Molecular and Cellular Responses of DNA Methylation and Thioredoxin System to Heat Stress in Meat-Type Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Determination of DNA Methylation (ng/mL)
2.3. Gene Expression of the Thioredoxin System
2.4. Determination of Thioredoxin Reductase Activity (µmol/min/mL)
2.5. Determination of Peroxiredoxin1 Levels (pg/mL)
2.6. Statistical Analysis
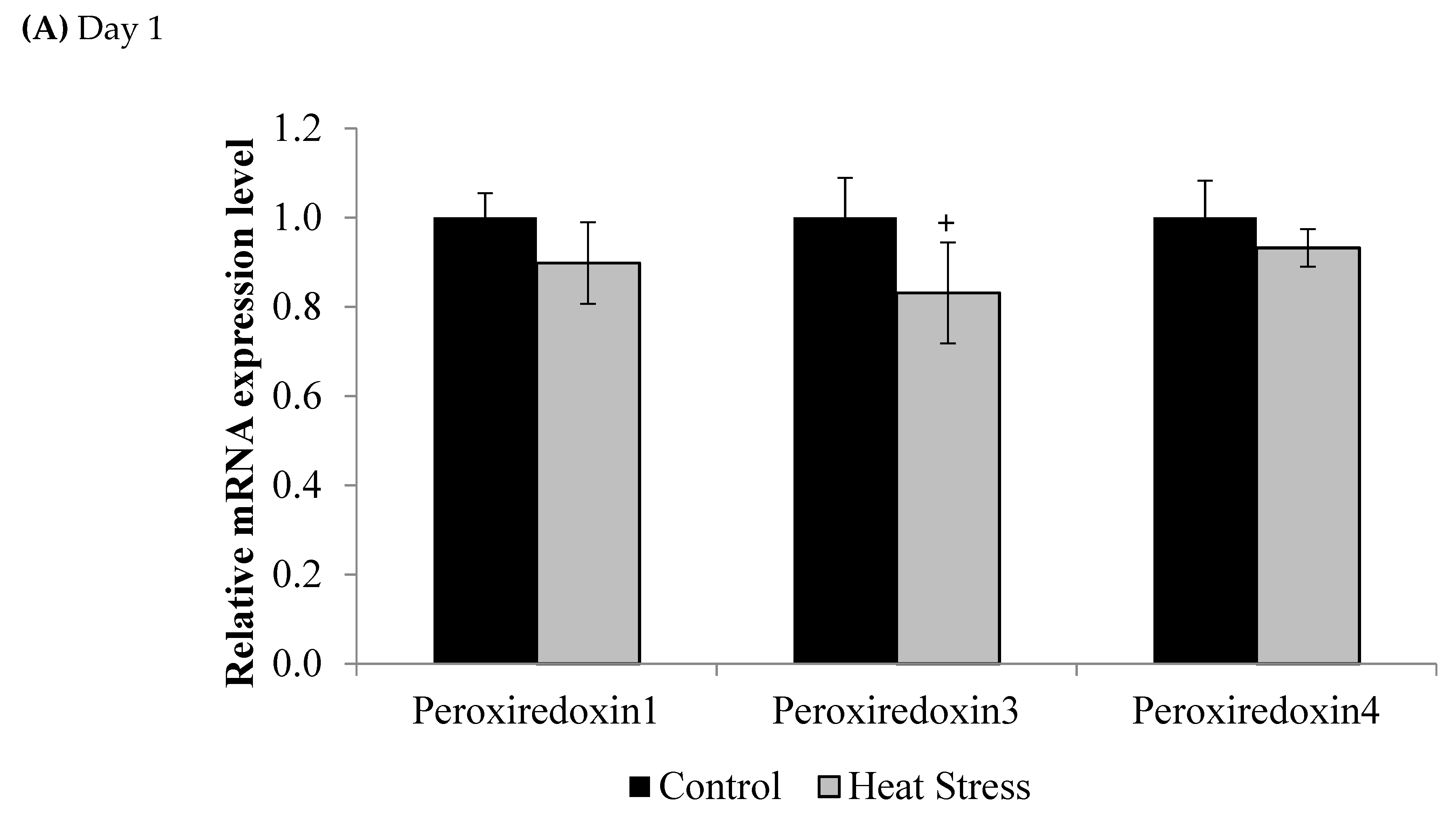
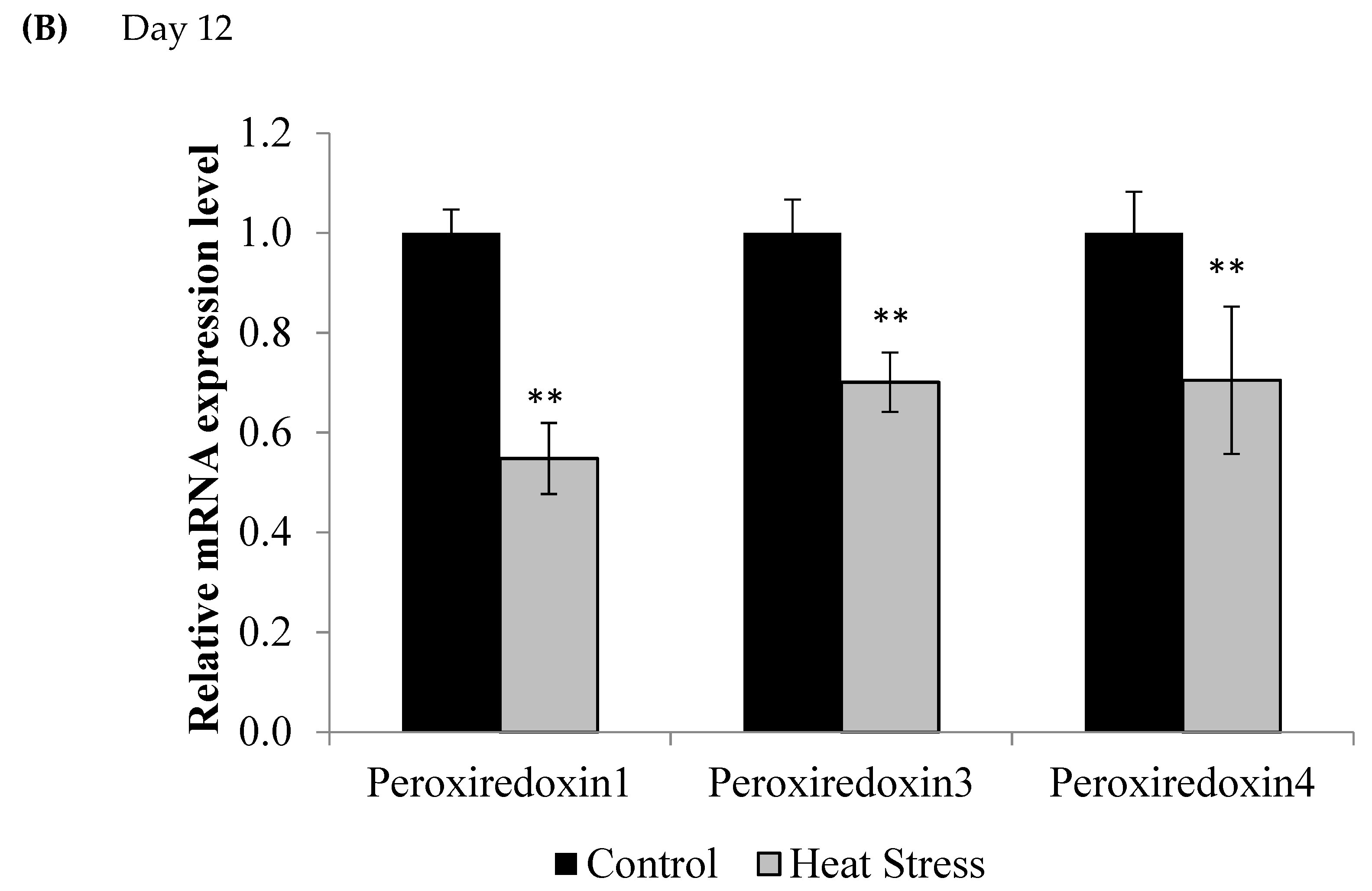
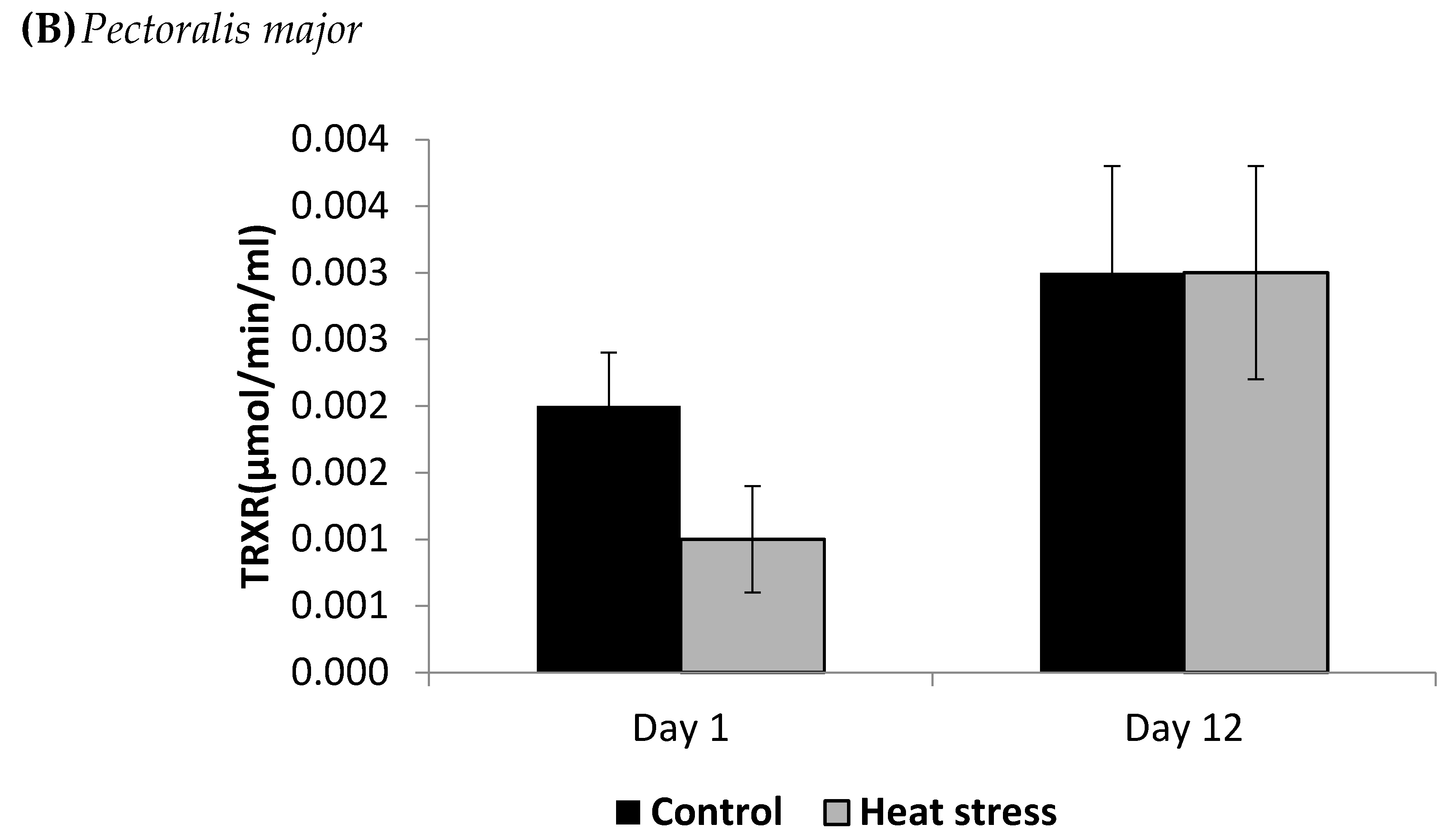
3. Results
4. Discussion
4.1. Heat Stress and Expression of the Thioredoxin System
4.2. Heat Stress and Cellular Activity of the Thioredoxin System
4.3. Heat Stress and DNA Methylation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Habashy, W.S.; Milfort, M.C.; Adomako, K.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult. Sci. 2017, 96, 2312–2319. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018, 45, 389–394. [Google Scholar] [CrossRef]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat stress impacts on broiler performance, a systematic review and meta-analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Akiba, Y.; Toyomizu, M. Acute heat stress induces oxidative stress and decrease adaptation in young White Leghorn cockerels by down regulation of avian uncoupling protein. Poult. Sci. 2007, 86, 364–371. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. Int. J. Biometeorol. 2019, 63, 1569–1584. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Malakhova, L.V.; Masalimov, Z.K.; Chernikov, A.V. Heat induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002, 30, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Ganaie, A.H.; Shanker, G.; Bumla, N.A.; Ghasura, R.S.; Mir, N.A. Biochemical and physiological changes during thermal stress in bovines. J. Vet. Sci. Technol. 2013, 4, 126. [Google Scholar]
