Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Collection and In Vitro Maturation
2.2. Determination of Oocytes Developmental Competence
2.3. Evaluation of Mitochondrial Activity and Reactive Oxygen Species (ROS) Intracellular Level
2.4. Tubulin Immunofluorescence and Confocal Microscope Evaluation
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Oocyte Developmental Competence
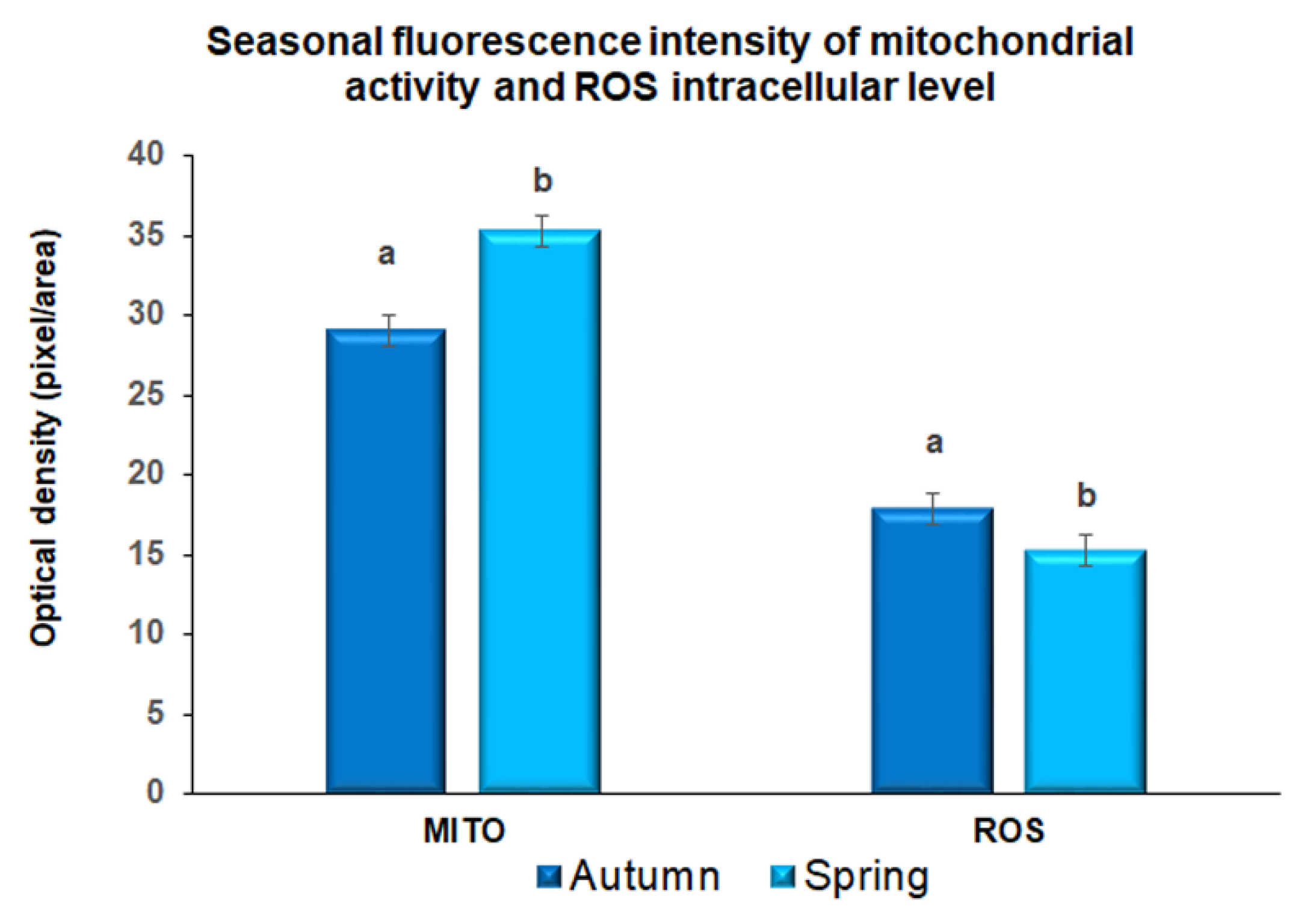
3.2. Evaluation of Mitochondrial Activity and ROS Intracellular Level
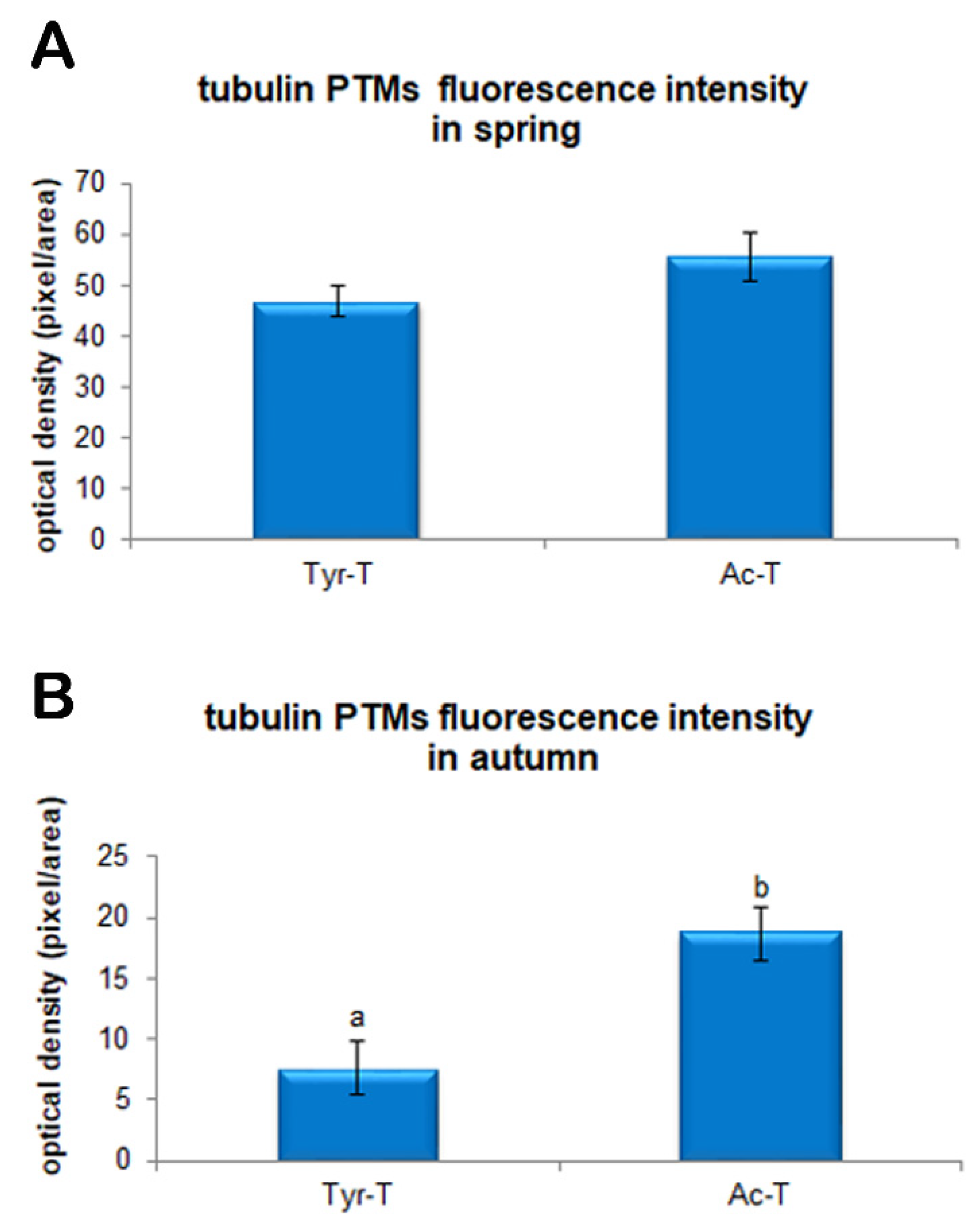
3.3. Evaluation of Tubulin Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, S.; Quinn, A.; Troupe, S.; Kingsland, C.; Lewis-Jones, I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum. Fertil. 2006, 9, 223–229. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ubuka, T. How to contribute to the progress of neuroendocrinology: Discovery of GnIH and progress of GnIH research. Front. Endocrinol. 2018, 9, 223. [Google Scholar] [CrossRef]
- Nestor, C.C.; Bedenbaugh, M.N.; Hileman, S.M.; Coolen, L.M.; Lehman, M.N.; Goodman, R.L. Regulation of GnRH pulsatility in ewes. Reproduction 2018, 156, R83–R99. [Google Scholar] [CrossRef]
- Robinson, T.J. Use of progestagen-impregnated sponges inserted intravaginally or subcutaneously for the control of the oestrous cycle in the sheep. Nature 1965, 206, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, B. Effects of reproductive season on embryo development in the buffalo. Reprod. Fertil. Dev. 2019, 31, 68–81. [Google Scholar] [CrossRef]
- Rutledge, J.; Monson, R.; Northey, D.; Leibfried-Rutledge, M. Seasonality of cattle embryo production in a temperate region. Theriogenology 1999, 51, 330. [Google Scholar] [CrossRef]
- Spindler, R.E.; Wildt, D.E. Circannual variations in intraovarian oocyte but not epididymal sperm quality in the domestic cat. Biol. Reprod. 1999, 61, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Si, W.; Wang, H.; Zou, R.; Bavister, B.D.; Ji, W. Effect of age and breeding season on the developmental capacity of oocytes from unstimulated and Follicle-Stimulating Hormone-stimulated rhesus monkeys. Biol. Reprod. 2001, 64, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Mara, L.; Sanna, D.; Casu, S.; Dattena, M.; Muñoz, I.M.M. Blastocyst rate of in vitro embryo production in sheep is affected by season. Zygote 2014, 22, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L. The effect of oocyte quality on development. J. Anim. Sci. 2004, 82, E14–E23. [Google Scholar]
