Short-Term Spirulina (Spirulina platensis) Supplementation and Laying Hen Strain Effects on Eggs’ Lipid Profile and Stability
Abstract
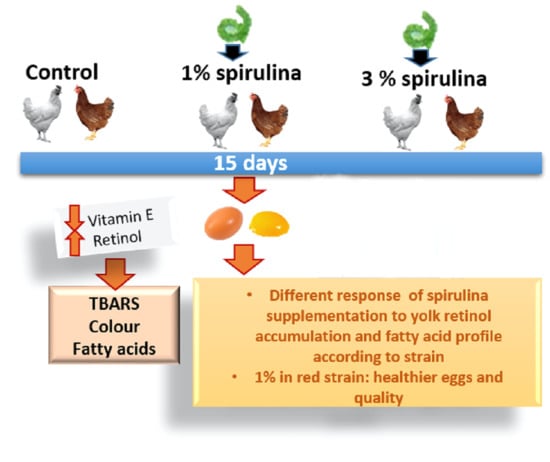
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Diets and Sample Collection
2.2. Laboratory Analysis
2.2.1. Extraction and Quantification of Total Fat and Fatty Acid Profile of Feed Samples
2.2.2. Instrumental Color Analysis.
2.2.3. Iron-Induced Lipid Oxidation of Yolk Samples.
2.2.4. Extraction and Quantification of Vitamins E and A in Feed and Yolk Samples
2.2.5. Extraction and Quantification of Total Fat and Fatty Acid Profile of Yolk Samples
2.2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, A.; Misra, N.; Srivastav, D.; Umapathy, D.; Kumar, S. Spirulina-the nature’s wonder: A review. Sch. J. App. Med. Sci. 2014, 2, 1334–1339. [Google Scholar]
- Shabana, K.A.; Arabi, M.S. Spirulina-an overview. Int. J. Pharm. Pharm. Sci. 2012, 4, 9–15. [Google Scholar]
- Vonshak, A. Use of spirulina biomass. In Spirulina platensis (Arthorospira): Physiology, Cell-Biology and Biotechnology; Voshak, A., Ed.; Taylor & Francis: Abingdon, UK, 1997; pp. 175–200. [Google Scholar]
- Anderson, D.W.; Chung-Shih, T.; Ross, E. The xanthophylls of spirulina and their effect on egg yolk pigmentation. Poult. Sci. 1991, 70, 115–119. [Google Scholar] [CrossRef]
- Mariey, Y.; Samak, A.H.R.; Ibrahem, M.A. Effect of using spirulina platensis algae as a feed additive for poultry diets: Productive and reproductive performances of local laying hens. Egypt. Poult. Sci. 2012, 32, 201–215. [Google Scholar]
- Selim, S.; Hussein, E.; Abou-Elkhair, R. Effect of spirulina platensis as a feed additive on laying performance, egg quality and hepatoprotective activity of laying hens. Eur. Poult. Sci. 2018, 82, 1612–9199. [Google Scholar] [CrossRef]
- Omri, B.; Amraoui, M.; Tarek, A.; Lucarini, M.; Durazzo, A.; Cicero, N.; Santini, A.; Kamoun, M. Arthrospira Platensis (Spirulina) supplementation on laying hens’ performance: Eggs physical, chemical, and sensorial qualities Besma. Foods 2019, 8, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.S.; Cintra, R.G.; Barros, S.B.; Mancini Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef]
- Cohen, Z. The Chemicals of Spirulina. In Spirulina platensis (Arthorospira): Physiology, Cell-Biology and Biotechnology; Voshak, A., Ed.; Taylor & Francis: Abingdon, UK, 1997; pp. 205–211. [Google Scholar]
- Kapoor, R.; Huang, Y.-S. Gamma Linolenic Acid: An Antiinflammatory Omega-6 Fatty Acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [Green Version]
- Tufarelli, V.; Baghban-Kanani, P.; Azimi-Youvalari, S.; Hosseintabar-Ghasemabad, B.; Slozhenkina, B.; Gorlov, I.; Seidavi, A.; Ayasan, T.; Laudadio, V. Effects of horsetail (Equisetum arvense) and spirulina (Spirulina platensis): Dietary Supplementation on laying hens productivity and oxidative status. Animals 2021, 11, 335. [Google Scholar] [CrossRef]