- Davies, K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–13. [Google Scholar] [PubMed]
- Cerda, S.; Weitzman, S.A. Influence of oxygen radical injury on DNA methylation. Mutat. Res. 1997, 386, 141–152. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics, a landscape takes shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [Green Version]
- García-Guede, Á.; Vera, O.; Ibáñez-de-Caceres, I. When Oxidative Stress Meets Epigenetics, Implications in Cancer Development. Antioxidants 2020, 9, 468. [Google Scholar] [CrossRef]
- Murphy, M.P. Mitochondrial thiols in antioxidant protection and redox signaling, distinct roles for glutathionylation and other thiol modifications. Antioxid. Redox Signal. 2012, 16, 476–495. [Google Scholar] [CrossRef]
- Mailloux, R.J.; McBride, S.L.; Harper, M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013, 38, 592–602. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pirson, M.; Clippe, A.; Knoops, B. The curious case of peroxiredoxin-5, what its absence in aves can tell us and how it can be used. BMC Evol. Biol. 2018, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Ghareeb, H.; Metanis, N. The Thioredoxin System, A Promising Target for Cancer Drug Development. Chemistry 2020, 26, 10175–10184. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Nanri, H.; Yoshitake, S.; Nagata-Kuno, K.; Minakami, S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur. J. Biochem. 