Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Management
2.2. Observations of Ranging Behavior
2.3. Gastrointestinal Tract Measurements
2.4. Separation of the Crop, Proventriculus and Gizzard Contents
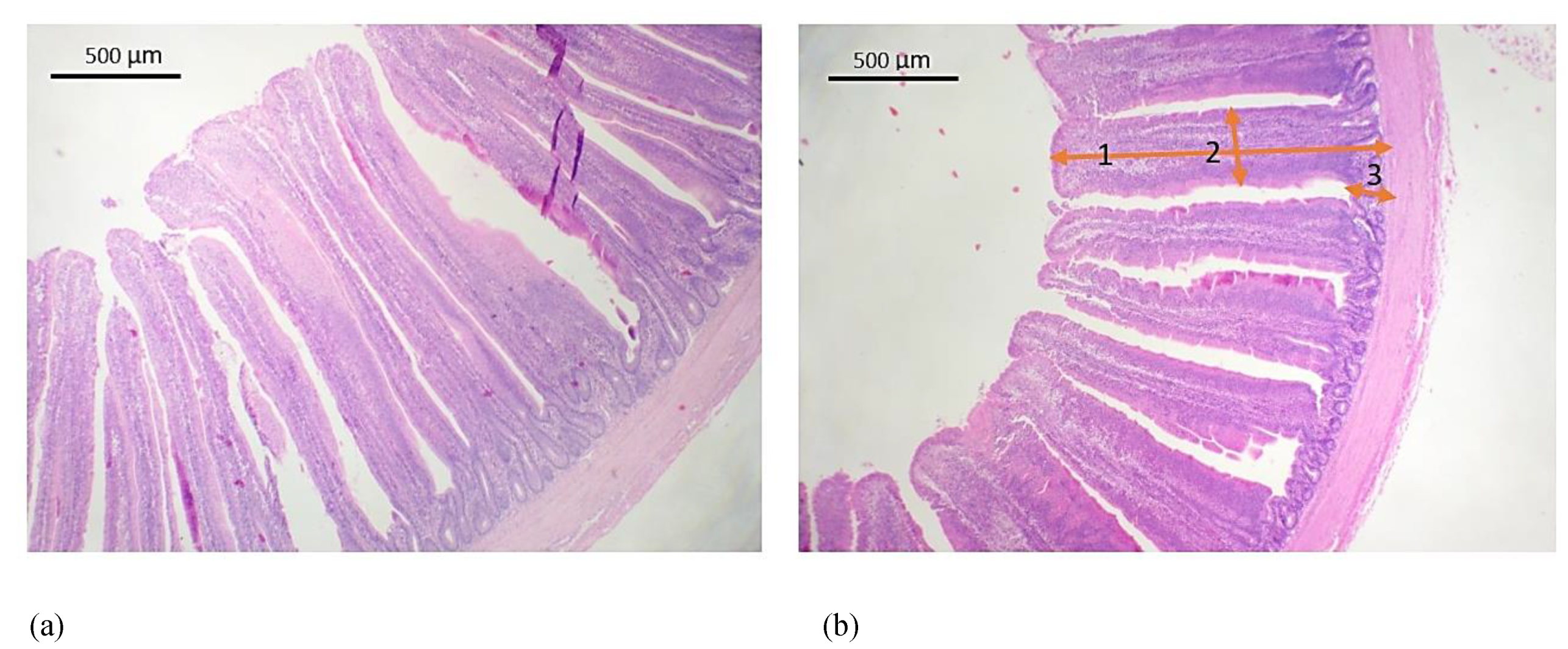
2.5. Histological Measurements
2.6. Statistical Analysis
3. Results
3.1. Ranging Profile Effect on Gastrointestinal Tract Morphometrics and Content of Green-Legged Partridges
3.2. Ranging Profile Effect on Gastrointestinal Tract Morphometrics and Content of Sasso
3.3. Correlations among Gastrointestinal Tract Morphometrics/Body Weight of Green-Legged Partridges
3.4. Correlations among Gastrointestinal Tract Morphometrics/Body Weight of Sasso
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hiemstra, S.J.; Ten Napel, J. Study of the Impact of Genetic Selection on the Welfare of Chickens Bred and Kept for Meat Production. Final Rep. a Proj. Comm. by Eur. Comm. (DG SANCO 2011/12254) 2013. Available online: https://www.responsiblebreeding.eu/uploads/2/3/1/3/23133976/final_report_impact_genetic_selection_welfare_broilers_2013_-_hiemstra_ten_napel.pdf (accessed on 24 June 2021).
