Ultrasound-Guided Lateral Transversus Abdominis Plane (TAP) Block in Rabbits: A Cadaveric Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
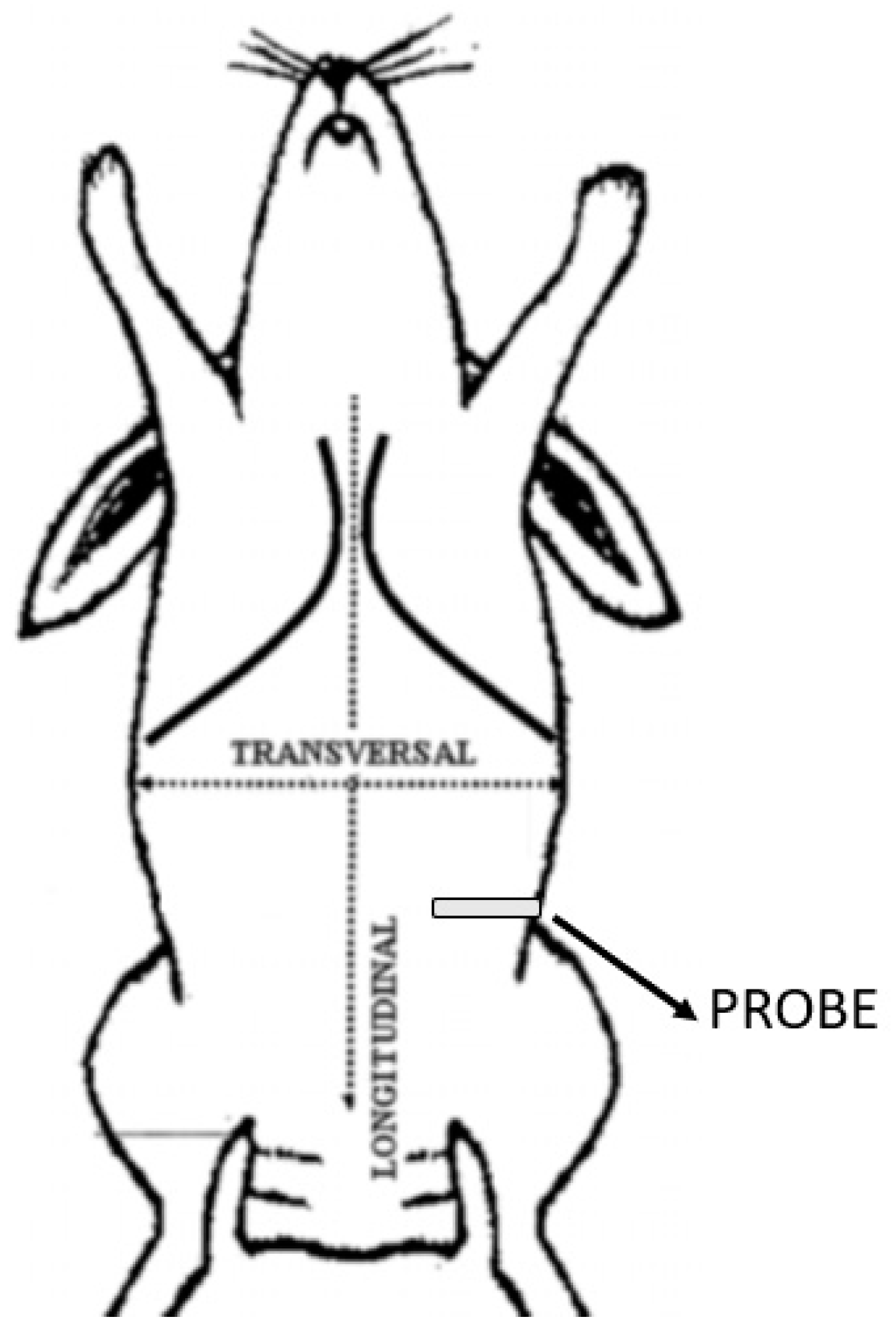
2.1. US-Guided TAP Block
2.2. Anatomical Dissection
3. Results
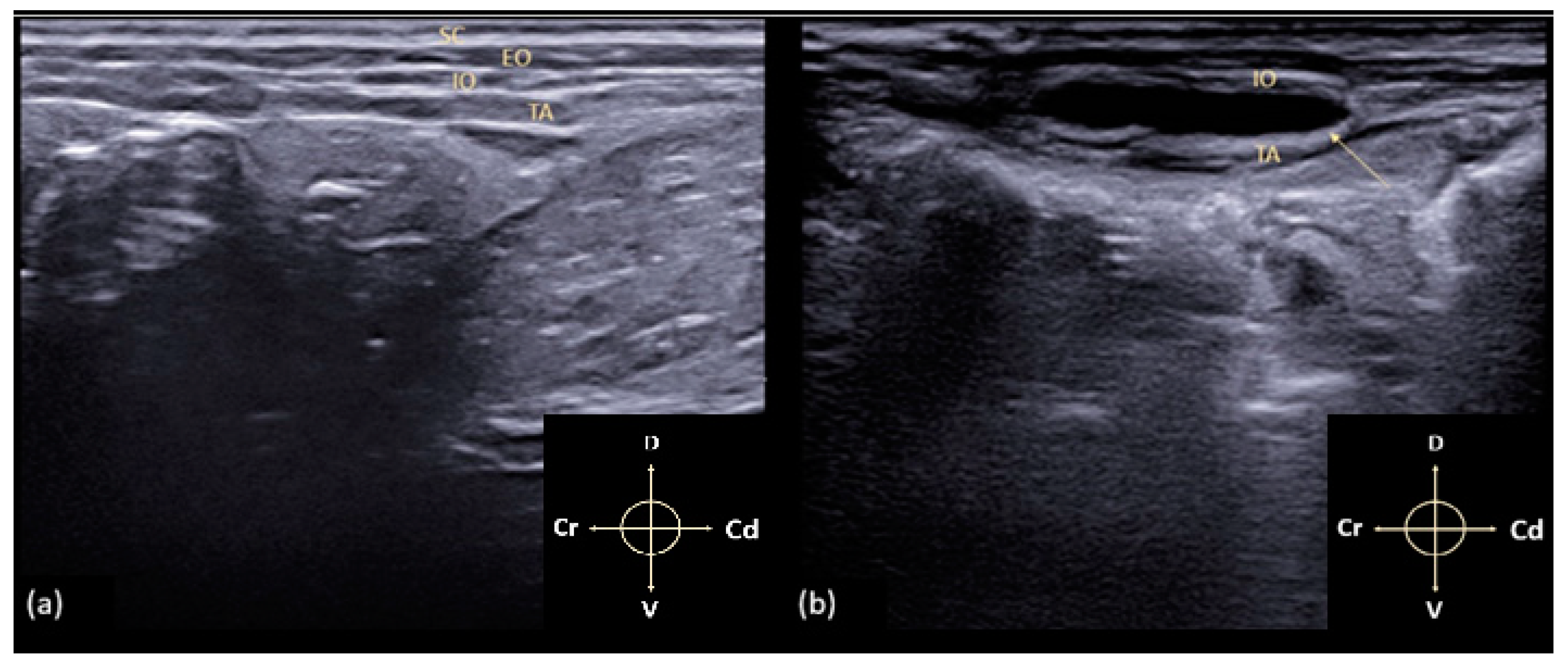
3.1. US-Guided TAP Block
3.2. Anatomical Dissection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonnell, J.G.; O’Donnell, B.D.; Farrell, T.; Gough, N.; Tuite, D.; Power, C.; Laffey, J.G. Transversus abdominis plane block: A cadaveric and radiological evaluation. RAMP J. 2007, 32, 399–404. [Google Scholar] [CrossRef]
- Barrington, M.J.; Ivanusic, J.J.; Rozen, W.M.; Hebbard, P. Spread of injectate after ultrasound-guided subcostal transversus abdominis plane block: A cadaveric study. Anaesthesia 2009, 64, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, K.; Xia, Y.; Zhang, X.; Papadimos, T.J.; Xu, X.; Wang, Q. Sensory assessment and regression rate of bilateral oblique subcostal transversus abdominis plane block in volunteers. RAMP J. 2018, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.A.; Schroeder, K.M.; Johnson, R.A. Transversus abdominis plane block for exploratory laparotomy in a Canadian lynx (Lynx canadensis). JZWM 2010, 41, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Herrera, F.E.; Buriticá-Gaviria, E.F.; Echeverry-Bonilla, D.F. Anatomical evaluation of the thoracolumbar nerves related to the transversus abdominis plane block technique in the dog. Anat. Histol. Embryol. 2017, 46, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mirra, A.; Von Rotz, A.; Schmidhalter, M.; Moser, L.; Casoni, D.; Spadavecchia, C. Ultrasound-guided lateral and subcostal transversus abdominis plane block in calves: A cadaveric study. Vet. Anaesth. Anal. 2018, 45, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, P.E.; Romano, M.; Zaccagnini, A.S.; Fuensalida, S.E.; Verdier, N.; Sanchez, F.; Portela, D.A. Transversus abdominis plane block in cat cadavers: Anatomical description and comparison of injectate spread using two-and three-point approaches. Vet. Anaesth. Analg. 2021, 48, 432–441. [Google Scholar] [CrossRef]
