Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Management
2.2. Growth Performance
2.3. Sample Collection
2.4. Serum Biochemical Analysis
2.5. Total RNA Isolation and MRNA Expression of Marker Genes
2.6. Evaluation of MiRNA Expression
2.7. Muscle Fiber Histological Characteristics
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Blood Biochemical Parameters
3.3. Muscle Development-Related Gene Expression
3.4. Integrated Analysis of MiRNAs Related to Muscle Mass Development
3.5. Muscle Fiber’s Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, V.; Sachdev, A.; Gopal, R.; Pramod, A. Roles of important candidate genes on broiler meat quality. Worlds Poult. Sci. J. 2009, 65, 37–50. [Google Scholar] [CrossRef]
- Ylihärsilä, H.; Kajantie, E.; Osmond, C.; Forsen, T.; Barker, D.J.; Eriksson, J.G. Birth size, adult body composition and muscle strength in later life. Int. J. Obes. 2007, 31, 1392–1399. [Google Scholar] [CrossRef] [Green Version]
- Hitachi, K.; Tsuchida, K. Role of microRNAs in skeletal muscle hypertrophy. Front. Physiol. 2014, 4, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Esser, K.A. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J. Appl. Physiol. 2007, 102, 306–313. [Google Scholar] [CrossRef]
- Allen, D.L.; Loh, A.S. Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. Am. J. Physiol. Cell Physiol 2011, 300, C124–C137. [Google Scholar] [CrossRef] [Green Version]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm Jr, R.J.; Olson, E.N. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanes, C.G. Perspectives on the endocrinology of poultry growth and metabolism. Gen. Comp. Endocrinol. 2009, 163, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, Z.; Peng, Q.; Zou, H.; Wang, H.; Yu, X.; Jing, X.; Wang, Y.; Cao, B.; Bao, S. Effects of GHRP-2 and cysteamine administration on growth performance, somatotropic axis hormone and muscle protein deposition in yaks (Bos grunniens) with growth retardation. PLoS ONE 2016, 11, e0149461. [Google Scholar] [CrossRef]
- Martinez, R.; Carpio, Y.; Morales, A.; Lugo, J.M.; Herrera, F.; Zaldívar, C.; Carrillo, O.; Arenal, A.; Pimentel, E.; Estrada, M.P. Oral administration of the growth hormone secretagogue-6 (GHRP-6) enhances growth and non-specific immune responses in tilapia (Oreochromis sp.). Aquaculture 2016, 452, 304–310. [Google Scholar] [CrossRef]
- Mehdi, Y.; Letourneau-Montminy, M.; Gaucher, M.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.; Cote, C.; Ramirez, A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, A.R.; Mohamed, D.I.; Arisha, A.H.; El-Hamid, M.I.A. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef]
- Hashizume, T.; Tanabe, Y.; Ohtsuki, K.; Mori, A.; Matsumoto, N.; Hara, S. Plasma growth hormone (GH) responses after administration of the peptidergic GH secretagogue KP102 into the oral cavity, rumen, abomasum and duodenum in adult goats. Domest. Anim. Endocrinol. 2001, 20, 37–46. [Google Scholar] [CrossRef]
- Hashizume, T.; Kawai, M.; Ohtsuki, K.; Ishii, A.; Numata, M. Oral administration of peptidergic growth hormone (GH) secretagogue KP102 stimulates GH release in goats. Domest. Anim. Endocrinol. 1999, 16, 31–39. [Google Scholar] [CrossRef]
- Walker, R.F.; Codd, E.E.; Barone, F.C.; Nelson, A.H.; Goodwin, T.; Campbell, S.A. Oral activity of the growth hormone releasing peptide His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 in rats, dogs and monkeys. Life Sci. 1990, 47, 29–36. [Google Scholar] [CrossRef]
- Beck, B.; Richy, S.; Stricker-Krongrad, A. Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci. 2004, 76, 473–478. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Dodo, K.-I.; Yahata, K.; Nishimoto, S.; Ueda, H.; Taneike, T.; Kitazawa, T.; Hosaka, Y.; Bungo, T. Intracerebroventricular administration of growth hormone releasing peptide-6 (GHRP-6) inhibits food intake, but not food retention of crop and stomach in neonatal chicks. J. Poult. Sci. 2006, 43, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, Y.; Lee, S.; Kuwayama, H.; Hidari, H.; You, S. Effects of dietary protein and growth hormone-releasing peptide (GHRP-2) on plasma IGF-1 and IGFBPs in Holstein steers. Domest. Anim. Endocrinol. 2005, 28, 134–146. [Google Scholar] [CrossRef]
- Aviagen, W. Ross 308: Broiler’s Management and Nutrition Specification. 2018. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 23 June 2021).