- Boiago, M.M.; Dilkin, J.D.; Kolm, M.; Barreta, M.; Souza, C.F.; Baldissera, M.D.; Dos Santos, I.D.; Wagner, R.; Tavernari, F.D.C.; Da Silva, M.L.B.; et al. Spirulina platensis in Japanese quail feeding alters fatty acid profiles and improves egg quality: Benefits to consumers. J. Food Biochem. 2019, 43, e12860. [Google Scholar] [CrossRef]
- Chen, G.; Cai, Y.; Su, Y.; Gao, B.; Wu, H.; Cheng, J. Effects of spirulina algae as a feed supplement on nutritional value and flavour components of silkie hens eggs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1408–1417. [Google Scholar] [CrossRef]
- Naber, E.C. Modifying vitamin composition of eggs: A review. Appl. Poult. Sci. Res. 1993, 2, 385–393. [Google Scholar] [CrossRef]
- Olivares, A.; Rey, A.I.; Daza, A.; López-Bote, C.J. High dietary vitamin A interferes with tissue -tocopherol concentrations in fattening pigs: A study that examines administration and withdrawal times. Animal 2009, 3, 1264–1270. [Google Scholar] [CrossRef]
- Grobas, S.; Méndez, J.; Lopez-Bote, C.; De Blas, C.; Mateos, G.G. Effect of Vitamin E and A Supplementation on Egg Yolk α-Tocopherol Concentration. Poult. Sci. 2002, 81, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; Amazan, D.; Cordero, G.; Olivares, A.; López-Bote, C.J. Lower oral doses of micellized α-tocopherol compared to α-tocopheryl acetate in feed modify fatty acid profiles and improve oxidative status in pigs. Int. J. Vitam. Nutr. Res. 2014, 84, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; Segura, J.; Olivares, A.; Cerisuelo, A.; Piñeiro, C.; López-Bote, C.J. Effect of micellized natural (D-α-tocopherol) vs. synthetic (DL-α-tocopheryl acetate) vitamin E supplementation given to turkeys on oxidative status and breast meat quality characteristics. Poult. Sci. 2015, 94, 1259–1269. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Grobas, S.; Méndez, J.; Lázaro, R.; De Blas, C.; Mateos, G.G. Influence of source and percentage of fat added to diet on performance and fatty acid composition of egg yolks of two Strains of laying hens. Poult. Sci. 2001, 80, 1171–1179. [Google Scholar] [CrossRef]
- Franco, D.; Rois, D.; Arias, A.; Justo, J.R.; Marti-Quijal, F.J.; Khubber, S.; Barba, F.J.; López-Pedrouso, M.; Lorenzo, J.M. Effect of breed and diet type on the freshness and quality of the eggs: A comparison between Mos (indigenous galician breed) and Isa brown hens. Foods 2020, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- BOE. RD3/2002 de 11 de Enero por el Que se Establecen las Normas Mínimas de Protección de las Gallinas Ponedoras; Boletín Oficial del Estado nº 13 de 15 de enero de; BOE: Madrid, Spain, 2002; pp. 1–7. [Google Scholar]
- BOE. RD 53/2013, de 21 de Octubre por la Que se Establecen las Normas Básicas Aplicables para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. In Bol. Of. Estado; 2013; Volume 252, pp. 34367–34391. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry; National Research Council, The National Academic Press: Washington, DC, USA, 1994. [Google Scholar]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- CIE (Commission Internationale de l’Eclairage). Recommendations on Uniform Colour Spaces, Color-Difference Equations, and Metric Color Terms; Suppl. 2 to CIE Publication No. 15 (E-1.3.1.) 1978, 1971/(TC-1-3); Commission Internationale de l’Eclairage: Paris, France, 1976. [Google Scholar]