- McCrea, B.A.; Mills, A.F.; Matthews, K.; Hutson, J. Performance and carcass characteristics of Delaware chickens in comparison with broilers. J. Appl. Poult. Res. 2014, 23, 586–592. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Cavitt, L.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Evaluation of slower-growing broiler genotypes grown with and without outdoor access: Meat quality. Poult. Sci. 2005, 84, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Fanatico, A.C.; Owens, C.M.; Emmert, J.L. Organic poultry production in the United States: Broilers. J. Appl. Poult. Res. 2009, 18, 355–366. [Google Scholar] [CrossRef]
- Rauw, W.M. Immune response from a resource allocation perspective. Front. Genet. 2012, 3, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawkins, M.S.; Cook, P.A.; Whittingham, M.J.; Mansell, K.A.; Harper, A.E. What makes free-range broiler chickens range? In situ measurement of habitat preference. Anim. Behav. 2003, 66, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Aurrekoetxea, A.; Estevez, I. Use of space and its impact on the welfare of laying hens in a commercial free-range system. Poult. Sci. 2016, 95, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.S.; Hemsworth, P.H.; Groves, P.J.; Rault, J.-L.; Gebhardt-Henrich, S.G. Ranging Behaviour of Commercial Free-Range Broiler Chickens 1: Factors Related to Flock Variability. Animals 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, G.J.; Wilkins, L.J.; Knowles, T.G.; Booth, F.; Toscano, M.J.; Nicol, C.; Brown, S.N. Continuous monitoring of pop hole usage by commercially housed free-range hens throughout the production cycle. Vet. Rec. 2011, 169, 338. [Google Scholar] [CrossRef]
- Gilani, A.-M.; Knowles, T.G.; Nicol, C. Factors affecting ranging behaviour in young and adult laying hens. Br. Poult. Sci. 2014, 55, 127–135. [Google Scholar] [CrossRef]
- Campbell, D.L.; Hinch, G.N.; Downing, J.A.; Lee, C. Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. Appl. Anim. Behav. Sci. 2016, 185, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Marchewka, J.; Sztandarski, P.; Zdanowska-Sąsiadek, Ż.; Damaziak, K.; Wojciechowski, F.; Riber, A.B.; Gunnarsson, S. Associations between welfare and ranging profile in free-range commercial and heritage meat-purpose chickens (Gallus gallus domesticus). Poult. Sci. 2020, 99, 4141–4152. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Yngvesson, J.; Gunnarsson, S.; Jönsson, L.; Wallenbeck, A. Feed efficiency, growth performance, and carcass characteristics of a fast- and a slower-growing broiler hybrid fed low- or high-protein organic diets. Org. Agric. 2018, 8, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, N.P.; Hott, J.M.; Kimbler, L.B.; Moritz, J.S. Nutrient Composition and Digestibility of Organic Broiler Diets and Pasture Forages. J. Appl. Poult. Res. 2007, 16, 13–21. [Google Scholar] [CrossRef]
- Sossidou, E.; Bosco, A.D.; Castellini, C.; Grashorn, M. Effects of pasture management on poultry welfare and meat quality in organic poultry production systems. World’s Poult. Sci. J. 2015, 71, 375–384. [Google Scholar] [CrossRef]