- Ibrahim, D.; Sewid, A.H.; Arisha, A.H.; Abd El-Fattah, A.H.; Abdelaziz, A.M.; Al-Jabr, O.A.; Kishawy, A.T. Influence of Glycyrrhiza glabra Extract on Growth, Gene Expression of Gut Integrity, and Campylobacter jejuni Colonization in Broiler Chickens. Front. Vet. Sci. 2020, 7, 612063. [Google Scholar] [CrossRef]
- Ibrahim, D.; El-Sayed, R.; Khater, S.I.; Said, E.N.; El-Mandrawy, S.A. Changing dietary n-6:n-3 ratio using different oil sources affects performance, behavior, cytokines mRNA expression and meat fatty acid profile of broiler chickens. Anim. Nutr. 2018, 4, 44–51. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Shahin, S.; Sherief, W.; Farag, M.; Nassan, M. Impact of fermented or enzymatically fermented dried olive pomace on growth, expression of digestive enzymes and glucose transporters genes, oxidative stability of frozen meat and economic efficiency of broiler chickens. Front. Vet. Sci. 2021, 8, 442. [Google Scholar] [CrossRef]
- ROH, S.-G.; MATSUNAGA, N.; HIDAKA, S.; HIDARI, H. Characteristics of growth hormone secretion responsiveness to growth hormone-releasing peptide-2 (GHRP-2 or KP102) in calves. Endocr. J. 1996, 43, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Czimmerer, Z.; Hulvely, J.; Simandi, Z.; Varallyay, E.; Havelda, Z.; Szabo, E.; Varga, A.; Dezso, B.; Balogh, M.; Horvath, A. A versatile method to design stem-loop primer-based quantitative PCR assays for detecting small regulatory RNA molecules. PLoS ONE 2013, 8, e55168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Arisha, A.H.; Abd El-Aziz, R.M.; Sherief, W.R.; Adil, S.H.; El Sayed, R.; Metwally, A.E. Impact of feeding anaerobically fermented feed supplemented with acidifiers on its quality and growth performance, intestinal villi and enteric pathogens of mulard ducks. Livest. Sci. 2020, 242, 104299. [Google Scholar] [CrossRef]
- Alba, M.; Fintini, D.; Bowers, C.Y.; Parlow, A.; Salvatori, R. Effects of long-term treatment with growth hormone-releasing peptide-2 in the GHRH knockout mouse. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E762–E767. [Google Scholar] [CrossRef] [PubMed]