- Kornbrust, D.J.; Mavis, R.D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: Correlation with vitamin E content. Lipids 1980, 15, 315–322. [Google Scholar] [CrossRef]
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Quantitative study of the α- and γ-tocopherols accumulation in muscle and back fat from Iberian pigs kept free-range as affected by time of free-range feeding or weight gain. Anim. Sci. 2006, 82, 901–908. [Google Scholar] [CrossRef]
- Segura, J.; Lopez-Bote, C.J. A laboratory efficient method for intramuscular fat analysis. Food Chem. 2014, 145, 821–825. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty acid and cholesterol profiles and hypocholesterolemic, atherogenic, and thrombogenic indices of table eggs in the retail market. Lipids Health Dis. 2015, 14, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korniluk, K.; Gabryszuk, M.; Kowalczyk, I.; Czauderna, M. Effect of diet supplementation with selenium, zinc and α-tocopherol on fatty acid composition in the liver and loin muscle of lambs. Anim. Sci. Pap. Rep. 2008, 26, 59–70. [Google Scholar]
- Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poult. Sci. 2012, 91, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina from growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Süleyman, C. The Important of Beta Carotene on Poultry Nutrition. Selcuk J. Agric. Food Sci. 2019, 33, 252–259. [Google Scholar]
- Pestana, J.M.; Puerta, B.; Santos, H.; Madeira, M.S.; Alfaia, C.M.; Lopes, P.A.; Pinto, R.M.A.; Lemos, J.P.C.; Fontes, C.M.G.A.; Lordelo, M.M.; et al. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020, 99, 2519–2532. [Google Scholar] [CrossRef]
- Ayuso, M.; Ovilo, C.; Fernández, A.; Nuñez, Y.; Isabel, B.; Daza, A.; López-Bote, C.J.; Rey, A.I. Effects of dietary vitamin A supplementation or restriction and its timing on retinol and α-tocopherol accumulation and gene expression in heavy pigs. Anim. Feed Sci. Technol. 2015, 202, 62–74. [Google Scholar] [CrossRef]
- Jiang, Y.H.; McGeachin, R.B.; Bailey, C.A. α-Tocopherol, β-Carotene, and Retinol Enrichment of Chicken Eggs. Poult. Sci. 1994, 73, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Surai, I.F.; Ionov, I.A.; Kuklenko, T.V.; Kostjuk, I.A.; Acpherson, A.M.; Speake, B.K.; Noble, R.C.; Sparks, N.H.C. Effect of supplementing the hen’s diet with vitamin A on the accumulation of vitamins A and E, ascorbic acid and carotenoids in the egg yolk and in the embryonic liver. Br. Poult. Sci. 1998, 39, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; López-Bote, C.J.; Kerry, J.P.; Lynch, P.B.; Buckley, D.J.; Morrissey, P.A. Modification of lipid composition and oxidation in porcine muscle and muscle microsomes as affectd by dietary supplementation of n-3 with either n-9 or n-6 fatty acids and α-tocopheryl acetate. Anim. Feed Sci. Technol. 2004, 113, 223–238. [Google Scholar] [CrossRef]