1992, 209, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.H.; Arscott, L.D.; Müller, S.; Lennon, B.W.; Ludwig, M.L.; Wang, P.F.; Veine, D.M.; Becker, K.; Schirmer, R.H. Thioredoxin reductase two modes of catalysis have evolved. Eur. J. Biochem. 2000, 267, 6110–6117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS® User’s Guide, version 9.4; Statistics; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Rimoldi, S.; Lasagna, E.; Sarti, F.M.; Marelli, S.P.; Cozzi, M.C.; Bernardini, G.; Terova, G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene 2015, 31, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and thioredoxin target proteins, from molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailloux, R.J. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid. Med. Cell Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef]
- Lopert, P.; Patel, M. Brain mitochondria from DJ-1 knockout mice show increased respiration-dependent hydrogen peroxide consumption. Redox Biol. 2014, 2, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Gao, J.; Zhu, Z.; Xiong, Y.; Liu, J. Mitochondrial Dysfunction Enhances Susceptibility to Oxidative Stress by Down-Regulation of Thioredoxin in Human Neuroblastoma Cells. Neurochem. Res. 2008, 33, 43–50. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins, Guardians Against Oxidative Stress and Modulators of Peroxide Signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.W.; Chae, H.Z.; Seo, M.S.; Kim, K.; Baines, I.C.; Rhee, S.G. Mammalian peroxidoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor- alpha. J. Biol. Chem. 1998, 273, 6297–6302. [Google Scholar] [CrossRef] [PubMed]
