Ruminal Fistulation and Cannulation: A Necessary Procedure for the Advancement of Biotechnological Research in Ruminants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Current Uses of Fistulation and Cannulation in Ruminants
2.1. The Search for New Nutritional Sources: Near the Area Where Livestock Is Raised and Can Replace Cereals or Forages Destined for Human Consumption
2.2. Control of Greenhouse Gas Emissions
2.3. Effect of the Ruminant Production System on Ruminal Balance
2.4. The Search for Solutions to Ruminal Diseases
2.5. The Study of Rumen Microbiota and Microbioma
- The search for sustainable livestock farming by taking advantage of local resources, and especially by using vegetable by-products that do not affect digestibility and rumen balance. For this, preliminary research through fistulation and cannulation is an essential step. These by-products, rich in antioxidants, contribute to what we know today as “food fortification”, adding value to the final product (milk/meat) through supplements of natural origin. In addition, through the constant control of antibiotics abuse in livestock farming, it has been demonstrated that antioxidants of natural origin have an antibacterial effect.
- From an environmental point of view, the control of greenhouse gas emissions has motivated the development of research in search of nutritional sources that minimize them, as well as the genetic study of the microbiome. Objective results cannot be obtained without in situ access to the rumen chamber.
- Finally, the study of rumen diseases, especially infectious diseases, contributes not only to the control of the farm’s economic losses but also to the prevention of such diseases, or even to the inhibition of new pathogens that end up becoming what is currently called “emerging diseases of animal origin”, which currently has such a great repercussion in the media as a result of the COVID-19 pandemic.
3. The Process of Ruminal Fistulation and Cannulation
How Do Fistulization and Cannulation Affect the General Health and Production of the Animals?
4. Alternatives to Rumen Fistulization and Cannulation
4.1. In Vivo
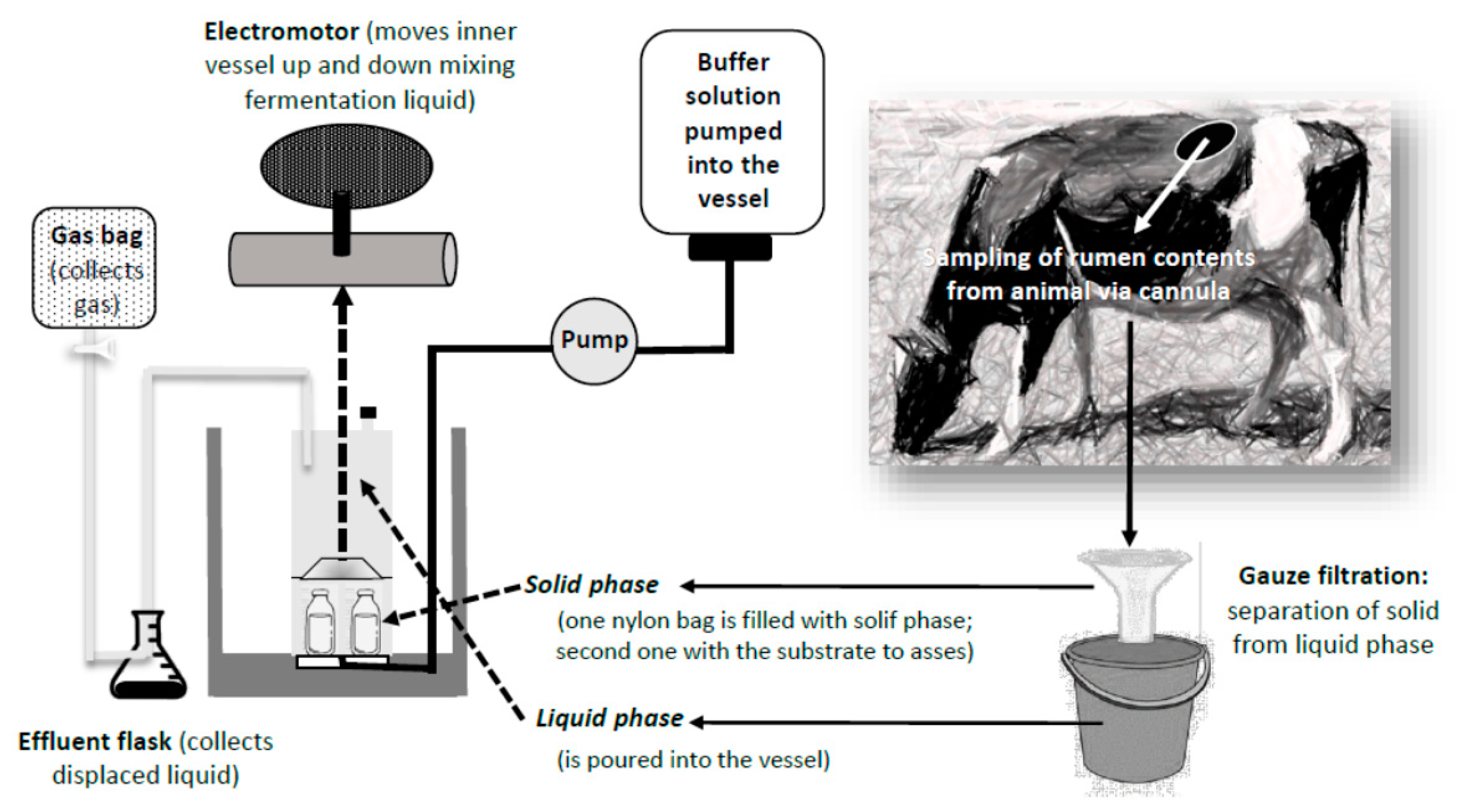
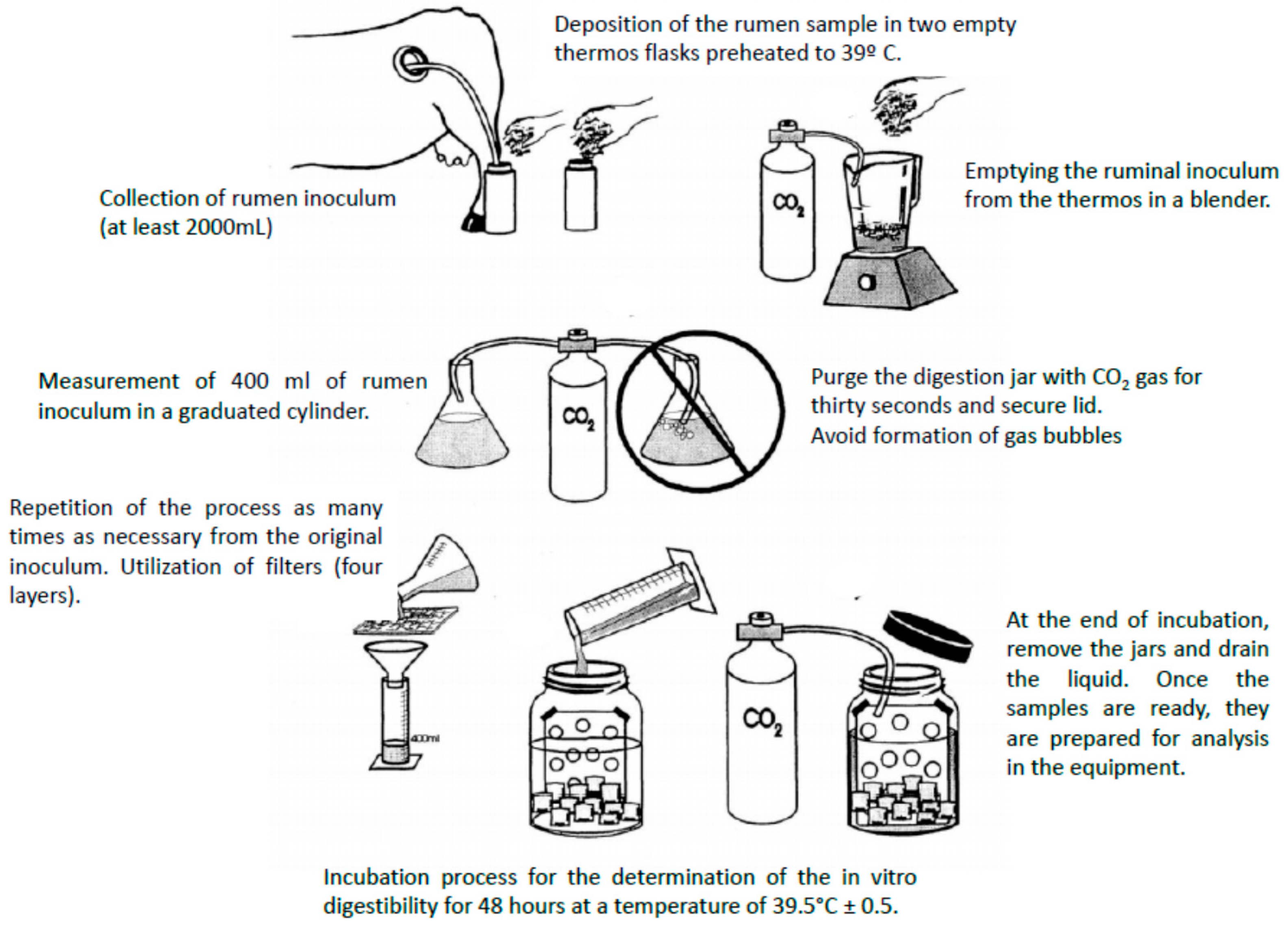
4.2. In Vitro: The Use of Fermenters