- Cuervo, M.; Corbalán, M.; Baladía, E.; Cabrerizo, L.; Formiguera, X.; Iglesias, C.; Lorenzo, H.; Polanco, I.; Quiles, J.; Romero de Ávila, M.D.; et al. Comparativa de las Ingestas Dietéticas de Referencia (IDR) de los diferentes países de la Unión Europea, de Estados Unidos (EEUU) y de la Organización Mundial de la Salud (OMS). Nutr. Hosp. 2009, 24, 384–414. [Google Scholar] [PubMed]
- Zahroojian, N.; Morave, H.; Shivazad, M. Effects of dietary marine algae (Spirulina platensis) on egg quality and production performance of laying hens. J. Agric. Sci. Technol. 2013, 15, 1353–1360. [Google Scholar]
- Ledvinka, Z.; Tůmová, E.; Englmaierová, M.; Podsedníček, M. Egg quality of three laying hen genotypes kept in conventional cages and on litter. Arch. Geflügelk. 2012, 76, 38–43. [Google Scholar]
- Rajesha, J.; Madhusudhan, B.; Swamy, M.M.; Rao, R.J.; Ravishankar, G.A.; Rangarao, A.; Karunakumar, M. Effects of flaxseed and spirulina biomass in layer diet on lipid profile and quality characteristics of egg yolk. J. Food Sci. Technol. Mys. 2009, 46, 509–514. [Google Scholar]
- Ahn, D.U.; Sunwoo, H.H.; Wolfe, F.H.; Sim, J.S. Effects of dietary α-linolenic acid and strain of hen on the fatty acid composition, storage ability, and flavor characteristics of chicken eggs. Poult. Sci. 1995, 74, 1540–1547. [Google Scholar] [CrossRef]
- Scheideler, S.E.; Jaroni, D.; Froning, G. Strain and age effects on egg composition from hens fed diets rich in n-3 fatty acids. Poult. Sci. 1998, 77, 192–196. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Dietary levels of chia: Influence on yolk cholesterol, lipid content and fatty acid composition for two strains of hens. Poult. Sci. 2000, 79, 724–739. [Google Scholar] [CrossRef]
- Olivares, A.; Daza, A.; Rey, A.I.; López-Bote, C. Dietary vitamin A concentration alters fatty acid composition in pigs. Meat Sci. 2009, 81, 291–299. [Google Scholar] [CrossRef]
- Daniel, Z.C.T.R.; Salter, A.M.; Buttery, P.J. Vitamin A regulation of stearoyl-CoA desaturase mRNA levels and fatty acid composition in sheep tissues. Anim. Sci. 2004, 78, 237–243. [Google Scholar] [CrossRef]
- Olivares, A.; Daza, A.; Rey, A.I.; López-Bote, C. Interactions between genotype, dietary fat saturation and vitamin A concentration on intramuscular fat content and fatty acid composition in pigs. Meat Sci. 2009, 89, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vajreswari, A.; Giridharan, N.V. Vitamin A regulates obesity in WNIN/Ob obese rat; independent of stearoyl-CoA desaturase-1. Biochem. Biophys. Res. Commun. 2008, 370, 243–247. [Google Scholar] [CrossRef]