- Nijland, E.A.; Strasburger, C.J.; Popp-Snijders, C.; Van der Wal Eduard, P.; Van der Veen, A. A five day treatment with daily subcutaneous injections of growth hormone-releasing peptide-2 causes response attenuation and does not stimulate insulin-like growth factor-I secretion in healthy young men. Eur. J. Endocrinol. 1998, 139, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, J. MicroRNAs in skeletal myogenesis. Cell Cycle 2011, 10, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.-Z. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, C.; Hennebry, A.; Thomas, M.; Plummer, E.; Ling, N.; Sharma, M.; Kambadur, R. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp. Cell Res. 2008, 314, 317–329. [Google Scholar] [CrossRef]
- McCroskery, S.; Thomas, M.; Maxwell, L.; Sharma, M.; Kambadur, R. Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 2003, 162, 1135–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crist, C.G.; Montarras, D.; Pallafacchina, G.; Rocancourt, D.; Cumano, A.; Conway, S.J.; Buckingham, M. Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc. Natl. Acad. Sci. USA 2009, 106, 13383–13387. [Google Scholar] [CrossRef] [Green Version]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential amino acids increase microRNA-499,-208b, and-23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J. Nutr. 2009, 139, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Kishawy, A.T.; Khater, S.I.; Hamed Arisha, A.; Mohammed, H.A.; Abdelaziz, A.S.; El-Rahman, A.; Ghada, I.; Elabbasy, M.T. Effect of dietary modulation of selenium form and level on performance, tissue retention, quality of frozen stored meat and gene expression of antioxidant status in ross broiler chickens. Animals 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963, 42, 283–290. [Google Scholar] [CrossRef]
- Iwamoto, H.; Hara, Y.; Ono, Y.; Takahara, H. Breed differences in the histochemical properties of the M. iliotibialis lateralis myofibre of domestic cocks. Br. Poult. Sci. 1992, 33, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Q.; Tang, M.; Ji, C. Development of breast muscle and meat quality in Arbor Acres broilers, Jingxing 100 crossbred chickens and Beijing fatty chickens. Meat Sci. 2007, 77, 220–227. [Google Scholar] [CrossRef]
- Koomkrong, N.; Theerawatanasirikul, S.; Boonkaewwan, C.; Jaturasitha, S.; Kayan, A. Breed-related number and size of muscle fibres and their response to carcass quality in chickens. Ital. J. Anim. Sci. 2015, 14, 4145. [Google Scholar] [CrossRef] [Green Version]