- Jankovic, Z.B.; Du Feu, F.M.; McConnell, P. An anatomical study of the transversus abdominis plane block: Location of the lumbar triangle of Petit and adjacent nerves. Anesth. Analg. 2009, 109, 981–985. [Google Scholar] [CrossRef]
- Küls, N.; Trujanovic, R.; Otero, P.E.; Larenza-Menzies, M.P. Ultrasound-Guided Transversus Abdominis Plane Block in Shetland Ponies: A Description of a Three-Point Injection Technique and Evaluation of Potential Analgesic Effects. J. Equine. Vet. Sci. 2020, 90, 102994. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Snyder, L.B.; Tearney, C.C.; Baker-Herman, T.L.; Schroeder, K.M. Ultrasound-guided transversus abdominis plane block in the dog: An anatomical evaluation. Vet. Anaesth. Analg. 2011, 38, 267–271. [Google Scholar] [CrossRef]
- Baldo, C.F.; Almeida, D.; Wendt-Hornickle, E.; Guedes, A. Transversus abdominis plane block in ponies: A preliminary anatomical study. Vet. Anaesth. Analg. 2018, 45, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Portela, D.A.; Romano, M.; Briganti, A. Retrospective clinical evaluation of ultrasound guided transverse abdominis plane block in dogs undergoing mastectomy. Vet. Anaesth. Analg. 2014, 41, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Yoshida, T.; Chuang, T.Y.; Yang, S.F.; Chang, C.C.; Yao, H.Y.; Chen, K.Y. Transversus abdominis plane block: An updated review of anatomy and techniques. BioMed Res. Int. 2017, 284363, 12. [Google Scholar] [CrossRef] [Green Version]