- Calvo, L.; Segura, J.; Toldra, F.; Flores, M.; Rodriguez, A.I.; López-Bote, C.J.; Rey, A.I. Meat quality, free fatty acid concentration, and oxidative stability of pork from animals fed diets containing different sources of selenium. Food Sci. Technol. Int. 2017, 23, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; Carmona, J.M.; Daza, A.; Sanz, M.; López-Bote, C.J. Quantitative study on fatty acid profile, oxidation and rheological properties of fat and breast muscle from broilers as affected by days of feeding a linoleic acid-enriched diet. Arch. Geflügelk. 2010, 74, 133–140. [Google Scholar]
- Michalak, I.; Andrys, M.; Korczyński, M.; Opaliński, S.; Łęska, B.; Konkol, D.; Wilk, R.; Rój, E.; Chojnacka, K. Biofortification of hens eggs with polyunsaturated fatty acids by new dietary formulation: Supercritical microalgal extract. Animals 2020, 10, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaie, S.; Zirak-Khattab, F.; Hosseini, S.A.; Donyaei-Darian, H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian Australas. J. Anim. Sci. 2017, 31, 556–563. [Google Scholar] [CrossRef]

| Ingredients (%). | Control | 1%-SP | 3%-SP |
|---|---|---|---|
| Corn grain | 63.80 | 64.60 | 65.80 |
| Soybean meal (44%CP) | 23.60 | 22.00 | 18.80 |
| Sunflower oil | 1.50 | 1.30 | 1.30 |
| Calcium carbonate | 8.90 | 8.90 | 8.80 |
| Monocalcium phosphate | 1.30 | 1.30 | 1.30 |
| DL-Methionine | 0.10 | 0.10 | 0.10 |
| L-Lysine 50 | 0.00 | 0.00 | 0.10 |
| Premix 2 | 0.50 | 0.50 | 0.50 |
| Spirulina 3 | 0.00 | 1.00 | 3.00 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vitamin E 50% (dl-α-tocopheryl acetate) | 0.04 | 0.04 | 0.04 |
| Nutrients (% Total Feed) | |||
| Metabolizable energy (kcal/kg) | 2763.50 | 2763.60 | 2763.90 |
| Crude protein | 16.50 | 16.50 | 16.50 |
| Crude fat | 3.90 | 3.80 | 3.50 |
| Crude fiber | 2.80 | 2.80 | 2.70 |
| Lys | 1.05 | 1.03 | 1.03 |
| Met+Cys | 0.69 | 0.69 | 0.69 |
| Trp | 0.19 | 0.19 | 0.19 |
| Calcium | 3.70 | 3.70 | 3.70 |
| Total phosphorous | 0.61 | 0.61 | 0.60 |
| Available phosphorous | 0.37 | 0.37 | 0.37 |
| C18:2 | 2.18 | 2.12 | 2.01 |
| Starch | 41.9 | 42.5 | 43.56 |
| Treatments | Control | 1%-SP | 3%-SP | |||
|---|---|---|---|---|---|---|
| Mean | SD 7 | Mean | SD | Mean | SD | |
| Fatty acids (g/100 g total fatty acids) | ||||||
| C14:0 | 0.089 | 0.007 | 0.092 | 0.002 | 0.124 | 0.011 |
| C15:1 | 0.182 | 0.023 | 0.137 | 0.046 | 0.148 | 0.002 |
| C16:0 | 12.184 | 0.800 | 12.501 | 0.105 | 14.265 | 0.156 |
| C17:0 | 0.414 | 0.044 | 0.465 | 0.023 | 0.463 | 0.032 |
| C17:1 | 0.068 | 0.003 | 0.075 | 0.011 | 0.080 | 0.001 |
| C18:0 | 3.149 | 0.149 | 2.973 | 0.041 | 3.047 | 0.003 |
| C18:1n-9 | 25.128 | 1.133 | 24.856 | 0.912 | 23.815 | 0.052 |
| C18:1n-7 | 0.824 | 0.009 | 0.866 | 0.015 | 0.844 | 0.010 |
| C18:2n-6 | 54.935 | 0.204 | 54.666 | 0.668 | 52.855 | 0.129 |
| C18:3n-6 | 0.035 | 0.001 | 0.364 | 0.010 | 1.264 | 0.003 |
| C18:3n-3 | 2.218 | 0.269 | 2.183 | 0.034 | 2.291 | 0.036 |
| C20:0 | 0.400 | 0.005 | 0.415 | 0.010 | 0.418 | 0.001 |
| C20:1 | 0.286 | 0.019 | 0.320 | 0.011 | 0.302 | 0.010 |
| C22:4n-6 | 0.036 | 0.016 | 0.031 | 0.004 | 0.029 | 0.000 |
| C22:5n-3 | 0.027 | 0.001 | 0.029 | 0.002 | 0.028 | 0.002 |
| C22:6n-3 | 0.024 | 0.004 | 0.028 | 0.003 | 0.028 | 0.000 |