- Dransfield, E.; Sosnicki, A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999, 78, 743–746. [Google Scholar] [CrossRef] [PubMed]


 Correspond to upregulation of miRNA expression relative to the control group (p < 0.05).
Correspond to upregulation of miRNA expression relative to the control group (p < 0.05).  Correspond to downregulation of miRNA expression relative to control group (p < 0.05).
Correspond to downregulation of miRNA expression relative to control group (p < 0.05).
 Correspond to upregulation of miRNA expression relative to the control group (p < 0.05).
Correspond to upregulation of miRNA expression relative to the control group (p < 0.05).  Correspond to downregulation of miRNA expression relative to control group (p < 0.05).
Correspond to downregulation of miRNA expression relative to control group (p < 0.05).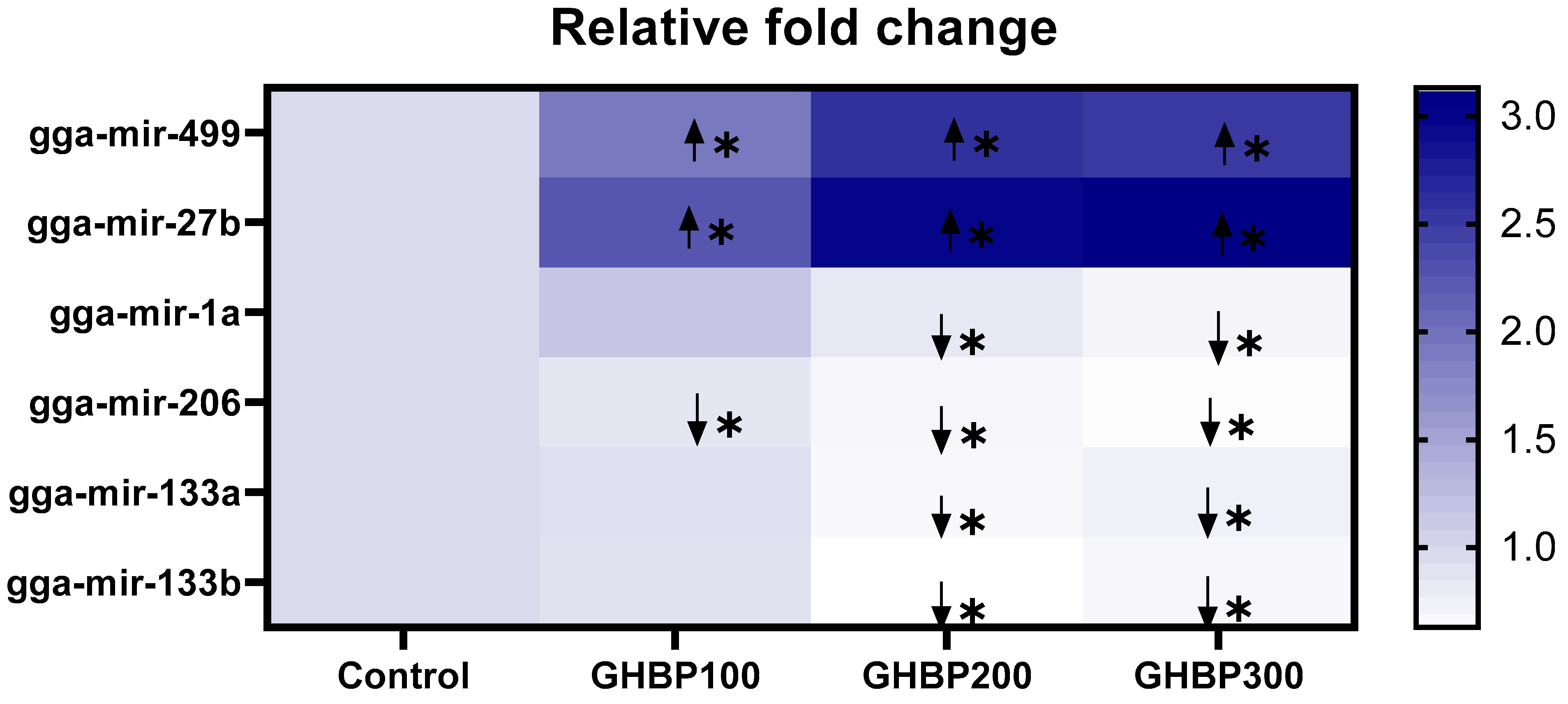

| Ingredients | Starter (0–10 days) | Grower (11–22 days) | Finisher (23–38 days) |
|---|---|---|---|
| Yellow corn | 53.03 | 56.6 | 59.90 |
| Soybean meal, 44% | 33.10 | 29.1 | 25.12 |
| Corn gluten, 60% | 7.04 | 7.05 | 7.00 |
| Soybean oil | 2.40 | 3.20 | 4.11 |
| Limestone | 1.52 | 1.36 | 1.28 |
| Monocalcium phosphate | 1.80 | 1.65 | 1.53 |
| Common salt | 0.38 | 0.38 | 0.38 |
| Premix * | 0.30 | 0.30 | 0.30 |
| DL-methionine, 98% | 0.13 | 0.11 | 0.11 |
| Lysine, Hcl, 78% | 0.25 | 0.21 | 0.22 |
| Antitoxin | 0.05 | 0.05 | 0.05 |
| Chemical composition | |||
| ME, Kcal/Kg | 3000.95 | 3100.02 | 3200.40 |
| CP% | 23.01 | 21.50 | 20.00 |
| EE% | 4.86 | 5.76 | 6.76 |
| CF% | 3.56 | 3.36 | 3.15 |
| Ca% | 0.96 | 0.87 | 0.81 |
| Available P% | 0.48 | 0.44 | 0.41 |
| Lysine% | 1.28 | 1.15 | 1.06 |
| Methionine% | 0.51 | 0.47 | 0.45 |
| Threonine% | 0.86 | 0.80 | 0.74 |
| Gene | Primer Sequence (5′-3′) | Accession No. |
|---|---|---|
| MSTN | F: ATGCAGATCGCGGTTGATC R: GCGTTCTCTGTGGGCTGACT | NM_001001461.1 |
| MyoD | F: CAGCAGCTACTACACGGAATCA R: GGAAATCCTCTCCACAATGCTT | NM_204214.2 |
| Myogenin | F: GGAGAAGCGGAGGCTGAAG R: GCAGAGTGCTGCGTTTCAGA | NM_204184.1 |
| mTOR | F: CATGTCAGGCACTGTGTCTATTCTC R: CTTTCGCCCTTGTTTCTTCACT | XM_417614.5 |
| IGF-1 | F: GCTGCCGGCCCAGAA R: ACGAACTGAAGAGCATCAACCA | NM_001004384.2 |
| Pax3 | F: ACTACCCTGACATTTATACTCG TGCCTGCTTCCTCCATCTAG | NM_204269.1 |