- Drożdżyńska, M.; Monticelli, P.; Neilson, D.; Viscasillas, J. Ultrasound-guided subcostal oblique transversus abdominis plane block in canine cadavers. Vet. Anaesth. Analg. 2017, 44, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.Q.; Bravo, D.; Leurcharusmee, P.; Neal, J.M. Transversus abdominis plane block: A narrative review. Anesthesia 2019, 131, 1166–1190. [Google Scholar] [CrossRef]
- Romano, M.; Portela, D.A.; Thomson, A.; Otero, P.E. Comparison between two approaches for the transversus abdominis plane block in canine cadavers. Vet. Anaesth. Analg. 2020, 48, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Bauquier, S.H.; Carter, J.E.; Whittem, T.; Beths, T. Two-point ultrasound-guided transversus abdominis plane injection in canine cadavers—A pilot study. Vet. Anaesth. Analg. 2018, 45, 871–875. [Google Scholar] [CrossRef]
- Freitag, F.V.; Muehlbauera, E.; Das Gaio, T.; Dos Santosa, A.; Machadoc, M.; Sanchezb, A.; Duque, C.J. Evaluation of injection volumes for the transversus abdominis plane block in dog cadavers: A preliminary trial. Vet. Anaesth. Analg. 2021, 48, 142–146. [Google Scholar] [CrossRef]
- Skouropoulou, D.; Lacitignola, L.; Centonze, P.; Simone, A.; Crovace, A.M.; Staffieri, F. Perioperative analgesic effects of an ultrasound-guided transversus abdominis plane block with a mixture of bupivacaine and lidocaine in cats undergoing ovariectomy. Vet. Anaesth. Analg. 2018, 45, 374–383. [Google Scholar] [CrossRef]
- IToh, T.; Kawabe, M.; Nagase, T.; KoIKe, T.; MIyoshI, M.; MIyahara, K. Measurements of body surface area and volume in laboratory rabbits (New Zealand White rabbits) using a computed tomography scanner. Exp. Anim. 2018, 67. [Google Scholar] [CrossRef] [Green Version]
- Aragón-Sánchez, J.; Quintana-Marrero, Y.; Aragón-Hernández, C.; Hernández-Herero, M.J. ImageJ: A Free, Easy, and Reliable Method to Measure Leg Ulcers Using Digital Pictures. Int. J. Low Extrem. Wounds 2017, 16, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, S.M.; Schroeder, K.M.; Baker-Herman, T.L.; Schroeder, C.A. Weight-based volume of injection influences cranial to caudal spread of local anesthetic solution in ultrasound-guided transversus abdominis plane blocks in canine cadavers. Vet. Surg. 2012, 41, 455–457. [Google Scholar] [CrossRef]