| ∑SAT 2 | 16.235 | 1.005 | 16.445 | 0.180 | 18.317 | 0.204 |
| ∑MUFA 3 | 26.489 | 1.186 | 26.254 | 0.996 | 25.189 | 0.076 |
| ∑PUFA 4 | 57.276 | 0.497 | 57.301 | 0.722 | 56.494 | 0.170 |
| ∑n-6 5 | 55.006 | 0.222 | 55.060 | 0.682 | 54.147 | 0.132 |
| ∑n-3 6 | 2.270 | 0.275 | 2.240 | 0.040 | 2.347 | 0.038 |
| Tocopherols (µg/g) | ||||||
| α-Tocopherol | 257.118 | 43.400 | 279.481 | 59.637 | 381.518 | 59.826 |
| γ-Tocopherol | 18.559 | 6.174 | 18.014 | 6.047 | 14.985 | 1.781 |
| Yolk Parameters | Treatment Effect | Strain Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 1% | 3% | WL | RIR | SEM 1 | SEM 2 | 3p Treatment | p Strain | p Treatment × Strain | |
| Egg weight, g | 61.4 | 63.1 | 62.5 | 61.6 | 63.1 | 0.81 | 0.83 | 0.3210 | 0.1107 | 0.0964 |
| Yolk composition | ||||||||||
| Humidity, % | 51.0 | 50.4 | 50.9A | 51.4 A | 50.2 B | 0.33 | 0.33 | 0.4060 | 0.0012 | 0.3475 |
| Fat % | 30.6 | 30.6 | 29.9 | 29.9 B | 30.9 A | 0.32 | 0.32 | 0.1706 | 0.0046 | 0.5091 |
| α-Tocopherol, µg/g | 227.7 a | 207.7 ba | 168.1 b | 165.5 B | 236.8 A | 11.44 | 12.27 | 0.0018 | 0.0001 | 0.7099 |
| γ-Tocopherol, µg/g | 7.5 | 6.5 | 6.5 | 6.7 | 6.9 | 0.38 | 0.38 | 0.1417 | 0.6175 | 0.5566 |
| Retinol, µg/g | 16.6 b | 17.6 ba | 19.0 a | 16.5 B | 18.9 A | 0.46 | 0.52 | 0.0023 | 0.0001 | 0.0068 |
| Color Measurements | Dietary Treatment | Strain Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 1% | 3% | WL | RIR | SEM 1 | SEM 2 | 3p Treatment | p Strain | p Treatment × strain | |
| CIE L*4 | 61.9 a | 59.4 b | 56.1 c | 60.2 A | 58.0 B | 0.49 | 0.67 | 0.0001 | 0.0003 | 0.7554 |
| CIE a*5 | 2.3 c | 6.2 b | 12.5 a | 6.6 | 7.4 | 0.38 | 0.98 | 0.0001 | 0.1983 | 0.4754 |
| CIE b*6 | 32.7 b | 37.3 a | 36.8 a | 35.9 | 35.3 | 1.07 | 0.98 | 0.0103 | 0.7228 | 0.3616 |
| Chroma | 32.8 b | 37.8 a | 38.9 a | 36.7 | 36.3 | 1.07 | 1.05 | 0.0007 | 0.8008 | 0.3526 |
| hue | 90.2 a | 80.7 b | 71.1 c | 81.7 A | 79.6 B | 0.86 | 1.99 | 0.0001 | 0.0299 | 0.1625 |
| hue angle | 0.3 b | 1.4 a | 1.2 a | 0.7 | 1.2 | 0.23 | 0.23 | 0.0033 | 0.0648 | 0.0286 |
| a/b | 0.1 c | 0.2 b | 0.3 a | 0.2 B | 0.2 A | 0.02 | 0.04 | 0.0001 | 0.0300 | 0.1836 |
| Fatty acids | Dietary Treatment | Strain Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 1% | 3% | WL | RIR | SEM 1 | SEM 2 | 3p Treatment | p Strain | p Treatment × Strain | |
| C14:0 | 0.34 | 0.35 | 0.34 | 0.35 | 0.33 | 0.008 | 0.006 | 0.7279 | 0.2578 | 0.0047 |
| C14:1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.003 | 0.002 | 0.8628 | 0.1246 | 0.3097 |
| C15:1 | 0.01 | 0.01 | 0.01 | 0.01 B | 0.01 A | 0.000 | 0.000 | 0.9263 | 0.0010 | 0.4418 |
| C16:0 | 26.33 | 26.53 | 26.31 | 26.91 A | 25.87 B | 0.210 | 0.173 | 0.7570 | 0.0001 | 0.0864 |
| C16:1n-9 | 0.54 a | 0.47 b | 0.45 b | 0.44 B | 0.53 A | 0.014 | 0.013 | 0.0001 | 0.0001 | 0.1416 |
| C16:1n-7 | 2.46 | 2.51 | 2.57 | 2.44 B | 2.59 A | 0.069 | 0.055 | 0.7751 | 0.0253 | 0.5226 |
| C17:0 | 0.17 b | 0.18 b | 0.20 a | 0.17 B | 0.19 A | 0.003 | 0.004 | 0.0001 | 0.0001 | 0.2363 |
| C17:1 | 0.12 b | 0.12 b | 0.14 a | 0.11 B | 0.14 A | 0.002 | 0.002 | 0.0001 | 0.0001 | 0.3266 |