- Ndelekwute, E.K.; Enyenihi, G.E.; Akpan, I.P. Potentials and challenges of utilizing ns: A review on forage resources for chicken production. J. Anim. Sci. Livest. Prod. 2018, 2, 1–6. [Google Scholar]
- Mateos, G.G.; Jiménez-Moreno, E.; Serrano, M.P.; Lázaro, R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J. Appl. Poult. Res. 2012, 21, 156–174. [Google Scholar] [CrossRef]
- Fölsch, D.W.; Hoffmann, R. Artgemäße Hühnerhaltung. Grundlagen und Beispiele aus der Praxis; Stiftung Ökologie & Landbau: München, Germany, 1999. [Google Scholar]
- Wood, G.M. Consumption of Forage by Chickens. Poult. Sci. 1956, 35, 1083–1089. [Google Scholar] [CrossRef]
- Horsted, K.; Hermansen, J.E.; Hansen, H. Botanical composition of herbage intake of free-range laying hens determined by microhistological analysis of faeces. Arch. Geflugelkd. 2007, 71, 145–151. [Google Scholar]
- Ponte, P.; Rosado, C.; Crespo, J.; Crespo, D.; Mourão, J.; Chaveiro-Soares, M.; Brás, J.; Mendes, I.; Gama, L.; Prates, J.; et al. Pasture Intake Improves the Performance and Meat Sensory Attributes of Free-Range Broilers. Poult. Sci. 2008, 87, 71–79. [Google Scholar] [CrossRef]
- de Verdal, H.; Mignon-Grasteau, S.; Jeulin, C.; Le Bihan-Duval, E.; Leconte, M.; Mallet, S.; Martin, C.; Narcy, A. Digestive tract measurements and histological adaptation in broiler lines divergently selected for digestive efficiency. Poult. Sci. 2010, 89, 1955–1961. [Google Scholar] [CrossRef]
- Wijtten, P.J.A.; Langhout, D.J.; Verstegen, M.W.A. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: A review. Acta Agric. Scand. Sect. A Anim. Sci. 2012, 62, 1–12. [Google Scholar] [CrossRef]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Khan, R.; Tufarelli, V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.D.; Butcher, G.D.; Henry, P.R.; Littell, R.C. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006, 85, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Rodrigues, E.; Marques, R.; Gravena, R.; Guandolini, G.; Moraes, V. Performance and morphology of intestinal mucosa of broilers fed mannan-oligosaccharides and enzymes. Arq. Bras. Med. Vet. Zootec. 2008, 60, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amerah, A.; Ravindran, V.; Lentle, R.; Thomas, D. Feed particle size: Implications on the digestion and performance of poultry. World’s Poult. Sci. J. 2007, 63, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.X.; Peng, K.M. Developmental Morphology of the Small Intestine of African Ostrich Chicks. Poult. Sci. 2008, 87, 2629–2635. [Google Scholar] [CrossRef]
- Siwek, M.; Wragg, D.; Sławińska, A.; Malek, M.; Hanotte, O.; Mwacharo, J.M. Insights into the genetic history of Green-legged Partridge like fowl: Mt DNA and genome-wide SNP analysis. Anim. Genet. 2013, 44, 522–532. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, J. Quality of eggs from native Greenleg and Yellowleg Partridge hens. Wiadomości Zootech. 2017, 3, 74–79. [Google Scholar]