| Pax7 | F: AGGCTGACTTCTCCATCTCTCCT R: TGTAACTGGTGGTGCTGTAGGTG | XM_015296832.1 |
| House keeping | ||
| GAPDH | F: CAACCCCCAATGTCTCTGTT R: TCAGCAGCAGCCTTCACTAC | NM205518 |
| U6 | TTCAGGCTCTTGGACGATTT CCGCTATTCCCAAGACTGAA | NM_001277862.1 |
| MicroRNA | Primer Name | Primer Sequence |
|---|---|---|
| gga-mir-1a | Stem-loop RT | 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTATGGG-3′ |
| gga-mir-1a | Forward | 5′-TGGGGGGGACATACTTCTTTATATG-3′ |
| gga-mir-27b | Stem-loop RT | 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTGTTCA-3′ |
| gga-mir-27b | Forward | 5′-GGTTTTTTTTAGAGCTTAGCTGATTGG-3′ |
| gga-mir-133a | Stem-loop RT | 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACGATTTG-3′ |
| gga-mir-133a | Forward | 5′-GGTGTTTTTAGCTGGTAAAATGGAAC-3′ |
| gga-mir-133b | Stem-loop RT | 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTAGCTG-3′ |
| gga-mir-133b | Forward | 5′-TTTTTGTTTTTTGGTCCCCTTCAAC-3′ |
| gga-mir-206 | Stem-loop RT | 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCCACAC-3′ |
| gga-mir-206 | Forward | 5′-GTTTGGTGTGGAATGTAAGGAAGT-3′ |
| gga-mir-499 | Stem-loop RT | 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCTAAAC-3′ |
| gga-mir-499 | Forward | 5′-TGGTTTTTTGGTTAAGACTTGTAGTGAT-3′ |
| Parameter | Control | GHBP100 | GHBP200 | GHBP300 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Starter (1–10 days) | ||||||
| BW, g | 328 b | 335 a | 339 a | 336 a | 5.64 | <0.001 |
| BWG, g | 283 b | 290 a | 295 a | 292 a | <0.001 | 0.01 |
| FI, g | 361 a | 361 a | 346 c | 353 b | 4.38 | 0.03 |
| FCR | 1.28 a | 1.23 b | 1.17 c | 1.21 b | 6.98 | 0.02 |
| Grower (11–22 days) | ||||||
| BW, g | 1216 d | 1240 c | 1281 a | 1269 b | 33.46 | 0.02 |
| BWG, g | 888 b | 905 b | 942 a | 933 a | 28.47 | <0.001 |
| FI, g | 1606 d | 1570 c | 1501 a | 1538 b | 45.31 | 0.03 |
| FCR | 1.81 a | 1.74 b | 1.59 d | 1.65 c | <0.001 | <0.001 |
| Finisher (23–38 days) | ||||||
| BW, g | 2321 d | 2456 c | 2714 a | 2576 b | 43.18 | <0.001 |
| BWG, g | 1105 d | 1216 c | 1433 a | 1307 b | 34.44 | <0.001 |
| FI, g | 2309 | 2321 | 2433 | 2380 | 39.18 | 0.30 |
| FCR | 2.09 a | 1.91 b | 1.70 c | 1.82 b | 0.03 | <0.001 |
| Overall performance (1–38 days) | ||||||
| BW, g | 2321 d | 2456 c | 2714 a | 2576 b | 43.18 | <0.001 |