- Zoff, A.; Laborda-Vidal, P.; Mortier, J.; Amengual, M.; Rioja, E. Comparison of the spread of two different volumes of contrast medium when performing ultrasound-guided transversus abdominis plane injection in dog cadavers. J. Small Anim. Pract. 2017, 58, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Garbin, M.; Portela, A.P.; Bertolizio, G.; Gallastegui, A.; Otero, P.E. A novel ultrasound-guided lateral quadratus lumborum block in dogs: A comparative cadaveric study of two approaches. Vet. Anaesth. Analg. 2020, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]




| Measurement | Labdomen (mm) | Habdomen (mm) | CCspread (mm) | DVspread (mm) | Lmethyl (%) | Hmethyl (%) | Area Stained (mm2) |
|---|---|---|---|---|---|---|---|
| MEAN | 101.615 | 98.630 | 48.916 | 44.485 | 48.17 | 43.41 | 2515 |
| SD | 8.63 | 9.43 | 7.56 | 9.26 | 6.78 | 8.98 | 595.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, C.; Pennasilico, L.; Staffieri, F.; Serino, F.; Palumbo Piccionello, A. Ultrasound-Guided Lateral Transversus Abdominis Plane (TAP) Block in Rabbits: A Cadaveric Study. Animals 2021, 11, 1953. https://doi.org/10.3390/ani11071953
Di Bella C, Pennasilico L, Staffieri F, Serino F, Palumbo Piccionello A. Ultrasound-Guided Lateral Transversus Abdominis Plane (TAP) Block in Rabbits: A Cadaveric Study. Animals. 2021; 11(7):1953. https://doi.org/10.3390/ani11071953
Chicago/Turabian StyleDi Bella, Caterina, Luca Pennasilico, Francesco Staffieri, Federica Serino, and Angela Palumbo Piccionello. 2021. "Ultrasound-Guided Lateral Transversus Abdominis Plane (TAP) Block in Rabbits: A Cadaveric Study" Animals 11, no. 7: 1953. https://doi.org/10.3390/ani11071953