- Nonn, L.; Berggren, M.; Powis, G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol. Cancer Res. 2003, 1, 682–689. [Google Scholar]
- Hwang, I.K.; Yoo, K.Y.; Kim, D.W.; Lee, C.H.; Choi, J.H.; Kwon, Y.G.; Kim, Y.M.; Choi, S.Y.; Won, M.H. Changes in the expression of mitochondrial peroxiredoxin and thioredoxin in neurons and glia and their protective effects in experimental cerebral ischemic damage. Free Radic. Biol. Med. 2010, 48, 1242–1251. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizy, M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS ONE 2013, 8, e64412. [Google Scholar] [CrossRef]
- Gao, F.; Luo, Y.; Li, S.; Li, J.; Lin, L.; Nielsen, A.l.; Sorensen, C.B.; Vaita, G.; Wang, J.; Zhang, X.; et al. Comparison of gene expression and genome-wide DNA methylation profiling between phenotypically normal cloned pigs and conventionally bred controls. PLoS ONE 2011, 6, e25901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalingaiah, P.K.; Ponnusamy, L.; Singh, K.P. Oxidative stress-induced epigenetic changes associated with malignant transformation of human kidney epithelial cells. Oncotarget 2017, 8, 11127–11143. [Google Scholar] [CrossRef] [PubMed]
- Mordaunt, C.E.; Shibata, N.M.; Kieffer, D.A.; Czlonkowska, A.; Litwin, T.; Weiss, K.H.; Gotthardt, D.N.; Olson, K.; Wei, D.; Cooper, S.; et al. Epigenetic changes of the thioredoxin system in the tx-j mouse model and in patients with Wilson disease. Hum. Mol. Genet. 2018, 27, 3854–3869. [Google Scholar] [CrossRef] [PubMed]





| Gene Name and Symbol | Gene Bank Accession Number | Product Size (bp) | Primer Sequence | |
|---|---|---|---|---|
| Thioredoxin (TXN) | NM_205453.1 | 82 | Forward | 5′CATGCCAACATTCCAGTTCTAC 3′ |