| C18:0 | 9.95 | 10.00 | 10.05 | 10.69 A | 9.30 B | 0.164 | 0.133 | 0.9654 | 0.0001 | 0.2591 |
| C18:1n-9 | 39.18 | 38.91 | 39.45 | 38.89 | 39.46 | 0.375 | 0.317 | 0.5502 | 0.3029 | 0.0248 |
| C18:1n-7 | 1.27 a | 1.12 b | 1.13 b | 1.09 B | 1.26 A | 0.040 | 0.035 | 0.0089 | 0.0005 | 0.1419 |
| C18:2n-6 | 15.03 | 15.18 | 14.78 | 14.32 B | 15.66 A | 0.190 | 0.155 | 0.3280 | 0.0001 | 0.3907 |
| C18:3n-6 | 0.14 | 0.15 | 0.16 | 0.15 | 0.15 | 0.005 | 0.004 | 0.2450 | 0.5470 | 0.2437 |
| C18:3n-3 | 0.24 | 0.24 | 0.25 | 0.22 B | 0.26 A | 0.005 | 0.004 | 0.7587 | 0.0001 | 0.4910 |
| C18:4n-3 | 0.07 | 0.07 | 0.07 | 0.07 B | 0.08 A | 0.002 | 0.002 | 0.4446 | 0.0001 | 0.1891 |
| C20:0 | 0.04 | 0.03 | 0.04 | 0.04 A | 0.03 B | 0.001 | 0.001 | 0.0901 | 0.0065 | 0.3448 |
| C20:1n-9 | 0.24 | 0.23 | 0.24 | 0.24 | 0.24 | 0.005 | 0.004 | 0.2545 | 0.5548 | 0.4459 |
| C20:3n-6 | 0.18 | 0.17 | 0.18 | 0.19 A | 0.17 B | 0.005 | 0.004 | 0.1654 | 0.0001 | 0.0602 |
| C20:4n-6 | 2.57 | 2.60 | 2.57 | 2.60 | 2.56 | 0.046 | 0.038 | 0.8378 | 0.3998 | 0.1817 |
| C20:5n-3 | 0.04 | 0.04 | 0.04 | 0.05 B | 0.04 A | 0.001 | 0.001 | 0.0937 | 0.0224 | 0.1254 |
| C22:1n-9 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.003 | 0.002 | 0.1448 | 0.1231 | 0.1275 |
| C22:4n-6 | 0.2 | 0.21 | 0.22 | 0.19 B | 0.23 A | 0.006 | 0.005 | 0.2220 | 0.0001 | 0.4521 |
| C22:5n-3 | 0.08 | 0.09 | 0.09 | 0.08 B | 0.10 A | 0.005 | 0.005 | 0.6841 | 0.0055 | 0.0052 |
| C22:6n-3 | 0.67 a | 0.65 ba | 0.61 b | 0.61 B | 0.67 A | 0.011 | 0.010 | 0.0047 | 0.0001 | 0.0989 |
| Fatty acids | Dietary Treatment | Strain Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 1% | 3% | WL | RIR | SEM 1 | SEM 2 | p Treatment 3 | p Strain | p Treatment × Strain | |
| ∑SAT 3 | 36.82 | 37.09 | 36.93 | 38.17 A | 35.73 B | 0.290 | 0.240 | 0.9159 | 0.0001 | 0.0691 |
| ∑MUFA 4 | 43.95 | 43.50 | 44.10 | 43.35 B | 44.35 A | 0.382 | 0.322 | 0.5468 | 0.0427 | 0.0309 |
| ∑PUFA 5 | 19.19 | 19.36 | 18.93 | 18.44 B | 19.88 A | 0.212 | 0.174 | 0.3453 | 0.0001 | 0.2573 |
| ∑n-6 6 | 18.12 | 18.31 | 17.91 | 17.46 B | 18.77 A | 0.204 | 0.168 | 0.3803 | 0.0001 | 0.2232 |
| ∑n-3 7 | 1.11 a | 1.09 ba | 1.06 b | 1.02 B | 1.15 A | 0.015 | 0.012 | 0.0539 | 0.0001 | 0.7162 |
| ∑n-6/∑n-3 8 | 16.39 | 16.83 | 17.01 | 17.11 A | 16.37 B | 0.205 | 0.173 | 0.1358 | 0.0034 | 0.0680 |
| Indices | ||||||||||
| Hypochol 9 | 2.19 | 2.17 | 2.19 | 2.10 B | 2.26 A | 0.028 | 0.023 | 0.8930 | 0.0001 | 0.0629 |
| Thrombogenic 10 | 0.72 | 0.72 | 0.72 | 0.75 A | 0.69 B | 0.007 | 0.006 | 0.7254 | 0.0001 | 0.2289 |
| Δ9−desat 11 | 0.52 | 0.52 | 0.52 | 0.51 B | 0.53 A | 0.004 | 0.003 | 0.7648 | 0.0001 | 0.0271 |
| Δ9−desat 14 12 | 0.15 | 0.14 | 0.15 | 0.14 B | 0.15 A | 0.004 | 0.003 | 0.4891 | 0.0107 | 0.9931 |
| Δ9−desat 16 13 | 0.02 a | 0.02 b | 0.02 b | 0.02 B | 0.02 A | 0.001 | 0.001 | 0.0003 | 0.0001 | 0.1409 |
| Δ9−desat 18 14 | 0.80 | 0.80 | 0.80 | 0.78 B | 0.81 A | 0.003 | 0.003 | 0.9643 | 0.0001 | 0.0582 |