| BWG, g | 2276 d | 2411 c | 2670 a | 2532 b | 55.65 | <0.001 |
| FI, g | 4276 | 4248 | 4280 | 4271 | 46.18 | 0.964 |
| FCR | 1.88 a | 1.76 b | 1.60 d | 1.69c | <0.001 | <0.001 |
| Parameter | Control | GHBP100 | GHBP200 | GHBP300 | SEM | p-Value |
|---|---|---|---|---|---|---|
| ALT, U/L | 37.9 | 36.8 | 36.2 | 39.1 | 0.520 | 0.217 |
| AST, U/L | 36.6 | 34.3 | 33.1 | 33.2 | 0.650 | 0.210 |
| Uric acid, μmol/L | 11.2 | 11.1 | 13.0 | 13.5 | 0.589 | 0.390 |
| Creatinine, mg/dL | 0.63 | 0.70 | 0.68 | 0.71 | 0.016 | 0.316 |
| Cholesterol, mg/dL | 98.4 a | 90.7 b | 89.9 b | 83.3c | 1.68 | <0.001 |
| TGs, mg/dL | 87.6 | 85.9 | 89.6 | 82.9 | 1.18 | 0.241 |
| HDL-C, mg/dL | 34.9 c | 41.1 b | 41.7 b | 52.0 a | 1.93 | <0.001 |
| LDL-C, mg/dL | 45.9 a | 32.4 b | 30.4 b | 14.7 c | 3.43 | <0.001 |
| VLDL-C, mg/dL | 17.5 | 17.2 | 17.9 | 16.6 | 0.235 | 0.241 |
| IGF-1 day 20, ng/mL | 9.53 c | 15.27 b | 18.50 a | 13.55 b | 1.01 | <0.001 |
| IGF-1 day 38, ng/mL | 10.73 c | 16.47 b | 19.70 a | 14.75 b | 1.00 | <0.001 |
| GH day 20, ng/mL | 6.87 b | 7.67 b | 10.07 a | 9.52 a | 0.438 | 0.003 |
| GH day 38, ng/mL | 8.80 c | 10.20 b | 12.30 a | 10.73 b | 0.390 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.; Al-Khalaifah, H.S.; Abdelfattah-Hassan, A.; Eldoumani, H.; Khater, S.I.; Arisha, A.H.; Mohamed, S.A.M.; Ismail, T.A.; Tolba, S.A. Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens. Animals 2021, 11, 1906. https://doi.org/10.3390/ani11071906
Ibrahim D, Al-Khalaifah HS, Abdelfattah-Hassan A, Eldoumani H, Khater SI, Arisha AH, Mohamed SAM, Ismail TA, Tolba SA. Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens. Animals. 2021; 11(7):1906. https://doi.org/10.3390/ani11071906
Chicago/Turabian StyleIbrahim, Doaa, Hanan S. Al-Khalaifah, Ahmed Abdelfattah-Hassan, Haitham Eldoumani, Safaa I. Khater, Ahmed H. Arisha, Sally A. M. Mohamed, Tamer Ahmed Ismail, and Samar A. Tolba. 2021. "Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens" Animals 11, no. 7: 1906. https://doi.org/10.3390/ani11071906
APA StyleIbrahim, D., Al-Khalaifah, H. S., Abdelfattah-Hassan, A., Eldoumani, H., Khater, S. I., Arisha, A. H., Mohamed, S. A. M., Ismail, T. A., & Tolba, S. A. (2021). Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens. Animals, 11(7), 1906. https://doi.org/10.3390/ani11071906






