitol. 2007, 37, 243–255. [Google Scholar] [CrossRef]
- Marchiori, E.; Negrisolo, E.; Cassini, R.; Garofalo, L.; Poppi, L.; Tessarin, C.; Marcer, F. Cardiovascular flukes (Trematoda: Spirorchiidae) in Caretta caretta Linnaeus, 1758 from the Mediterranean Sea. Parasites Vectors 2017, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef]
- Marcer, F.; Marchiori, E.; Centelleghe, C.; Ajzenberg, D.; Gustinelli, A.; Meroni, V.; Mazzariol, S. Parasitological and pathological findings in fin whales Balaenoptera physalus stranded along Italian coastlines. Dis. Aquat. Organ. 2019, 133, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Giovannini, A.; Raga, J.A.; Fernández, M. Intestinal helminth fauna of bottlenose dolphin Tursiops truncatus and common dolphin Delphinus delphis from the western Mediterranean. J. Parasitol. 2013, 99, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Raga, J.A.; Aznar, F.J.; Balbuena, J.A.; Fernandez, M. Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Wursig, B., Tewissen, H.G.M., Eds.; Accademic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Terracciano, G.; Fichi, G.; Comentale, A.; Ricci, E.; Mancusi, C.; Perrucci, S. Dolphins stranded along the tuscan coastline (Central Italy) of the “pelagos sanctuary”: A parasitological investigation. Pathogens 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- de Quirós, Y.B.; Hartwick, M.; Rotstein, D.S.; Garner, M.M.; Bogomolni, A.; Greer, W.; Niemeyer, M.E.; Early, G.; Wenzel, F.; Moore, M. Discrimination between bycatch and other causes of cetacean and pinniped stranding. Dis. Aquat. Org. 2018, 127, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Animals ID | Species | Stranding Date and Location | Estimated Age | Cause of Death Hypothesis | DCC | Organs Tested | Conservation |
|---|---|---|---|---|---|---|---|
| 12694 | Z. cavirostris | 22/12/17 Donoratico (LI) | Adult | Infectious (viral/parasitic) | 2 | Stomach | Frozen |
| 12878 | S. coeruleoalba | 03/01/19 Alassio (SV) | Adult | Infectious (viral) | 2 | Stomach and intestine | Frozen |
| 12755 | G. melas | 17/03/18 Aglientu (OR) | Adult | n.d. | 3 | Stomach and intestine | Frozen |
| 12948 | T. truncatus | 14/04/19 Pellestrina (VE) | Young | n.d. | 3 | Stomach and intestine | Frozen |
| 13065 | T. truncatus | 15/9/2019 Pellestrina (VE) | Adult | n.d. | 3 | Stomach and intestine | Frozen |
| Mesh Dimension | Justification for Choice | Investigations |
|---|---|---|
| 20 mm | Retains undigested macro-food; separates the biggest marine debris items present. | Diet analysis, macro-litter assessment, parasitology |
| 10 mm | Increases the content subdivision and improves its visualization. | |
| 5 mm | Cut-off dimension between macro/meso- and micro-litter; increases the content subdivision and improves its visualization [28]. | Diet analysis, meso-litter assessment, parasitology |
| 2 mm | Increases the content subdivision and improves its visualization. | |
| 1000 μm | Cut-off dimension for MSFD protocol for the analysis of micro-litter: in this way, the MSFD protocol is included in the new meshes device protocol [29]. | Diet analysis, micro-litter assessment, parasitology |
| 500 μm | The minimum size required for diet studies; increases the content subdivision and avoid clogging problems [6]. | |
| 250 μm | Size required for diet analysis which considers the presence of otoliths and beaks of small species and for collecting gastro-intestinal helminthes; increases the content subdivision and avoid clogging problems [6,30]. | |
| 100 μm | Collects only micro-litter items. | Micro-litter assessment |
| 53 μm | Collects only micro-litter items. |
| Mesh Sequence | Problems | Solutions |
|---|---|---|
| 20 mm–10 mm–5 mm–2 mm-1000 µm–500 µm–53 µm | 1. The time of execution of the protocol based on this number of sieves was too slow; 2. There was clogging problems at the 53 µm mesh level. | 1. The number of square sieves decreased (the 10 mm mesh was eliminated); 2. The 500 µm mesh was substituted with a smaller one (250 µm) to have less material in the 53 µm mesh. |