| Δ9−desat 20 15 | 6.67 | 7.03 | 7.21 | 6.47 B | 7.47 A | 0.253 | 0.211 | 0.0968 | 0.0036 | 0.3511 |
| Δ5+Δ6-desat 16 | 0.20 | 0.19 | 0.20 | 0.20 A | 0.19 B | 0.003 | 0.002 | 0.7949 | 0.0005 | 0.9616 |
| Elongase 17 | 0.26 | 0.26 | 0.26 | 0.27 A | 0.25 B | 0.003 | 0.002 | 0.8933 | 0.0001 | 0.4822 |
| Elongase (18/16) 18 | 0.38 | 0.38 | 0.38 | 0.40 A | 0.36 B | 0.007 | 0.005 | 0.8841 | 0.0001 | 0.4679 |
| Thioesterase 19 | 79.32 | 77.07 | 77.72 | 78.04 | 78.04 | 1.493 | 1.272 | 0.8192 | 0.7171 | 0.0097 |
| Dietary Treatment | Strain Effect | SEM 1 | SEM 2 | 3p Treatment | p Strain | p Treatment × Strain | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 1% | 3% | WL | RIR | ||||||
| 0 min | 0.036b | 0.075a | 0.07a | 0.068 | 0.054 | 0.010 | 0.008 | 0.0209 | 0.2704 | 0.4981 |
| 60 min | 0.237 | 0.266 | 0.273 | 0.245 | 0.272 | 0.017 | 0.015 | 0.1658 | 0.2809 | 0.3688 |
| 90 min | 0.289 | 0.291 | 0.335 | 0.275 | 0.332 | 0.025 | 0.021 | 0.2338 | 0.0947 | 0.8427 |
| 180 min | 0.29 | 0.313 | 0.386 | 0.327 | 0.333 | 0.019 | 0.016 | 0.1173 | 0.9467 | 0.2935 |
| Variable y | Intercept | S.D. 1 | Slope | S.D. | Variable x | r | R 2 | p Linear |
|---|---|---|---|---|---|---|---|---|
| % Fat | 63.123 ± 67.819 ± −5.399 ± 1.567 ± 0.976 ± | 5.19 | −0.649 ± −4.826 ± 7.443 ± −0.002 ± 2.973 ± | 0.10 | Humidity | −0.72 | 51.4 | 0.0001 |
| CIE L* | 2.50 | 1.39 | Retinol | −0.46 | 21.0 | 0.0012 | ||
| CIE a* | 3.91 | 2.17 | Retinol | 0.49 | 23.7 | 0.0015 | ||
| Total TBARS | 0.14 | 0.00 | α-tocopherol | −0.42 | 17.3 | 0.0129 | ||
| Total TBARS | 0.11 | 1.26 | C22:5n-3 | 0.39 | 14.8 | 0.0245 | ||
| Total TBARS | 0.703 ± | 0.23 | 2.508 ± | 1.07 | C22:4n-6 | 0.38 | 14.6 | 0.0256 |
| SAT 2 | 41.469 ± | 1.62 | −2.542 ± | 0.90 | Retinol | −0.38 | 14.7 | 0.0072 |
| C18:0 | 13.422 ± | 0.84 | −1.931 ± | 0.47 | Retinol | −0.52 | 26.7 | 0.0002 |
| Δ9−des 18 3 | 0.7348 ± | 0.02 | 0.034 ± | 0.01 | Retinol | 0.47 | 21.8 | 0.0008 |
| Elongase 4 | 0.3148 ± | 0.01 | −0.031 ± | 0.01 | Retinol | −0.51 | 26.0 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, A.I.; de-Cara, A.; Rebolé, A.; Arija, I. Short-Term Spirulina (Spirulina platensis) Supplementation and Laying Hen Strain Effects on Eggs’ Lipid Profile and Stability. Animals 2021, 11, 1944. https://doi.org/10.3390/ani11071944
Rey AI, de-Cara A, Rebolé A, Arija I. Short-Term Spirulina (Spirulina platensis) Supplementation and Laying Hen Strain Effects on Eggs’ Lipid Profile and Stability. Animals. 2021; 11(7):1944. https://doi.org/10.3390/ani11071944
Chicago/Turabian StyleRey, Ana I., Almudena de-Cara, Almudena Rebolé, and Ignacio Arija. 2021. "Short-Term Spirulina (Spirulina platensis) Supplementation and Laying Hen Strain Effects on Eggs’ Lipid Profile and Stability" Animals 11, no. 7: 1944. https://doi.org/10.3390/ani11071944
APA StyleRey, A. I., de-Cara, A., Rebolé, A., & Arija, I. (2021). Short-Term Spirulina (Spirulina platensis) Supplementation and Laying Hen Strain Effects on Eggs’ Lipid Profile and Stability. Animals, 11(7), 1944. https://doi.org/10.3390/ani11071944