- Sirard, M.A.; Richard, F.; Blondin, P.; Robert, C. Contribution of the oocyte to embryo quality. Theriogenology 2006, 65, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.G.; Palmerini, M.G.; Satta, V.; Succu, S.; Pasciu, V.; Zinellu, A.; Carru, C.; Macchiarelli, G.; Nottola, S.A.; Naitana, S.; et al. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS ONE 2015, 10, e0124911. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Mabuchi, T.; Hirata, S.; Shoda, T.; Kasai, T.; Yokota, S.; Shitara, H.; Yonekawa, H.; Hoshi, K. Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku J. Exp. Med. 2006, 210, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Succu, S.; Gadau, S.D.; Serra, E.; Zinellu, A.; Carru, C.; Porcu, C.; Naitana, S.; Berlinguer, F.; Leoni, G.G. A recovery time after warming restores mitochondrial function and improves developmental competence of vitrified ovine oocytes. Theriogenology 2018, 110, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Blerkom, J. Van Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Nixon, B.; Jones, K.T.; Aitken, R.J. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol. Reprod. 2013, 88, 67. [Google Scholar] [CrossRef]
- Wang, T.; Lessman, C.A. Isoforms of soluble α-tubulin in oocytes and brain of the frog (genus Rana): Changes during oocyte maturation. Cell. Mol. Life Sci. 2002, 59, 2216–2223. [Google Scholar] [CrossRef]
- Combelles, C.M.H.; Albertini, D.F. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of γ-tubulin. Dev. Biol. 2001, 239, 281–294. [Google Scholar] [CrossRef]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Succu, S.; Berlinguer, F.; Porcu, C.; Leoni, G.G.; Naitana, S.; Gadau, S.D. Tubulin posttranslational modifications in in vitro matured prepubertal and adult ovine oocytes. Theriogenology 2018, 114, 237–243. [Google Scholar] [CrossRef]
- Fukushima, N.; Furuta, D.; Hidaka, Y.; Moriyama, R.; Tsujiuchi, T. Post-translational modifications of tubulin in the nervous system. J. Neurochem. 2009, 109, 683–693. [Google Scholar] [CrossRef]
- Serra, E.; Gadau, S.D.; Berlinguer, F.; Naitana, S.; Succu, S. Morphological features and microtubular changes in vitrified ovine oocytes. Theriogenology 2020, 148, 216–224. [Google Scholar] [CrossRef]