| 20 mm–5 mm–2 mm–1000 μm–250 μm–53 μm | 1. The time of execution of the protocol based on this number of sieves was still too slow (the separation and visibility of the content were optimal); 2. The clogging problem was partially but not completely solved. | 1. The 2 mm mesh was removed; 2. The 500 µm mesh was re-insert (in addition to the 250 µm) to have less material in the last sieve. The 53 µm mesh was substituted for a 100 µm one. |
| 20 mm–5 mm–1000 μm–500 μm–250 μm–100 μm | 1. With the re-insertion of the 500 µm mesh, the time of execution of the protocol remain too slow; 2. The problem of clogging was present only in large amounts of content, depending on the content composition. | 1. The 500 µm became optional, to be used when the amount of gastro-intestinal content is abundant and a better separation of the material is necessary; 2. To totally solve this problem, the application of a shaking system or a manual shaking of the sieves for the smaller meshes could be necessary in some cases. |
| 20 mm–5 mm–1000 μm–500 μm–250 μm–100 μm | With this sequence, the main problems encountered were completely solved and it became the definitive mesh sequence. |
| DCC 1 | DCC 2 | DCC 3 | DCC 4 | DCC 5 | Conservation (Refrigerated/Frozen) | |
|---|---|---|---|---|---|---|
| Microbiological analysis (Organ and content) | X | X | * | Refrigerated/ frozen Δ | ||
| Virological analysis (Organ) | X | X | * | Refrigerated/frozen | ||
| Anatomo-pathological macroscopic evaluation (Organ) | X | X | X | * | Refrigerated/frozen | |
| Histopathological evaluation (Organ) | X | X | * | Refrigerated/frozen * | ||
| Parasitological analysis (Organ and content) | X | X | X | * | Refrigerated/frozen | |
| Diet analysis (Content) | X | X | X | X | * | Refrigerated/frozen |
| Macro- and meso-litter presence assessment (Content) | X | X | X | X | * | Refrigerated/frozen |
| Micro-litter presence assessment (Content) | X | X | X | X | Refrigerated/frozen | |
| Algal biotoxins analysis (Content) | X | X | * | Refrigerated |
| ID/Organ | Pathological Evaluation | Diet Analysis | Parasitological Investigations | Marine Litter Assessment |
|---|---|---|---|---|
| 12694/S | Gross findings: abundant content was present. Histological findings: severe diffuse autolysis of the tissue. | Presence of macro- and micro-food residues (squid beaks, eye lens and otoliths). More in details, 273 upper and 262 lower cephalopod beaks were present. Regarding the upper beaks, 1 belonged to the family Ommastrephidae, 262 belonged to the genus Histioteuthis and 2 belonged to Octopoteuthis sicula. Regarding the lower ones, the majority (257) belonged to the genus Histioteuthis. Remains of eye lens and shellfish were found. | Nematode larvae belonging to the Family Anisakidae: 7 specimens. | Macro-litter: 5 items; Meso-litter: 5 items; Micro-litter: 49 items. |
| 12878/S | Gross finding: scarce content was present. 1° stomach: multifocal, mild, chronic ulcerative gastritis. 2° stomach showed multifocal, mild, chronic gastritis; several nodules caused by Pholeter gastrophilus were observed in main and pyloric chambers. Multifocal thickening of the mucosa at conjunction of the two stomachs were recorded. Histological findings: severe diffuse autolysis of the tissue. | Presence of macro- and micro-food residues (cephalopod beaks and eye lens) More in details, 8 upper and 11 lower cephalopod beaks were present, belonging to the family Ommastrephidae (n = 10) and one Loliginid squid. All food remains were completely digested, suggesting a non-recent meal. Some remains of the marine seagrass Cymodocea nodosa were also found, probably accidentally ingested in shallow waters before stranding. | Adult stage of digeneans (whole specimens and fragments) belonging to the Family Brachycladiidae: 38 specimens. | Macro-litter: 1 items; Meso-litter: 1 items; Micro-litter: 8 items. |
| 12878/I | Gross findings: no macroscopic content was present. No lesions were recorded. Histological findings: severe diffuse autolysis of the tissue. | Several small cephalopod beaks (7 upper and 9 lower) were found. The analysis of lower beaks allowed identification of 6 ommastrephids squid 2 specimens of the pelagic sepiolidae Heteroteuthis dipar; one beak was not identified. | Adult stage of digeneans belonging to the Family Brachycladiidae (fragments): 4 specimens. Adult cestodes (whole specimens and scolexes) belonging to the Family Tetrabothriidae: 159 specimens. | Micro-litter: 4 items. |