- Commission, E. Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and co. Off. J. Eur. Union 2008, 250, 1–84. [Google Scholar]
- Council of the European Commission. Council Regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing Regulation (EEC) No 2092/91. Off. J. Eur. Union 2007, 189, 1–23. [Google Scholar]
- Sanchez, C.; Estevez, I. The Chickitizer Software Program; The University of Maryland: College Park, MD, USA, 1998. [Google Scholar]
- Lorenz, C.; Kany, T.; Grashorn, M.A. Method to estimate feed intake from pasture in broilers and laying hens. Arch. Geflügelkd. 2013, 77, 160–165. [Google Scholar]
- Nain, S.; Renema, R.; Zuidhof, M.; Korver, D. Effect of metabolic efficiency and intestinal morphology on variability in n-3 polyunsaturated fatty acid enrichment of eggs. Poult. Sci. 2012, 91, 888–898. [Google Scholar] [CrossRef]
- Browne, T. Some Observations on the Digestive System of the Fowl. J. Comp. Pathol. 1922, 35, 12–32. [Google Scholar] [CrossRef]
- Heuser, G.F. The Rate of Passage of Feed from the Crop of the Hen. Poult. Sci. 1945, 24, 20–24. [Google Scholar] [CrossRef]
- Bolton, W. Digestion in the crop of the fowl. Br. Poult. Sci. 1965, 6, 97–102. [Google Scholar] [CrossRef]
- Duke, G.E. Alimentary Canal: Anatomy, Regulation of Feeding, and Motility. In Marine Ecological Processes; Springer: Berlin, Germany, 1986; pp. 269–288. [Google Scholar]
- Branciari, R.; Mugnai, C.; Mammoli, R.; Miraglia, D.; Ranucci, D.; Bosco, A.D.; Castellini, C. Effect of genotype and rearing system on chicken behavior and muscle fiber characteristics1. J. Anim. Sci. 2009, 87, 4109–4117. [Google Scholar] [CrossRef]
- Damaziak, K.; Michalczuk, M.; Adamek, D.; Czapliński, M.; Niemiec, J.; Goryl, A.; Pietrzak, D. Influence of housing system on the growth and histological structure of duck muscles. S. Afr. J. Anim. Sci. 2014, 44, 97. [Google Scholar] [CrossRef] [Green Version]
- Kocher, A.; Choct, M.; Porter, M.D.; Broz, J. The effects of enzyme addition to broiler diets containing high concentrations of canola or sunflower meal. Poult. Sci. 2000, 79, 1767–1774. [Google Scholar] [CrossRef]
- Elwinger, K.; Tufvesson, M.; Lagerkvist, G.; Tauson, R. Feeding layers of different genotypes in organic feed environments. Br. Poult. Sci. 2008, 49, 654–665. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Van Reenen, C.G. Animal behavior and well-being symposium: Interaction between coping style/personality, stress, and welfare: Relevance for domestic farm animals1. J. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Starck, J. Phenotypic flexibility of the avian gizzard: Rapid, reversible and repeated changes of organ size in response to changes in dietary fibre content. J. Exp. Biol. 1999, 202, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Steenfeldt, S.; Kjaer, J.; Engberg, R. Effect of feeding silages or carrots as supplements to laying hens on production performance, nutrient digestibility, gut structure, gut microflora and feather pecking behaviour. Br. Poult. Sci. 2007, 48, 454–468. [Google Scholar] [CrossRef] [Green Version]