- Ledda, S.; Bogliolo, L.; Calvia, P.; Leoni, G.; Naitana, S. Meiotic progression and developmental competence of oocytes collected from juvenile and adult ewes. J. Reprod. Fertil. 1997, 109, 73–78. [Google Scholar] [CrossRef]
- Walker, S.K.; Hill, J.L.; Kleemann, D.O.; Nancarrow, C.D. Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations. Biol. Reprod. 1996, 55, 703–708. [Google Scholar] [CrossRef][Green Version]
- Gadau, S.D. Tubulin post-translational modifications in developing dog primary neurons obtained with methods according to the 3Rs principles. Res. Vet. Sci. 2019, 122, 56–63. [Google Scholar] [CrossRef]
- Gadau, S.D. Morphological and quantitative analysis on α-tubulin modifications in glioblastoma cells. Neurosci. Lett. 2018, 687, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rosa, H.J.D.; Bryant, M.J. Seasonality of reproduction in sheep. Small Rumin. Res. 2003, 48, 155–171. [Google Scholar] [CrossRef]
- Leboeuf, B.; Delgadillo, J.A.; Manfredi, E.; Piacère, A.; Clément, V.; Martin, P.; Pellicer, M.; Boué, P.; Cremoux, R. De Management of Goat Reproduction and Insemination for Genetic Improvement in France. Reprod. Domest. Anim. 2008, 43, 379–385. [Google Scholar] [CrossRef]
- Colleoni, S.; Luciano, A.M.; Gandolfi, F. Cumulus-oocyte communications in the horse: Role of the breeding season and of the maturation medium. Reprod. Domest. Anim. 2004, 39, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, K.; Schmidt, A.L. Meiotic competence in horse oocytes: Interactions among chromatin configuration, follicle size, cumulus morphology, and season. Biol. Reprod. 2000, 62, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Davachi, N.D.; Shahneh, A.Z.; Kohram, H.; Zhandi, M.; Dashti, S.; Shamsi, H.; Moghadam, R. In vitro ovine embryo production: The study of seasonal and oocyte recovery method effects. Iran. Red Crescent Med. J. 2014, 16, e20749. [Google Scholar]
- Ahmadi, E.; Nazari, H.; Hossini-Fahraji, H. Low developmental competence and high tolerance to thermal stress of ovine oocytes in the warm compared with the cold season. Trop. Anim. Health Prod. 2019, 51, 1611–1618. [Google Scholar] [CrossRef]
- Stenbak, T.K.; Redmer, D.A.; Berginski, H.R.; Erickson, A.S.; Navanukraw, C.; Toutges, M.J.; Bilski, J.J.; Kirsch, J.D.; Kraft, K.C.; Reynolds, L.P.; et al. Effects of follicle stimulating hormone (FSH) on follicular development, oocyte retrieval, and in vitro fertilization (IVF) in ewes during breeding season and seasonal anestrus. Theriogenology 2001, 56, 51–64. [Google Scholar] [CrossRef]