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Harmon, D.L.; Richards, C.J. Considerations for gastrointestinal cannulations in ruminants. J. Anim. Sci. 1997, 75, 2248–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DePeters, E.J.; George, L.W. Rumen transfaunation. Immunol. Lett. 2014, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.N.; Basalan, M. Ruminal Fermentation. In Rumenology; Domingues Millen, D., Mario De Beni Arrigoni, M., Pacheco, R.D.L., Eds.; Springer International Publishing: Geneva, Switzerland, 2016; pp. 63–102. [Google Scholar] [CrossRef]
- Durmic, Z.; McGrath, P.; Wilmot, M.; Adams, N.; Tan, T.; Callahand, L.; Mayberry, C. Surgical and postoperative events during permanent fistulation of sheep rumen by the Schalk and Amadon method. Aust. Vet. J. 2015, 93, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Resillez, A.; González, M. Nota Técnica: Modificación en la y/o nueva técnica quirúrgica de implantación de cánula ruminal. Rev. Prod. Anim. 2009, 21, 177–179. Available online: https://revistas.reduc.edu.cu/index.php/rpa/article/view/2993 (accessed on 25 April 2021).
- Kristensen, N.B.; Engbæk, M.; Vestergaard, M.; Harmon, D.L. Technical note: Ruminal cannulation technique in young Holstein calves: Effects of cannulation on feed intake, body weight gain, and ruminal development at six weeks of age. J. Dairy Sci. 2010, 93, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Schramm, H.H.; Gleason, C.B.; White, R.R. Influence of two rumen cannulation techniques on postoperative recovery in sheep. Vet. Surg. 2021, 50, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Abuelo, A.; Hernández, J. Biotechnological Approaches to Improve Sustainable Milk and Meat Yield in Bovines. Ref. Mod. Food Sci. 2017, 1–27. [Google Scholar] [CrossRef]
- Janssen, P.H.; Kirs, M. Structure of the archaeal community of the rumen. Appl. Environ. Microbiol. 2007, 74, 3619–3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Perez, G.A.; Ominski, K.H.; McAllister, T.A.; Krause, D.O. Effect of environmental factors and influence of rumen and hindgut biogeography on bacterial communities in steers. Appl. Environ. Microbiol. 2011, 7, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, R.W. Permanent stomach and intestinal fistulas in ruminants: Some modifications and simplifications. Cornell Vet. 1955, 45, 331–357. [Google Scholar] [PubMed]