- Hünigen, H.; Mainzer, K.; Hirschberg, R.M.; Custodis, P.; Gemeinhardt, O.; Al Masri, S.; Richardson, K.C.; Hafez, H.M.; Plendl, J. Structure and age-dependent development of the turkey liver: A comparative study of a highly selected meat-type and a wild-type turkey line. Poult. Sci. 2016, 95, 901–911. [Google Scholar] [CrossRef]
- Al Masri, S.; Kattanek, M.; Richardson, K.C.; Hafez, H.M.; Plendl, J.; Hünigen, H. Comparative Quantitative Studies on the Microvasculature of the Heart of a Highly Selected Meat-Type and a Wild-Type Turkey Line. PLoS ONE 2017, 12, e0170858. [Google Scholar] [CrossRef] [Green Version]
- Incharoen, T.; Yamauchi, K.-E.; Erikawa, T.; Gotoh, H. Histology of intestinal villi and epithelial cells in chickens fed low-crude protein or low-crude fat diets. Ital. J. Anim. Sci. 2010, 9, e82. [Google Scholar] [CrossRef]
- Svihus, B.; Hetland, H. Ileal starch digestibility in growing broiler chickens fed on a wheat-based diet is improved by mash feeding, dilution with cellulose or whole wheat inclusion. Br. Poult. Sci. 2001, 42, 633–637. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Lee, B.-H.; Chang, P.-S.; Lee, H.G.; Yoo, S.-H. Improved quantitative analysis of oligosaccharides from lichenase-hydrolyzed water-soluble barley β-glucans by high-performance anion-exchange chromatography. J. Agric. Food Chem. 2007, 55, 1656–1662. [Google Scholar] [CrossRef]
- Praes, M.; Pereira, A.; Sgavioli, S.; Duarte, K.; Alva, J.; Domingues, C.D.F.; Puzzoti, M.; Junqueira, O.M. Small intestine development of laying hens fed different fiber sources diets and crude protein levels. Braz. J. Poult. Sci. 2011, 13, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Amat, C.; Planas, J.M.; Moretó, M. Kinetics of hexose uptake by the small and large intestine of the chicken. Am. J. Physiol. Content 1996, 271, R1085–R1089. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Ravindran, V.; Morel, P.C.H.; Hendriks, W.H.; Pierce, J. Evaluation of a Microbial Phytase, Produced by Solid-State Fermentation, in Broiler Diets. 1. Influence on Performance, Toe Ash Contents, and Phosphorus Equivalency Estimates. J. Appl. Poult. Res. 2004, 13, 373–383. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Ding, S.; He, F.; Beier, R.C.; Li, J.; Jiang, H.; Feng, C.; Wan, Y.; Zhang, S.; et al. Development of a Monoclonal Antibody-Based Broad-Specificity ELISA for Fluoroquinolone Antibiotics in Foods and Molecular Modeling Studies of Cross-Reactive Compounds. Anal. Chem. 2007, 79, 4471–4483. [Google Scholar] [CrossRef]
- Yamauchi, K.-E. Review on Chicken Intestinal Villus Histological Alterations Related with Intestinal Function. J. Poult. Sci. 2002, 39, 229–242. [Google Scholar] [CrossRef] [Green Version]
| Variable | Ranging Profile of Green-Legged Partridge (n = 60) | Pooled SEM | F Value | p Value | |||
|---|---|---|---|---|---|---|---|