| 12755/S | Gross findings: scarce content was present. Nodules caused by Pholeter gastrophilus were recorded in glandular stomach. Histological findings: severe diffuse autolysis of the tissue. | Presence of macro-food residues (vertebrae and otoliths of bony fish and cephalopod beaks and eye lenses). Four vertebrae and one otolith were encountered. The four lower cephalopod beaks were represented by one large Octopoteuthis sicula, 1 Histioteuthis reversa and 1 small H. bonnellii. All food remains were completely digested, suggesting a non-recent meal. | Fragments of Nematoda parasites, not morphologically identified. | No items. |
| 12755/I | Gross findings: no macroscopic content was present. No lesions were recorded. Histological findings: severe diffuse autolysis of the tissue. | Absence of food evidence. | Adult stage of digeneans belonging to the Family Brachycladiidae: 4 specimens. Adult cestodes belonging to the Family Tetrabothriidae: 2 specimens. | Micro-litter: 41 items. |
| 12948/S | Gross findings: abundant content was present. 1° stomach: multifocal, mild, chronic ulcerative gastritis. Histological findings: severe diffuse autolysis of the tissue. | Among the macro-food residues, the presence of an undigested octopus (Octopus vulgaris) provided evidence of a recent meal. Other remains were highly digested; in de-tails, a large amount of fish bones and vertebrae were found, together with 63 otoliths, all belonging to the poor cod Trisopterus minutus, representing a total amount of about 38 specimens. Additionally, cephalopod beaks were present, 5 upper and 6 lower octopod beaks and one lower Ommastrephid beak. | Negative. | Micro-litter: 7 items. |
| 12948/I | Gross findings: no macroscopic content was present. Histological findings: moderate diffuse autolysis, multifocal reactivity of the gastro-intestinal associated lymphoid tissue (GALT) of the submucosa. | Absence of food evidence. | Negative. | Micro-litter: 8 items. |
| 13065/S | Gross findings: no macroscopic content was present. 2° stomach: multifocal, mild, chronic catarrhal gastritis (attributable to parasites). Histological findings: moderate diffuse autolysis, in the submucosa of 2° stomach multifocal aggregates of parasitic elements (larvae and eggs) are recorded, attributable to Pholeter gastrophilus. | Absence of food evidence. | Fragments of Nematoda parasites, not morphologically identified. | Micro-litter: 8 items. |
| 13065/I | Gross findings: no macroscopic content was present. No lesions were recorded. Histological findings: severe diffuse autolysis of the tissue. | Absence of food evidence. | Negative. | Micro-litter: 36 items. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corazzola, G.; Baini, M.; Grattarola, C.; Panti, C.; Marcer, F.; Garibaldi, F.; Berio, E.; Mancusi, C.; Galli, M.; Mazzariol, S.; et al. Analysis of the Gastro-Intestinal Tract of Marine Mammals: A Multidisciplinary Approach with a New Multi-Sieves Tool. Animals 2021, 11, 1824. https://doi.org/10.3390/ani11061824
Corazzola G, Baini M, Grattarola C, Panti C, Marcer F, Garibaldi F, Berio E, Mancusi C, Galli M, Mazzariol S, et al. Analysis of the Gastro-Intestinal Tract of Marine Mammals: A Multidisciplinary Approach with a New Multi-Sieves Tool. Animals. 2021; 11(6):1824. https://doi.org/10.3390/ani11061824
Chicago/Turabian StyleCorazzola, Giorgia, Matteo Baini, Carla Grattarola, Cristina Panti, Federica Marcer, Fulvio Garibaldi, Enrica Berio, Cecilia Mancusi, Matteo Galli, Sandro Mazzariol, and et al. 2021. "Analysis of the Gastro-Intestinal Tract of Marine Mammals: A Multidisciplinary Approach with a New Multi-Sieves Tool" Animals 11, no. 6: 1824. https://doi.org/10.3390/ani11061824
APA StyleCorazzola, G., Baini, M., Grattarola, C., Panti, C., Marcer, F., Garibaldi, F., Berio, E., Mancusi, C., Galli, M., Mazzariol, S., Fossi, M. C., Centelleghe, C., & Casalone, C. (2021). Analysis of the Gastro-Intestinal Tract of Marine Mammals: A Multidisciplinary Approach with a New Multi-Sieves Tool. Animals, 11(6), 1824. https://doi.org/10.3390/ani11061824