- Lopes Muzzi, L.A.; Lázaro Muzzi, R.A.; Alves Gabellini, E.L. Rumen fistulation and cannulation technique in cattle and sheep. Ciênc Agrotec. Lavras. 2009, 33, 2059–2064. [Google Scholar] [CrossRef]
- Berean, K.J.; Adetutu, E.M.; Ou, J.Z.; Nour, M.; Nguyen, E.P.; Paull, D.; Mcleod, J.; Ramanathan, R.; Bansal, V.; Latham, K.; et al. A unique in vivo approach for investigating antimicrobial materials utilizing fistulated animals. Sci. Rep. 2015, 5, 11515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, X.; Dang, S.; Liu, S.; Jing, L.; Wang, Y.; Zhang, W. Determination of the appropriate ratio of sample size to nylon bag area for in situ nylon bag technique evaluation of rumen digestibility of feedstuffs in sheep. Liv. Sci. 2020, 241, 1413–1871. [Google Scholar] [CrossRef]
- Frey, J.C.; Pell, A.N.; Berthiaume, R.; Lapierre, H.; Lee, S.; Ha, J.K.; Mendell, J.E.; Angert, E.R. Comparative studies of microbial populations in the rumen, duodenum, ileum and faeces of lactating dairy cows. J. Appl. Microbiol. 2010, 108, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Fagodiya, R.K.; Hiloidhari, M.; Dahiya, R.P.; Kumar, A. Methane production and estimation from livestock husbandry: A mechanistic understanding and emerging mitigation options. Sci. Total Environm. 2020, 709, 136135. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.S.; Han, Y.; Cao, S.; Wen, J.; Zhang, K.; Yuan, H.; Wang, X.C. Cosubstrate strategy for enhancing lignocellulose degradation during rumen fermentation in vitro: Characteristics and microorganism composition. Chemosphere 2020, 250, 126104. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sust. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffield, T.; Plaizier, J.C.; Fairfield, A.; Bagg, R.; Vessie, G.; Dick, P.; Wilson, J.; Aramini, J.; McBride, B. Comparison of techniques for measurement of rumen pH in lactating dairy cows. J. Dairy Sci. 2004, 87, 59–66. [Google Scholar] [CrossRef]
- Riede, S.; Toboldt, A.; Breves, G.; Metzner, M.; Köhler, B.; Bräunig, J.; Schafft, H.M.; Lahrssen-Wiederholt, M.; Niemann, L. Investigations on the possible impact of a glyphosate-containing herbicide on ruminal metabolism and bacteria in vitro by means of the ‘Rumen Simulation Technique’. J. Appl. Microbiol. 2016, 121, 644–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laflin, S.L.; Gnad, D.P. Rumen Cannulation: Procedure and Use of a Cannulated Bovine. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 335–340. [Google Scholar] [CrossRef]