| Outdoor-Preferring (n = 24) | Moderate-Indoor (n = 21) | Indoor-Preferring (n = 15) | |||||
| Mean | |||||||
| Body weight (kg) | 1.2 | 1.1 | 1.1 | 0.1 | 1.53 | 0.2322 | |
| Crop (g) | |||||||
| Empty | 4.9 | 5.0 | 4.6 | 0.4 | 0.22 | 0.8074 | |
| Full | 10.3 | 13.3 | 9.4 | 2.2 | 0.81 | 0.4536 | |
| Feed | 4.7 | 7.9 | 3.7 | 1.9 | 1.08 | 0.3533 | |
| Pasture matter | 0.05 B | 0.3 A | 0.03 B | 0.05 | 6.26 | 0.0451 | |
| Feather | 0.07 | 0.1 | 0.02 | 0.03 | 0.81 | 0.4796 | |
| Gizzard (muscular stomach) (g) | |||||||
| Empty | 34.5 | 31.7 | 31.8 | 1.9 | 0.59 | 0.5604 | |
| Full | 53.1 | 48.4 | 53.5 | 2.4 | 0.74 | 0.4862 | |
| Fraction 1 (600–1000 µm) | 8.2 | 6.9 | 7.3 | 1.1 | 0.39 | 0.6794 | |
| Fraction 2 (1000–1400 µm) | 7.6 B | 7.5 B | 11.2 A | 1.1 | 3.06 | 0.0517 | |
| Fraction 3 (1400–1800 µm) | 1.7 | 2.1 | 2.4 | 0.3 | 1.81 | 0.1817 | |
| Digestive tract measurements (cm) | |||||||
| Small intestine length (duodenum + jejunum + ileum) | 140.1 | 131.4 | 138.4 | 5.2 | 0.74 | 0.4858 | |
| Caeca length | 33.9 | 34.3 | 33.5 | 1.1 | 0.10 | 0.9087 | |
| Colon length | 11.8 | 11.6 | 10.8 | 0.3 | 2.53 | 0.0960 | |
| Histological measurements (μm) | |||||||
| Villi height | 1445.2 | 1558.2 | 1522.8 | 70.2 | 0.74 | 0.4862 | |
| Villi width | 165.4 | 158.3 | 161.7 | 8.0 | 0.22 | 0.8036 | |
| Crypt depth | 236.1 | 239.4 | 239.6 | 10.1 | 0.04 | 0.9630 | |
| Villus area (µm2) | 236,713.9 | 247,802.4 | 247,540.9 | 14,745.4 | 0.06 | 0.9378 | |
| Villus height/crypt depth | 6.2 | 6.6 | 6.4 | 0.3 | 0.35 | 0.7098 | |
| Variable | Ranging Profile of Sasso (n = 60) | Pooled SEM | F Value | p Value | |||
|---|---|---|---|---|---|---|---|
| Outdoor-Preferring (n = 14) | Moderate-Indoor (n = 19) | Indoor-Preferring (n = 27) | |||||
| Mean | |||||||
| Body weight (kg) | 3.0 | 2.8 | 3.1 | 0.5 | 0.82 | 0.4517 | |
| Crop (g) | |||||||
| Empty | 9.5 | 8.0 | 9.3 | 0.7 | 1.33 | 0.2830 | |
| Full | 32.8 | 20.2 | 39.1 | 6.5 | 1.80 | 0.1852 | |
| Feed | 22.1 | 11.9 | 27.6 | 5.4 | 1.52 | 0.2378 | |
| Pasture matter | 0.1 | 0.1 | 0.2 | 0.07 | 0.82 | 0.4524 | |
| Feather | 0.01 | 0.01 | 0.02 | 0.008 | 0.57 | 0.6745 | |
| Gizzard (muscular stomach) (g) | |||||||
| Empty | 49.3 | 42.8 | 49.5 | 4.2 | 0.84 | 0.4433 | |
| Full | 67.9 | 64.7 | 74.5 | 8.5 | 0.33 | 0.7186 | |
| Fraction 1 (600–1000 µm) | 9.6 | 12.2 | 12.3 | 2.7 | 0.13 | 0.8785 | |
| Fraction 2 (1000–1400 µm) | 2.8 | 4.7 | 5.1 | 1.2 | 0.75 | 0.4838 | |
| Fraction 3 (1400–1800 µm) | 2.3 | 2.1 | 2.4 | 0.7 | 0.06 | 0.9464 | |
| Digestive tract measurements (cm) | |||||||
| Small intestine length (duodenum + jejunum + ileum) | 223.6 | 216.7 | 231.2 | 12.3 | 0.49 | 0.6161 | |
| Caeca length | 47.4 | 49.1 | 48.6 | 2.1 | 0.19 | 0.8308 | |
| Colon length | 14.4 | 13.8 | 14.7 | 0.6 | 0.55 | 0.5839 | |
| Histological measurements (μm) | |||||||
| Villi height (µm) | 1204.3 A | 1153.7 AB | 1038.7 B | 45.2 | 3.60 | 0.0393 | |
| Villi width (µm) | 158.1 | 146.9 | 148.6 | 4.9 | 1.47 | 0.2456 | |
| Crypt depth (µm) | 198.8 | 216.3 | 199 | 7.1 | 1.97 | 0.1565 | |