- Mitchell, L.M.; Mylne, M.J.A.; Hunton, J.; Matthews, K.; McEvoy, T.G.; Robinson, J.J.; Dingwall, W.S. Ovum recovery from ewes during the peak breeding season and transition to anoestrus. BSAP Occas. Publ. 2004, 30, 327–329. [Google Scholar] [CrossRef]
- Berlinguer, F.; Gonzalez-Bulnes, A.; Succu, S.; Leoni, G.; Mossa, F.; Bebbere, D.; Ariznavarreta, C.; Tresguerres, J.A.F.; Veiga-Lopez, A.; Naitana, S. Effects of progestagens on follicular growth and oocyte developmental competence in FSH-treated ewes. Domest. Anim. Endocrinol. 2007, 32, 303–314. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Veiga-Lopez, A.; Garcia, P.; Garcia-Garcia, R.M.; Ariznavarreta, C.; Sanchez, M.A.; Tresguerres, J.A.F.; Cocero, M.J.; Flores, J.M. Effects of progestagens and prostaglandin analogues on ovarian function and embryo viability in sheep. Theriogenology 2005, 63, 2523–2534. [Google Scholar] [CrossRef]
- Catala, M.G.; Roura, M.; Soto-Heras, S.; Menéndez, I.; Contreras-Solis, I.; Paramio, M.T.; Izquierdo, D. Effect of season on intrafollicular fatty acid concentrations and embryo production after in vitro fertilization and parthenogenic activation of prepubertal goat oocytes. Small Rumin. Res. 2018, 168, 82–86. [Google Scholar] [CrossRef]
- Gou, K.M.; Guan, H.; Bai, J.H.; Cui, X.H.; Wu, Z.F.; Yan, F.X.; An, X.R. Field evaluation of juvenile in vitro embryo transfer (JIVET) in sheep. Anim. Reprod. Sci. 2009, 112, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Brevini Gandolfi, T.A.L.; Gandolfi, F. The maternal legacy to the embryo: Cytoplasmic components and their effects on early development. Theriogenology 2001, 55, 1255–1276. [Google Scholar] [CrossRef]
- Sirard, M.A. Resumption of meiosis: Mechanism involved in meiotic progression and its relation with developmental competence. Theriogenology 2001, 55, 1241–1254. [Google Scholar] [CrossRef]
- Soom, A.V.; Ysebaert, M.T.; Kruif, A. De Relationship between timing of development, morula morphology, and cell allocation to inner cell mass and trophectoderm in in vitro-produced bovine embryos. Mol. Reprod. Dev. 1997, 47, 47–56. [Google Scholar] [CrossRef]
- Leoni, G.G.; Succu, S.; Berlinguer, F.; Rosati, I.; Bebbere, D.; Bogliolo, L.; Ledda, S.; Naitana, S. Delay on the in vitro kinetic development of prepubertal ovine embryos. Anim. Reprod. Sci. 2006, 92, 373–383. [Google Scholar] [CrossRef]