- Steiner, S.; Nina Linhart, N.; Neidl, A.; Walter Baumgartner, W.; Alexander Tichy, A.; Wittek, T. Evaluation of the therapeutic efficacy of rumen transfaunation. J. Anim. Physiol. Anim. Nutr. 2019, 00, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Karim, S.A. Rumen cannulation in sheep and goats: Fabrication of cannula and surgical procedure for its implantation. Indian J. Anim. Sci. 2002, 72, 978–980. [Google Scholar]
- Wetzels, S.U.; Eger, M.; Burmester, M.; Kreienbrock, L.; Abdulmawjood, A.; Pinior, B.; Wagner, M.; Breves, G.; Mann, E. The application of rumen simulation technique (RUSITEC) for studying dynamics of the bacterial community and metabolome in rumen fluid and the effects of a challenge with Clostridium perfringens. PLoS ONE 2018, 13, e0192256. [Google Scholar] [CrossRef] [Green Version]
- Loor, J.J.; Elolimy, A.A.; McCann, J.C. Dietary impacts on rumen microbiota in beef and dairy production. Anim. Front. 2016, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, M.; Zhang, X.M.; Wen, J.N.; Ma, Z.Y.; Long, D.L.; Deng, J.P.; Tan, Z.L. Effects of rumen cannulation on dissolved gases and methanogen community in dairy cows. J. Dairy Sci. 2019, 102, 2275–2282. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Choi, H.; Jeong, J.Y.; Lee, S.; Lee, H.J.; Baek, Y.; Ji, S.Y.; Kim, M. Effects of sampling techniques and sites on rumen microbiome and fermentation parameters in Hanwoo steers. J. Mocrobiol. Biotechnol. 2018, 28, 1700–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indugu, N.; Bittinger, K.; Kumar, S.; Vecchiarelli, B.; Pitta, D. A comparison of rumen microbial profiles in dairy cows as retrieved by 454-Roche and Ion-Torrent (PGM) sequencing platforms. Peer J. 2016, 4, e1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmuthuge, N.; Guan, L.L. Understanding host-microbial interactions in rumen: Searching the best opportunity for microbiota manipulation. J. Anim. Sci. Biotechnol. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Directive 2010/63/EU of The European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276/33–L276/79. Available online: EUR-Lex-32010L0063-EN-EUR-Lex (accessed on 25 April 2021). (europa.eu) .