| Villus area (µm2) | 188,655.9 A | 169,771.2 B | 154,128.2 C | 7237.1 | 5.05 | 0.0126 | |
| Villus height/crypt depth | 6.2 | 5.4 | 5.3 | 0.3 | 2.65 | 0.0866 | |
| Crop (g) | Gizzard (Muscular Stomach) (g) | Digestive Tract Measurements (cm) | Small Intestine Histological Measurements | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full | Feed Content | Pasture Matter | Feather | Empty | Full | Fraction 1 (600–1000 µm) | Fraction 2 (1000–1400 µm) | Fraction 3 (1400–1800 µm) | Small Intestine Length (Duodenum + Jejunum + Ileum) | Caeca Length | Colon Length | Villus Height (µm) | Villus Width (µm) | Crypt Depth (µm) | Villus Area (µm2) | VILLUS Height/Crypt Depth | |
| Crop (g) | |||||||||||||||||
| Empty | 0.23 | 0.09 | 0.06 | 0.16 | 0.32 | 0.25 | 0.17 | 0.07 | −0.07 | 0.47 ** | 0.03 | 0.27 | 0.07 | −0.20 | 0.07 | −0.07 | 0.01 |
| Full | 0.96 **** | 0.19 | 0.10 | −0.02 | 0.11 | 0.15 | 0.16 | 0.08 | 0.15 | 0.03 | 0.24 | −0.08 | 0.00 | 0.21 | −0.06 | −0.18 | |
| Feed content | 0.14 | 0.06 | −0.04 | 0.11 | 0.15 | 0.19 | 0.09 | 0.11 | 0.09 | 0.25 | −0.09 | −0.01 | 0.15 | −0.08 | −0.15 | ||
| Pasture matter | −0.07 | 0.00 | −0.15 | 0.13 | −0.24 | −0.03 | 0.04 | −0.18 | 0.24 | 0.07 | −0.15 | 0.58 *** | −0.03 | −0.27 | |||
| Feather | −0.03 | 0.05 | 0.01 | 0.12 | −0.10 | 0.07 | −0.13 | −0.03 | −0.22 | −0.19 | −0.07 | −0.28 | −0.15 | ||||
| Gizzard (g) | |||||||||||||||||
| Empty | 0.88 **** | 0.41 * | 0.13 | 0.13 | 0.42 * | 0.36 * | 0.39 * | 0.05 | 0.40 * | −0.16 | 0.31 | 0.10 | |||||
| Full | 0.57 *** | 0.47 ** | 0.26 | 0.38 * | 0.15 | 0.26 | −0.05 | 0.35 * | −0.24 | 0.19 | 0.08 | ||||||
| Fraction 1 (500–1000 µm) | −0.01 | 0.11 | 0.13 | −0.18 | 0.17 | −0.04 | 0.19 | 0.05 | 0.11 | −0.03 | |||||||
| Fraction 2 (1000–1500 µm) | 0.15 | 0.21 | −0.15 | −0.10 | −0.07 | −0.10 | −0.31 | −0.12 | 0.12 | ||||||||
| Fraction 3 (1500–2000 µm) | −0.04 | −0.06 | 0.15 | −0.18 | 0.37 * | 0.02 | 0.09 | −0.15 | |||||||||
| Digestive tract mes. (cm) | |||||||||||||||||
| Small intestine length (duodenum + jejunum + ileum) | 0.48 ** | 0.48 ** | 0.26 | −0.16 | 0.01 | 0.09 | 0.20 | ||||||||||
| Caeca length | 0.16 | 0.07 | 0.05 | −0.26 | 0.06 | 0.19 | |||||||||||
| Colon length | 0.08 | 0.30 | 0.18 | 0.27 | −0.03 | ||||||||||||
| Small intestine hist. mes. | |||||||||||||||||
| Villus height (µm) | −0.08 | 0.04 | 0.74 **** | 0.76 **** | |||||||||||||
| Villus width (µm) | −0.24 | 0.61 *** | 0.07 | ||||||||||||||
| Crypt depth (µm) | −0.12 | 0.59 *** | |||||||||||||||
| Villus area (µm2) | 0.66 **** | ||||||||||||||||
| Crop (g) | Gizzard (Muscular Stomach) (g) | Digestive Tract Measurements (cm) | Small Intestine Histological Measurements | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full | Feed Content | Pasture Matter | Feather | Empty | Full | Fraction 1 (600–1000 µm) | Fraction 2 (1000–1400 µm) | Fraction 3 (1400–1800 µm) | Small Intestine Length (Duodenum + Jejunum + Ileum) | Caeca Length | Colon Length | Villus Height (µm) | Villus Width (µm) | Crypt Depth (µm) | Villus Area (µm2) | Villus Height/Crypt Depth | |