- Torres-Rovira, L.; Gonzalez-Bulnes, A.; Succu, S.; Spezzigu, A.; Manca, M.E.; Leoni, G.G.; Sanna, M.; Pirino, S.; Gallus, M.; Naitana, S.; et al. Predictive value of antral follicle count and anti-Müllerian hormone for follicle and oocyte developmental competence during the early prepubertal period in a sheep model. Reprod. Fertil. Dev. 2014, 26, 1094–1106. [Google Scholar] [CrossRef]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Fulka, J.; First, N.L.; Moor, R.M. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol. Hum. Reprod. 1998, 4, 41–49. [Google Scholar] [CrossRef]
- Leoni, G.G.; Bebbere, D.; Succu, S.; Berlinguer, F.; Mossa, F.; Galioto, M.; Bogliolo, L.; Ledda, S.; Naitana, S. Relations between relative mRNA abundance and developmental competence of ovine oocytes. Mol. Reprod. Dev. 2007, 74, 249–257. [Google Scholar] [CrossRef]
- Bebbere, D.; Bogliolo, L.; Ariu, F.; Fois, S.; Leoni, G.G.; Succu, S.; Berlinguer, F.; Ledda, S. Different temporal gene expression patterns for ovine pre-implantation embryos produced by parthenogenesis or in vitro fertilization. Theriogenology 2010, 74, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Emidio, G.D.; Vento, M.; Ciriminna, R.; Artini, P.G. Cryopreservation and oxidative stress in reproductive cells. Gynecol. Endocrinol. 2010, 26, 563–567. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Blerkom, J.V.; Davis, P.W.; Lee, J. Fertilization and early embryolgoy: ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Zeng, H.T.; Ren, Z.; Yeung, W.S.B.; Shu, Y.M.; Xu, Y.W.; Zhuang, G.L.; Liang, X.Y. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.S.; Wang, Q.; Li, M.; Shi, L.H.; Ola, S.I.; Xiong, B.; Yin, S.; Chen, D.Y.; Sun, Q.Y. Roles of microtubules and microfilaments in spindle movements during rat oocyte meiosis. J. Reprod. Dev. 2008, 54, 391–396. [Google Scholar] [CrossRef]
- Schatten, H.; Sun, Q.Y. Centrosome dynamics during mammalian oocyte maturation with a focus on meiotic spindle formation. Mol. Reprod. Dev. 2011, 78, 757–768. [Google Scholar] [CrossRef]
- Gundersen, G.G.; Bulinski, J.C. Distribution of tyrosinated and nontyrosinated α-tubulin during mitosis. J. Cell Biol. 1986, 102, 1118–1126. [Google Scholar] [CrossRef]
- Viklický, V.; Dráber, P.; Hašek, J.; Bártek, J. Production and characterization of a monoclonal antitubulin antibody. Cell Biol. Int. Rep. 1982, 6, 725–731. [Google Scholar] [CrossRef]