- Ghazy, A. One-Stage technique for ruminal fistulation in rams. AJVS 2017, 52, 109–117. [Google Scholar] [CrossRef]
- Szakács, J.; Chrastinová, L.; Chrenková, M. Innovated surgery protocol for rumen cannulation in ruminants. Slovak J. Anim. Sci. 2021, 54, 1–6. [Google Scholar]
- Tapio, I.; Shingfield, K.J.; McKain, N.; Bonin, A.; Fischer, D.; Bayat, A.R.; Vilkki, J.; Taberlet, P.; Snelling, T.J.; Wallace, R.J. Oral Samples as non-invasive proxies for assessing the composition of the rumen microbial community. PLoS ONE 2016, 11, e0151220. [Google Scholar] [CrossRef] [Green Version]
- Rands, S.A. Ethical policies on animal experiments are not compromised by whether a journal is freely accessible or charges for publication. Animal 2009, 3, 1591–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moate, P.J.; Williams, R.R.O.; Deighton, M.H.; Hannah, M.C.; Ribaux, B.E.; Morris, G.L.; Jacobs, J.L.; Hill, J.; Wales, W.J. Effects of feeding wheat or corn and of rumen fistulation on milk production and methane emissions of dairy cows. Anim. Prod. Sci. 2017, 59, 891–905. [Google Scholar] [CrossRef]
- Mbaya, Y.P.; Kibon, A.; Yahaya, M.S.; Gworgwor, Z.A. Fistulation and cannulation of goat single stage technique using locally improvised cannula. Glob. J. Agric. Sci. 2011, 10, 83–88. [Google Scholar]
- Kebamo, M. Traumatic ruminal fistula in cow: Case report. IJVSAH 2021, 6, 23–25. [Google Scholar]
- Shen, J.S.; Chai, Z.; Song, L.J.; Liu, J.X.; Wu, Y.M. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, M.N.; Minson, D.J. Large particle breakdown by cattle eating ryegrass and alfalfa. J. Anim. Sci. 1988, 66, 992–999. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.A.; Bull, I.D.; Yan, T.; Dewhurst, R.J. Assessment of archaeol as a molecular proxy for methane production in cattle. J. Dairy Sci. 2013, 96, 1211–1217. [Google Scholar] [CrossRef] [Green Version]
- Kittelmann, S.; Kirk, M.R.; Jonker, A.; McCulloch, A.; Janssen, P.H. Buccal swabbing as a noninvasive method to determine bacterial, archaeal, and eukaryotic microbial community structures in the rumen. Appl. Environ. Microbiol. 2015, 81, 7470–7483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerkawski, J.W.; Breckenridge, G. Design and development of a long-term rumen simulation technique (RUSITEC). Br. J. Nutr. 1977, 38, 371–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, L.A.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Influence of exogenous fibrolytic enzymes and fumarate on methane production, microbial growth and fermentation in RUSITEC fermenters. Br. J. Nutr. 2007, 98, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. Comparison of fermentation of diets of variable composition and microbial populations in the rumen of sheep and RUSITEC fermenters. I. Digestibility, fermentation parameters, and microbial growth. J. Dairy Sci. 2010, 93, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Tassone, S.; Fortina, R.; Peiretti, P.G. In Vitro Techniques Using the DaisyII Incubator for the Assessment of Digestibility: A Review. Animals 2020, 10, 775. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, C.; Hernández, J. Ruminal Fistulation and Cannulation: A Necessary Procedure for the Advancement of Biotechnological Research in Ruminants. Animals 2021, 11, 1870. https://doi.org/10.3390/ani11071870
Castillo C, Hernández J. Ruminal Fistulation and Cannulation: A Necessary Procedure for the Advancement of Biotechnological Research in Ruminants. Animals. 2021; 11(7):1870. https://doi.org/10.3390/ani11071870
Chicago/Turabian StyleCastillo, Cristina, and Joaquin Hernández. 2021. "Ruminal Fistulation and Cannulation: A Necessary Procedure for the Advancement of Biotechnological Research in Ruminants" Animals 11, no. 7: 1870. https://doi.org/10.3390/ani11071870
APA StyleCastillo, C., & Hernández, J. (2021). Ruminal Fistulation and Cannulation: A Necessary Procedure for the Advancement of Biotechnological Research in Ruminants. Animals, 11(7), 1870. https://doi.org/10.3390/ani11071870