| Crop (g) | |||||||||||||||||
| Empty | 0.63 *** | 0.54 ** | 0.34 | 0.10 | 0.24 | 0.22 | 0.02 | 0.08 | 0.24 | 0.26 | 0.23 | 0.23 | 0.33 | −0.02 | 0.18 | 0.23 | 0.13 |
| Full | 0.99 ** | 0.51 ** | 0.11 | 0.26 | 0.35 | 0.25 | 0.18 | 0.37 | 0.11 | 0.16 | −0.10 | 0.18 | 0.26 | 0.16 | 0.33 | 0.01 | |
| Feed content | 0.50 ** | 0.10 | 0.26 | 0.35 | 0.26 | 0.17 | 0.37 | 0.06 | 0.13 | −0.15 | 0.16 | 0.29 | 0.15 | 0.33 | 0.00 | ||
| Pasture matter | −0.17 | 0.13 | 0.13 | 0.01 | 0.05 | 0.36 | 0.41 ** | 0.23 | 0.15 | 0.13 | −0.20 | −0.36 | −0.04 | 0.38 * | |||
| Feather | −0.28 | −0.20 | −0.06 | −0.13 | −0.15 | −0.14 | −0.31 | 0.03 | −0.15 | 0.13 | 0.20 | −0.03 | −0.19 | ||||
| Gizzard (g) | |||||||||||||||||
| Empty | 0.83 **** | 0.39 * | 0.38 * | 0.46 * | 0.05 | 0.07 | 0.07 | 0.10 | 0.04 | −0.05 | 0.12 | 0.07 | |||||
| Full | 0.81 **** | 0.69 **** | 0.53 ** | −0.02 | 0.03 | −0.12 | 0.09 | 0.21 | −0.03 | 0.23 | 0.05 | ||||||
| Fraction 1 (500–1000 µm) | 0.65 **** | 0.29 | −0.10 | 0.01 | −0.30 | 0.00 | 0.29 | 0.01 | 0.21 | −0.03 | |||||||
| Fraction 2 (1000–1500 µm) | 0.01 ** | −0.25 | 0.03 | −0.04 | 0.23 | 0.04 | 0.04 | 0.21 | 0.12 | ||||||||
| Fraction 3 (1500–2000 µm) | 0.00 | −0.13 | −0.11 | 0.27 | 0.34 | −0.07 | 0.42 * | 0.24 | |||||||||
| Digestive tract mes. (cm) | |||||||||||||||||
| Small intestine length (duodenum + jejunum + ileum) | 0.64 *** | 0.52 ** | 0.06 | −0.03 | −0.13 | 0.04 | 0.13 | ||||||||||
| Caeca length | 0.47 * | 0.36 | −0.19 | −0.04 | 0.14 | 0.28 | |||||||||||
| Colon length | 0.24 | −0.40 * | −0.15 | −0.11 | 0.32 | ||||||||||||
| Small intestine hist. mes. | |||||||||||||||||
| Villus height (µm) | 0.00 | 0.28 | 0.71 **** | 0.61 *** | |||||||||||||
| Villus width (µm) | 0.47 | 0.70 **** | 0.39 * | ||||||||||||||
| Crypt depth (µm) | 0.56 ** | −0.58 *** | |||||||||||||||
| Villus area (µm2) | 0.13 | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchewka, J.; Sztandarski, P.; Zdanowska-Sąsiadek, Ż.; Adamek-Urbańska, D.; Damaziak, K.; Wojciechowski, F.; Riber, A.B.; Gunnarsson, S. Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles. Animals 2021, 11, 1881. https://doi.org/10.3390/ani11071881
Marchewka J, Sztandarski P, Zdanowska-Sąsiadek Ż, Adamek-Urbańska D, Damaziak K, Wojciechowski F, Riber AB, Gunnarsson S. Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles. Animals. 2021; 11(7):1881. https://doi.org/10.3390/ani11071881
Chicago/Turabian StyleMarchewka, Joanna, Patryk Sztandarski, Żaneta Zdanowska-Sąsiadek, Dobrochna Adamek-Urbańska, Krzysztof Damaziak, Franciszek Wojciechowski, Anja B. Riber, and Stefan Gunnarsson. 2021. "Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles" Animals 11, no. 7: 1881. https://doi.org/10.3390/ani11071881
APA StyleMarchewka, J., Sztandarski, P., Zdanowska-Sąsiadek, Ż., Adamek-Urbańska, D., Damaziak, K., Wojciechowski, F., Riber, A. B., & Gunnarsson, S. (2021). Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles. Animals, 11(7), 1881. https://doi.org/10.3390/ani11071881