- Schaletzky, J.; Rape, M. Getting a Grip on Microtubules. Cell 2016, 164, 836–837. [Google Scholar] [CrossRef][Green Version]
- Pavin, N.; Tolić-Nørrelykke, I.M. Swinging a sword: How microtubules search for their targets. Syst. Synth. Biol. 2014, 8, 179–186. [Google Scholar] [CrossRef][Green Version]
- Howe, K.; FitzHarris, G. Recent insights into spindle function in mammalian oocytes and early embryos. Biol. Reprod. 2013, 3, 71–89. [Google Scholar]
- Gorbsky, G.J.; Simerly, C.; Schatten, G.; Borisy, G.G. Microtubules in the metaphase-arrested mouse oocyte turn over rapidly. Proc. Natl. Acad. Sci. USA 1990, 87, 6049–6053. [Google Scholar] [CrossRef]
- Hayden, J.H.; Bowser, S.S.; Rieder, C.L. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: Direct visualization in live newt lung cells. J. Cell Biol. 1990, 111, 1039–1045. [Google Scholar] [CrossRef]
- Portran, D.; Schaedel, L.; Xu, Z.; Théry, M.; Nachury, M.V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 2017, 19, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dai, X.; Sun, Y.; Lu, Y.; Zhou, C.; Miao, Y.; Wang, Y.; Xiong, B. Stag3 regulates microtubule stability to maintain euploidy during mouse oocyte meiotic maturation. Oncotarget 2017, 8, 1593–1602. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Molle, G.; Decandia, M.; Ligios, S.; Fois, N.; Treacher, T.T.; Sitzia, M. Grazing management and stocking rate with particular reference to the Mediterranean environment. In Dairy Sheep Nutrition; CABI Publishing: Wallingford, UK, 2009; pp. 191–211. [Google Scholar]
- Ramirez, R.G.; Haenlein, G.F.W.; Núñez-González, M.A. Seasonal variation of macro and trace mineral contents in 14 browse species that grow in northeastern Mexico. Small Rumin. Res. 2001, 39, 153–159. [Google Scholar] [CrossRef]
- Cabiddu, A.; Decandia, M.; Addis, M.; Piredda, G.; Pirisi, A.; Molle, G. Managing Mediterranean pastures in order to enhance the level of beneficial fatty acids in sheep milk. Small Rumin. Res. 2005, 59, 169–180. [Google Scholar] [CrossRef]
- Requena, R.; Molina, P.; Fernandez, N.; Rodriguez, M.; Peris, C.; Torres, A. Changes in Milk and Cheese Composition Throughout Lactation in Manchega Sheep. In Proceedings of the 6th International Symposium on the Milking of Small Rumunants, Athens, Greece, 26 September–1 October 1990; EAAP Publ. No. 95; Wageningen Pers: Wageningen, The Netherlands, 1990. [Google Scholar]