| Reverse | 5′GGTCTCTTCCAGCTTCTCTTT 3′ | |||
| Thioredoxin reductase1 (TXNRD1) | NM_001030762.2 | 126 | Forward | 5′AGAGCATGACCCAGCTTTATT 3′ |
| Reverse | 5′GTGTGAAGGAAGCCTCAGTATC 3′ | |||
| Peroxiredoxin 1 (PRDX1) | NM_001271932.1 | 84 | Forward | 5′GTACAGTGACAGAGCTGATGAA 3′ |
| Reverse | 5′GCAAGGTGACAGAAGTGAGA3′ | |||
| Peroxiredoxin 3 (PRDX3) | XM_426543.5 | 95 | Forward | 5′GGAAATACCTCGTGCTCTTCTT 3′ |
| Reverse | 5′GTGGAACTCATTCGCTTTGTTAC 3′ | |||
| Peroxiredoxin 4 (PRDX4) | XM_416800.5 | 100 | Forward | 5′GGACTCGGACCAATGAAGATT3′ |
| Reverse | 5′CCCTAAGTGCATGTCCTTGAT 3′ | |||
| β-actin | NM_205518.1 | 125 | Forward | 5′AGACATCAGGGTGTGATGGTTGGT3′ |
| Reverse | 5′TCCCAGTTGGTGACAATACCGTGT3′ | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Molecular and Cellular Responses of DNA Methylation and Thioredoxin System to Heat Stress in Meat-Type Chickens. Animals 2021, 11, 1957. https://doi.org/10.3390/ani11071957
Habashy WS, Milfort MC, Rekaya R, Aggrey SE. Molecular and Cellular Responses of DNA Methylation and Thioredoxin System to Heat Stress in Meat-Type Chickens. Animals. 2021; 11(7):1957. https://doi.org/10.3390/ani11071957
Chicago/Turabian StyleHabashy, Walid S., Marie C. Milfort, Romdhane Rekaya, and Samuel E. Aggrey. 2021. "Molecular and Cellular Responses of DNA Methylation and Thioredoxin System to Heat Stress in Meat-Type Chickens" Animals 11, no. 7: 1957. https://doi.org/10.3390/ani11071957
APA StyleHabashy, W. S., Milfort, M. C., Rekaya, R., & Aggrey, S. E. (2021). Molecular and Cellular Responses of DNA Methylation and Thioredoxin System to Heat Stress in Meat-Type Chickens. Animals, 11(7), 1957. https://doi.org/10.3390/ani11071957