- Barron, L.J.R.; Fernández de Labastida, E.; Perea, S.; Chávarri, F.; Vega, C.D.; Soledad, V.M.; Isabel, T.M.; Isabel, N.A.; Virto, M.; Santisteban, A.; et al. De Seasonal changes in the composition of bulk raw ewe’s milk used for Idiazabal cheese manufacture. Int. Dairy J. 2001, 11, 771–778. [Google Scholar] [CrossRef]
- Nudda, A.; McGuire, M.A.; Battacone, G.; Pulina, G. Seasonal variation in conjugated linoleic acid and vaccenic acid in milk fat of sheep and its transfer to cheese and ricotta. J. Dairy Sci. 2005, 88, 1311–1319. [Google Scholar] [CrossRef]
- Pulina, G.; Nudda, A.; Battacone, G.; Cannas, A. Effects of nutrition on the contents of fat, protein, somatic cells, aromatic compounds, and undesirable substances in sheep milk. Anim. Feed Sci. Technol. 2006, 131, 255–291. [Google Scholar] [CrossRef]
- Mazzone, G.; Giammarco, M.; Vignola, G.; Sardi, L.; Lambertini, L. Effects of the rearing season on carcass and meat quality of suckling Apennine light lambs. Meat Sci. 2010, 86, 474–478. [Google Scholar] [CrossRef]

| Season | No. of Collected Oocytes | In Vitro Matured and Inseminated Oocytes (%) | Cleavage (%) | Blastocyst (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot * | 7 dpf | 8 dpf | 9 dpf | Tot ** | Tot *** | |||||
| Autumn | 898 | 624 | 351 (75.6) | 113 (24.4) | 464 a (74.4) | 8 a (13.8) | 44 a (75.9) | 6 (10.3) | 58 a (12.5) | 58 a |
| (69.5) | (6.5) | |||||||||
| Spring | 1373 | 1005 | 575 (71.4) | 230 (28.6) | 805 b (80.1) | 74 b (46.5) | 71 b (44.6) | 14 (8.8) | 159 b (19.8) | 159 b (11.6) |
| (73.2) | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, E.; Gadau, S.D.; Leoni, G.G.; Naitana, S.; Succu, S. Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte. Animals 2021, 11, 1886. https://doi.org/10.3390/ani11071886
Serra E, Gadau SD, Leoni GG, Naitana S, Succu S. Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte. Animals. 2021; 11(7):1886. https://doi.org/10.3390/ani11071886
Chicago/Turabian StyleSerra, Elisa, Sergio Domenico Gadau, Giovanni Giuseppe Leoni, Salvatore Naitana, and Sara Succu. 2021. "Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte" Animals 11, no. 7: 1886. https://doi.org/10.3390/ani11071886
APA StyleSerra, E., Gadau, S. D., Leoni, G. G., Naitana, S., & Succu, S. (2021). Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte. Animals, 11(7), 1886. https://doi.org/10.3390/ani11071886








